Anti-Photoaging Effects of Antioxidant Peptide from Seahorse (Hippocampus abdominalis) in In Vivo and In Vitro Models
Abstract
:1. Introduction
2. Results
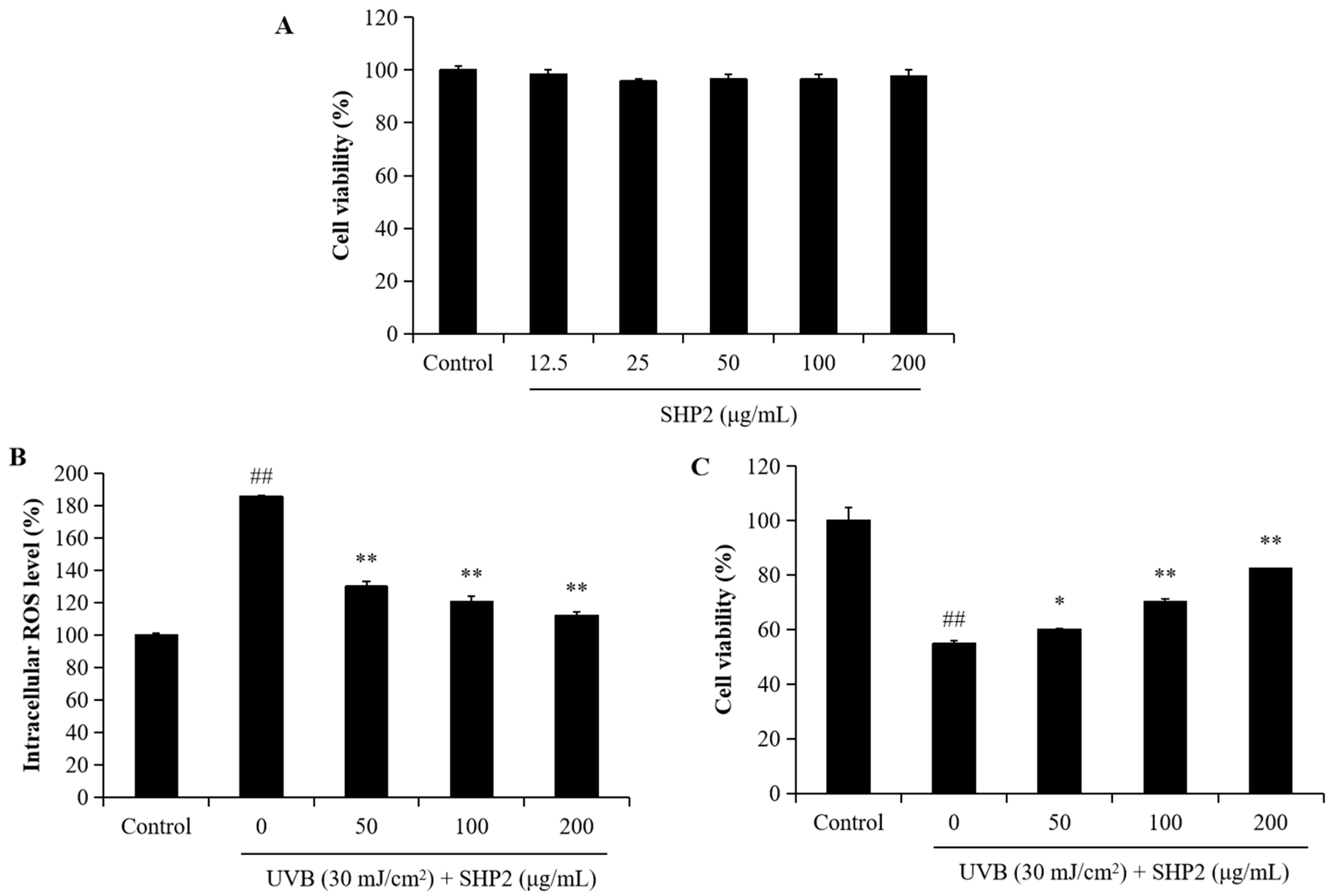
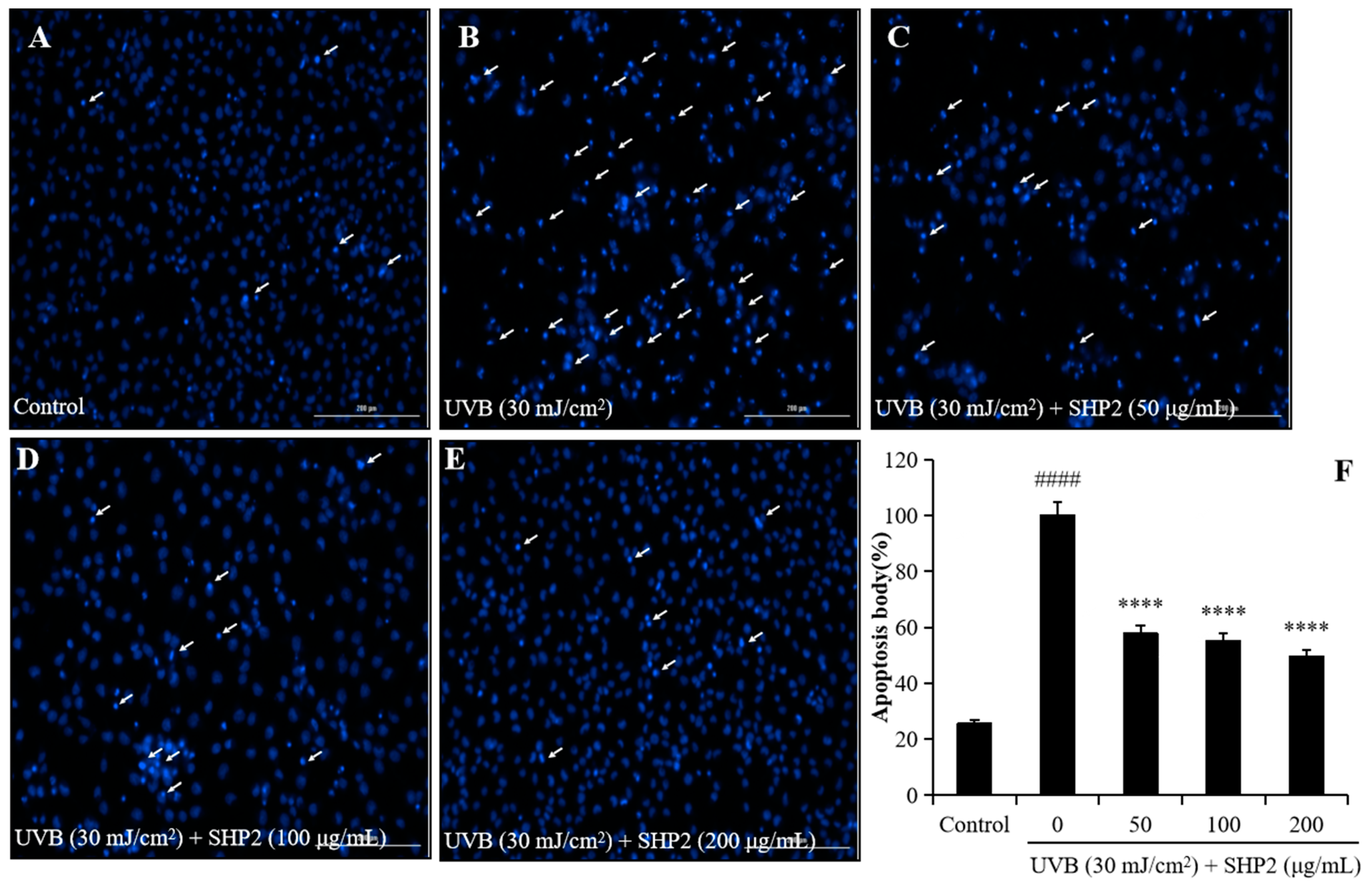
2.1. Protective Effect of SHP2 against UVB-Irradiated HaCaT Cell Skin Damage
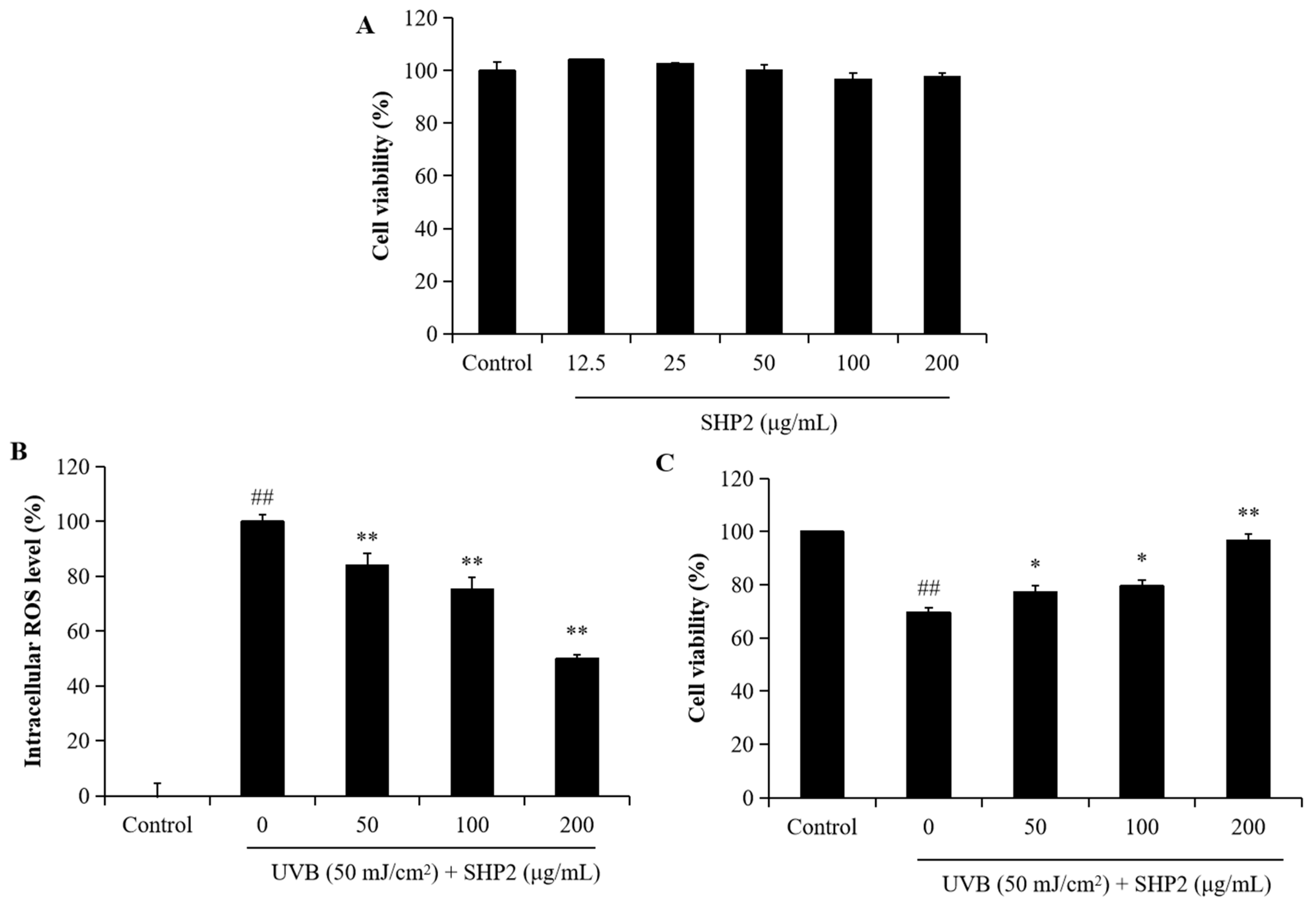
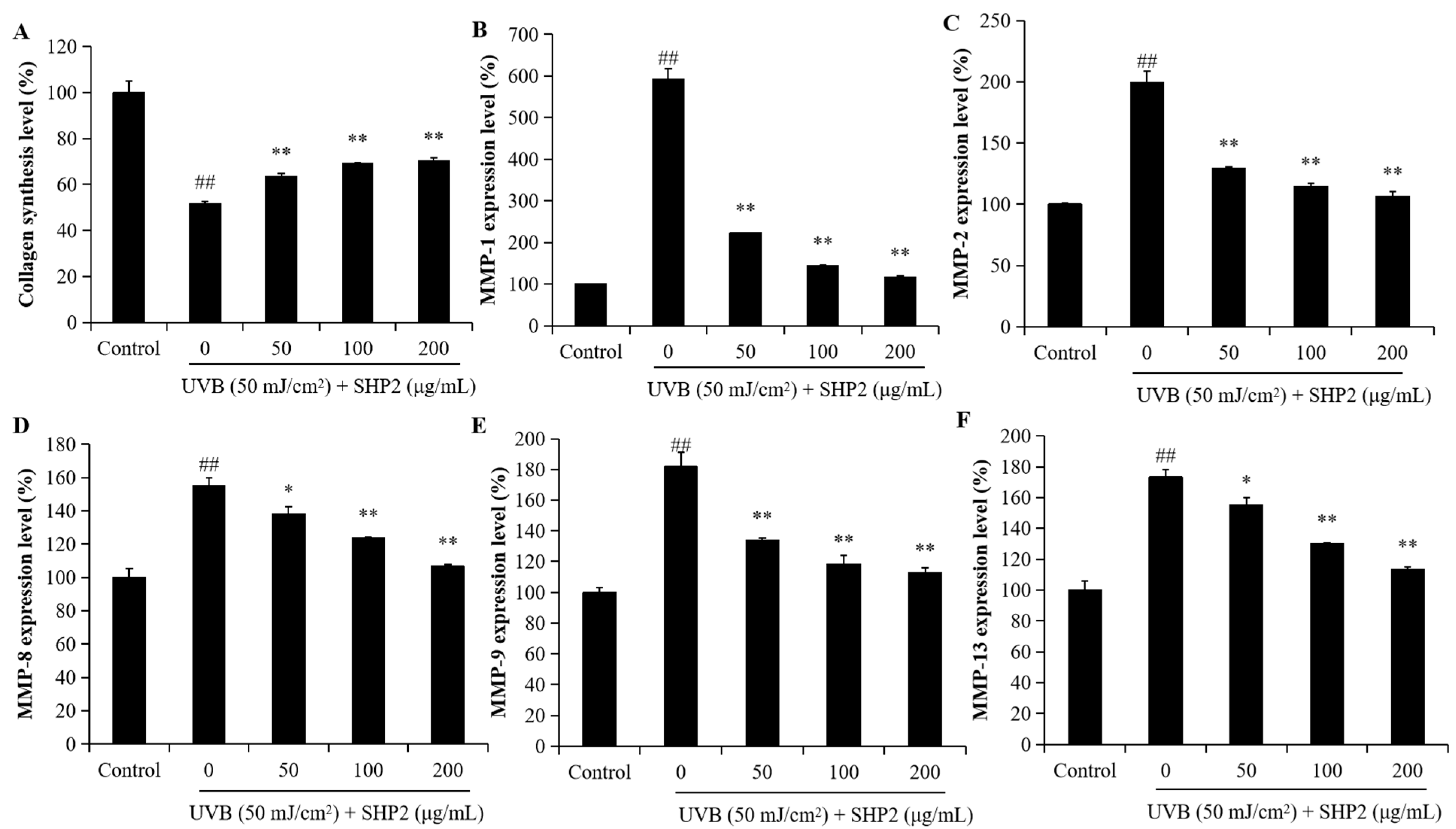
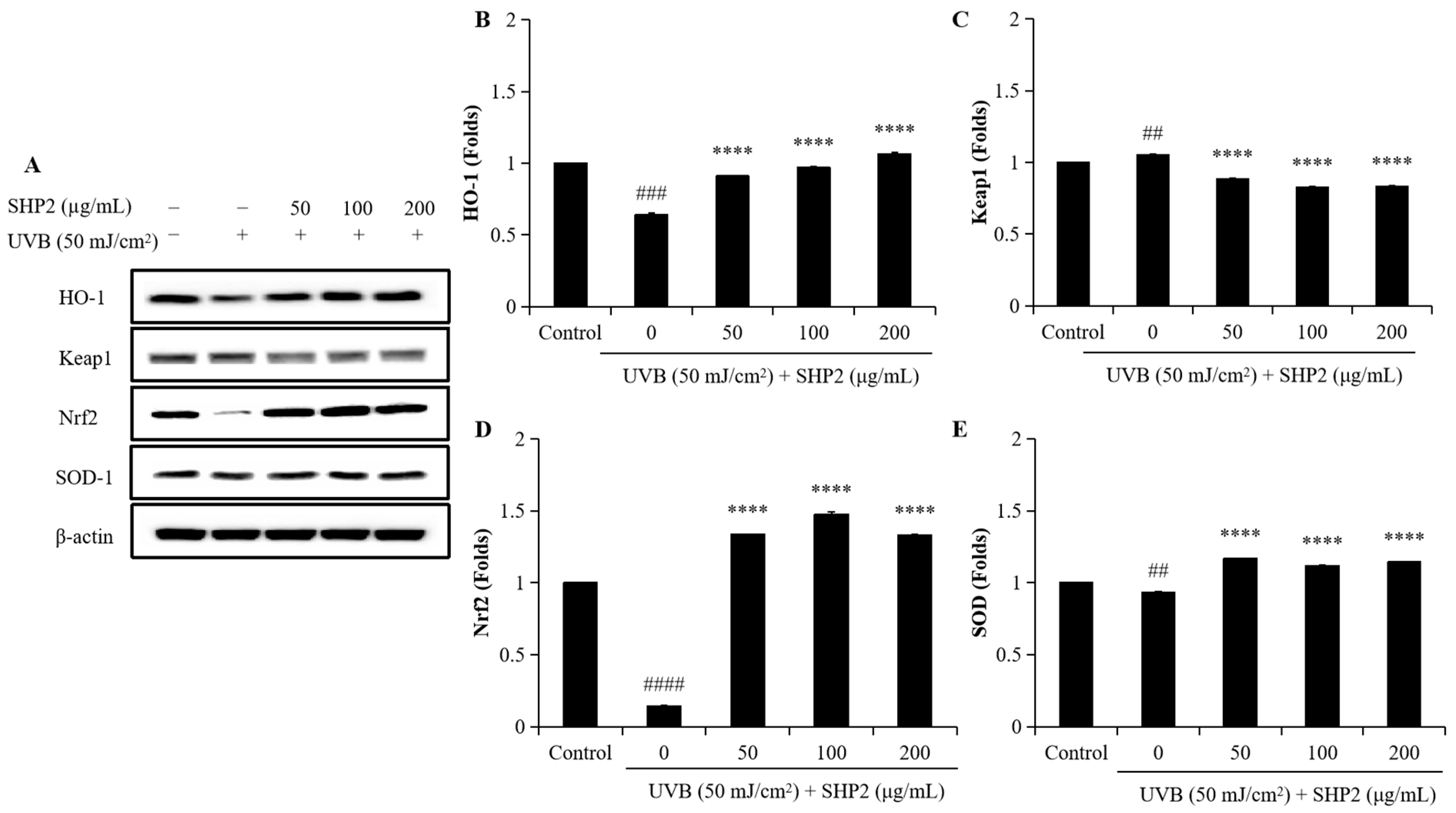
2.2. Protective Effect of SHP2 against UVB-Induced HDF Cell Model
2.3. Protective Effect of SHP2 in UVB-Irradiated Zebrafish Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Purification of Bioactive Peptides from H. abdominalis
4.3. Cell Culture and Cell Viability Analysis
4.4. Determination of the Anti-UVB-Induced Skin Damage Effects on HaCaT Cells
4.5. Determination of the Anti-UVB-Induced Skin Damage Effects on HDF Cells
4.6. Western Blotting
4.7. In Vivo Studies in Zebrafish
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loo, Y.C.; Hu, H.-C.; Yu, S.-Y.; Tsai, Y.-H.; Korinek, M.; Wu, Y.-C.; Chang, F.-R.; Chen, Y.-J. Development on potential skin anti-aging agents of Cosmos caudatus Kunth via inhibition of collagenase, MMP-1 and MMP-3 activities. Phytomedicine 2023, 110, 154643. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Yang, J.H.; Lee, J.H.; Kim, K.M.; Cho, S.S.; Baek, J.S.; Kim, J.M.; Choi, M.-H.; Shin, H.-J.; Ki, S.H. Protective Effect of Castanopsis sieboldii Extract against UVB-Induced Photodamage in Keratinocytes. Molecules 2023, 28, 2842. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hyun, J.; Nagahawatta, D.P.; Kim, Y.M.; Heo, M.-S.; Jeon, Y.-J. Cosmeceutical Effects of Ishige okamurae Celluclast Extract. Antioxidants 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Geng, R.; Kang, S.-G.; Li, X.; Huang, K. Revitalizing Photoaging Skin through Eugenol in UVB-Exposed Hairless Mice: Mechanistic Insights from Integrated Multi-Omics. Antioxidants 2024, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Domingues, M.R.; Calado, R. Marine Animal Co-Products—How Improving Their Use as Rich Sources of Health-Promoting Lipids Can Foster Sustainability. Mar. Drugs 2024, 22, 73. [Google Scholar] [CrossRef]
- Abdo, A.A.A.; Al-Dalali, S.; Hou, Y.; Aleryani, H.; Shehzad, Q.; Asawmahi, O.; Al-Farga, A.; Mohammed, B.; Liu, X.; Sang, Y. Modification of Marine Bioactive Peptides: Strategy to Improve the Biological Activity, Stability, and Taste Properties. Food Bioprocess Technol. 2023, 17, 1412–1433. [Google Scholar] [CrossRef]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryu, B.; Cui, Y.; Li, C.; Zhou, C.; Hong, P.; Lee, B.; Qian, Z.-J. A peptide isolated from Hippocampus abdominalis improves exercise performance and exerts anti-fatigue effects via AMPK/PGC-1α pathway in mice. J. Funct. Food. 2019, 61, 103489. [Google Scholar] [CrossRef]
- Li, K.; Yan, L.; Zhang, Y.; Yang, Z.; Zhang, C.; Li, Y.; Kalueff, A.V.; Li, W.; Song, C. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J. Ethnopharmacol. 2020, 250, 112487. [Google Scholar] [CrossRef]
- Lee, H.-G.; Nagahawatta, D.P.; Yang, F.; Jayawardhana, H.H.A.C.K.; Liyanage, N.M.; Lee, D.-S.; Lee, J.M.; Yim, M.-J.; Ko, S.-C.; Kim, J.-Y.; et al. Antioxidant potential of hydrolysate-derived seahorse (Hippocampus abdominalis) peptide: Protective effects against AAPH-induced oxidative damage in vitro and in vivo. Food Chem. 2023, 407, 135130. [Google Scholar] [CrossRef]
- Kodagoda, Y.K.; Liyanage, D.S.; Omeka, W.K.M.; Kim, G.; Kim, J.; Lee, J. Identification, expression profiling, and functional characterization of cystatin C from big-belly seahorse (Hippocampus abdominalis). Fish Shellfish Immunol. 2023, 138, 108804. [Google Scholar] [CrossRef] [PubMed]
- Wijerathna, H.M.S.M.; Nadarajapillai, K.; Udayantha, H.M.V.; Kasthuriarachchi, T.D.W.; Shanaka, K.A.S.N.; Kwon, H.; Wan, Q.; Lee, J. Molecular delineation, expression profiling, immune response, and anti-apoptotic function of a novel clusterin homolog from big-belly seahorse (Hippocampus abdominalis). Fish Shellfish Immunol. 2022, 124, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Je, J.-G.; Ryu, B.; Kang, N.; Shanura Fernando, I.P.; Jayawardena, T.U.; Asanka Sanjeewa, K.K.; Oh, J.-Y.; Lee, T.-G.; Jeon, Y.-J. Antioxidant and angiotensin-I converting enzyme inhibitory peptides from Hippocampus abdominalis. Eur. Food Res. Technol. 2019, 245, 479–487. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, S.-Y.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Wang, L.; Lee, S.-H.; Ko, S.-C.; Kang, M.C.; Jayawardena, T.U.; Jeon, Y.-J. Free radical scavenging activity of the peptide from the Alcalase hydrolysate of the edible aquacultural seahorse (Hippocampus abdominalis). J. Food Biochem. 2019, 43, e12833. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, J.; Du, R.; Gao, Y.; Zhao, P. Collagen study advances for photoaging skin. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12931. [Google Scholar] [CrossRef]
- Yang, F.; Nagahawatta, D.P.; Yang, H.-W.; Ryu, B.; Lee, H.-G.; Je, J.-G.; Heo, M.-S.; Jeon, Y.-J. In vitro and in vivo immuno-enhancing effect of fucoidan isolated from non-edible brown seaweed Sargassum thunbergii. Int. J. Biol. Macromol. 2023, 253, 127212. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y.-J. Antioxidant and anti-photoaging effects of a fucoidan isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 303. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a New Light on Skin Aging, Iron- and Redox-Homeostasis and Emerging Natural Antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, B.; Zhao, Y.; Wang, N. Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs 2023, 21, 144. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.-B.; Je, J.-Y. Cytoprotective Role of Edible Seahorse (Hippocampus abdominalis)-Derived Peptides in H2O2-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Mar. Drugs 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-G.; Kim, H.-S.; Lee, H.-G.; Oh, J.-Y.; Lu, Y.A.; Wang, L.; Rho, S.; Jeon, Y.-J. Low-molecular weight peptides isolated from seahorse (Hippocampus abdominalis) improve vasodilation via inhibition of angiotensin-converting enzyme in vivo and in vitro. Process Biochem. 2020, 95, 30–35. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.-M.; Cai, W.-W.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, J.; Qin, X.; Peng, Z.; Lin, H. Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Mar. Drugs 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme against Ultraviolet B-Induced Skin Damage by Regulating NF-κB, AP-1, and MAPKs Signaling Pathways In vitro in Human Dermal Fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Yang, H.-W.; Kim, H.S.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from a Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced photoaging in vitro in human keratinocytes and in vivo in zebrafish. Mar. Life Sci. Tech. 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Oh, C.; Park, C.-H.; Aliya, S.; Kim, H.-S.; Bajpai, V.K.; Huh, Y.S. Antioxidant and anti-aging potential of a peptide formulation (Gal 2–Pep) conjugated with gallic acid. RSC Adv. 2021, 11, 29407–29415. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Q.; Liu, Y.; Jin, L.; Peng, R. Research Progress on the Construction and Application of a Diabetic Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 5159. [Google Scholar] [CrossRef]
- Shimizu, N.; Shiraishi, H.; Hanada, T. Zebrafish as a Useful Model System for Human Liver Disease. Cells 2023, 12, 2246. [Google Scholar] [CrossRef]
- Tanveer, M.A.; Rashid, H.; Nazir, L.A.; Archoo, S.; Shahid, N.H.; Ragni, G.; Umar, S.A.; Tasduq, S.A. Trigonelline, a plant derived alkaloid prevents ultraviolet-B-induced oxidative DNA damage in primary human dermal fibroblasts and BALB/c mice via modulation of phosphoinositide 3-kinase-Akt-Nrf2 signalling axis. Exp. Gerontol. 2023, 171, 112028. [Google Scholar] [CrossRef] [PubMed]
- Santhakumaran, I.; Shanuja, S.K.; Narayanaswamy, R.; Gnanamani, A. Asperyellone prevents HDF cells from UVB irradiation damages: An elaborated study. J. Cell. Biochem. 2019, 120, 7560–7572. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Wang, K.; Ahn, G.; Cha, S.-H.; Jeon, Y.-J. Protective effect of the brown seaweed Padina boryana against UVB-induced photoaging in vitro in skin cells and in vivo in zebrafish. Algal Res. 2023, 76, 103316. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yang, Y.; Xiao, D.; Kim, P.; Lee, J.; Jeon, Y.-J.; Wang, L. Anti-Photoaging Effects of Antioxidant Peptide from Seahorse (Hippocampus abdominalis) in In Vivo and In Vitro Models. Mar. Drugs 2024, 22, 471. https://doi.org/10.3390/md22100471
Yang F, Yang Y, Xiao D, Kim P, Lee J, Jeon Y-J, Wang L. Anti-Photoaging Effects of Antioxidant Peptide from Seahorse (Hippocampus abdominalis) in In Vivo and In Vitro Models. Marine Drugs. 2024; 22(10):471. https://doi.org/10.3390/md22100471
Chicago/Turabian StyleYang, Fengqi, Yang Yang, Dandan Xiao, Poongho Kim, Jihee Lee, You-Jin Jeon, and Lei Wang. 2024. "Anti-Photoaging Effects of Antioxidant Peptide from Seahorse (Hippocampus abdominalis) in In Vivo and In Vitro Models" Marine Drugs 22, no. 10: 471. https://doi.org/10.3390/md22100471
APA StyleYang, F., Yang, Y., Xiao, D., Kim, P., Lee, J., Jeon, Y.-J., & Wang, L. (2024). Anti-Photoaging Effects of Antioxidant Peptide from Seahorse (Hippocampus abdominalis) in In Vivo and In Vitro Models. Marine Drugs, 22(10), 471. https://doi.org/10.3390/md22100471







