Characterization of Phytoplankton-Derived Amino Acids and Tracing the Source of Organic Carbon Using Stable Isotopes in the Amundsen Sea
Abstract
1. Introduction
2. Results
2.1. Hydrography
2.2. Phytoplankton Biomass and Structure
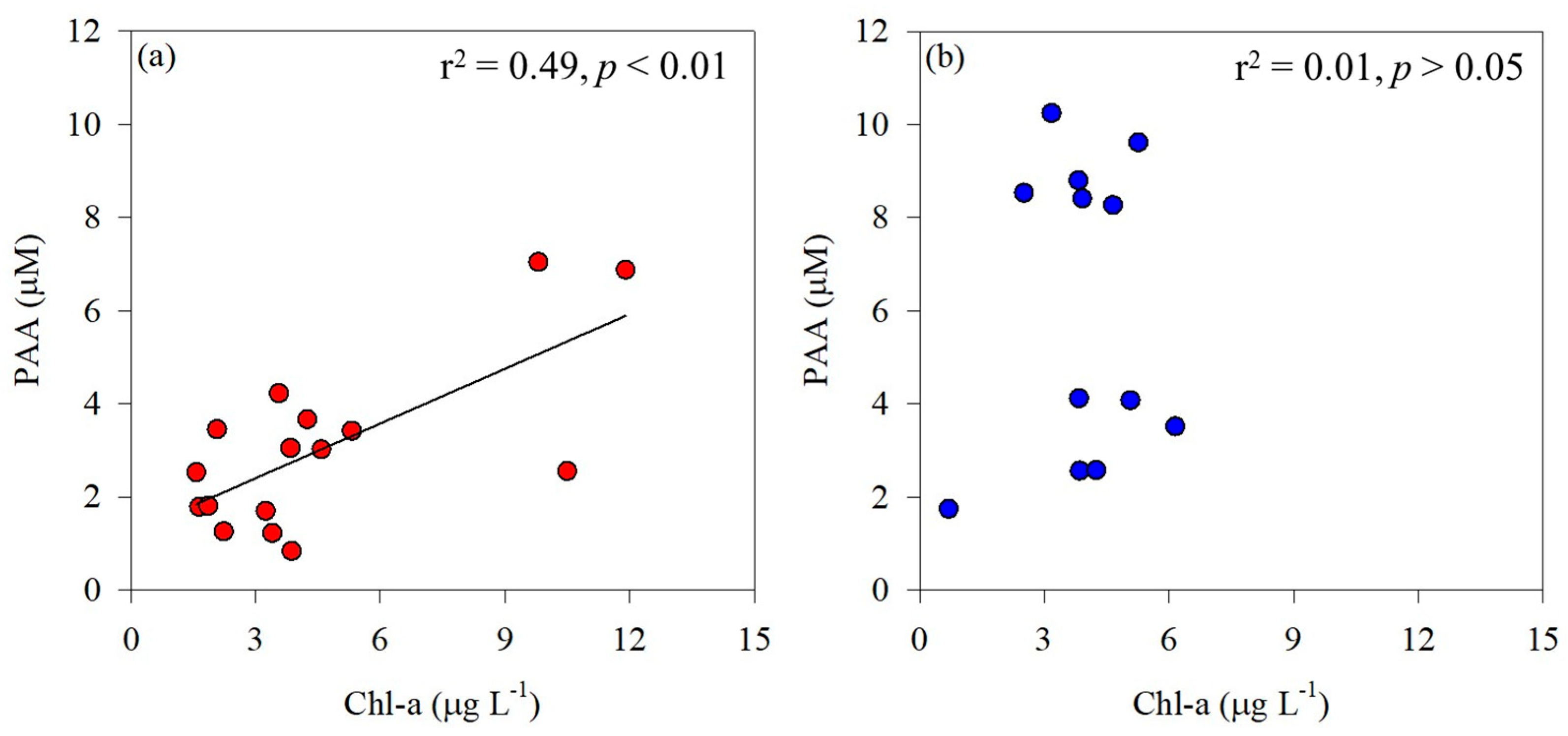
2.3. Chemical Parameters
2.4. Amino Acid Composition in Particulate Organic Matter
2.4.1. Amino Acid Composition
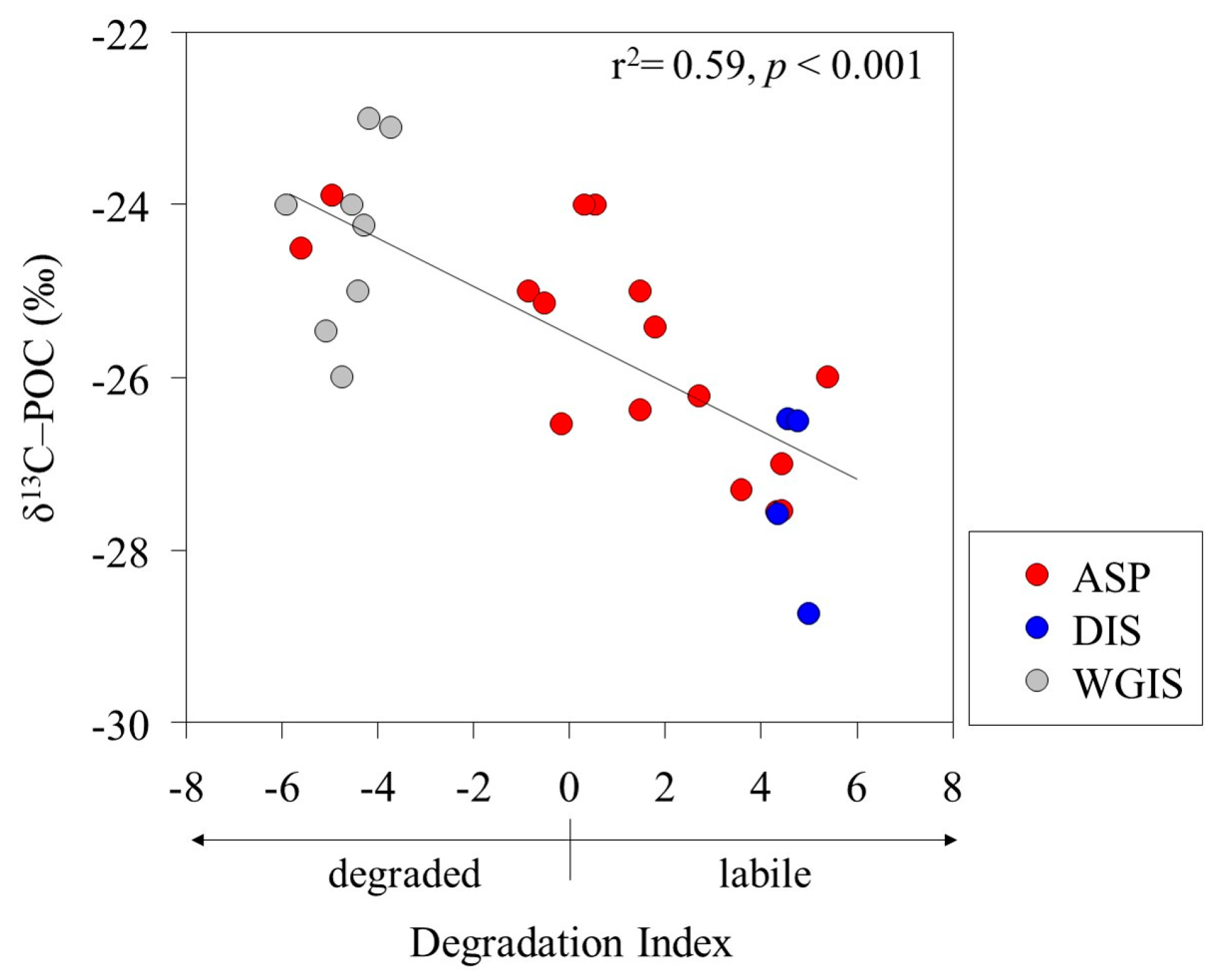
2.4.2. Indicators of Particulate Organic Matter Diagenesis
3. Discussion
3.1. Sources and Stable Isotope Characteristics of Particulate Organic Matter
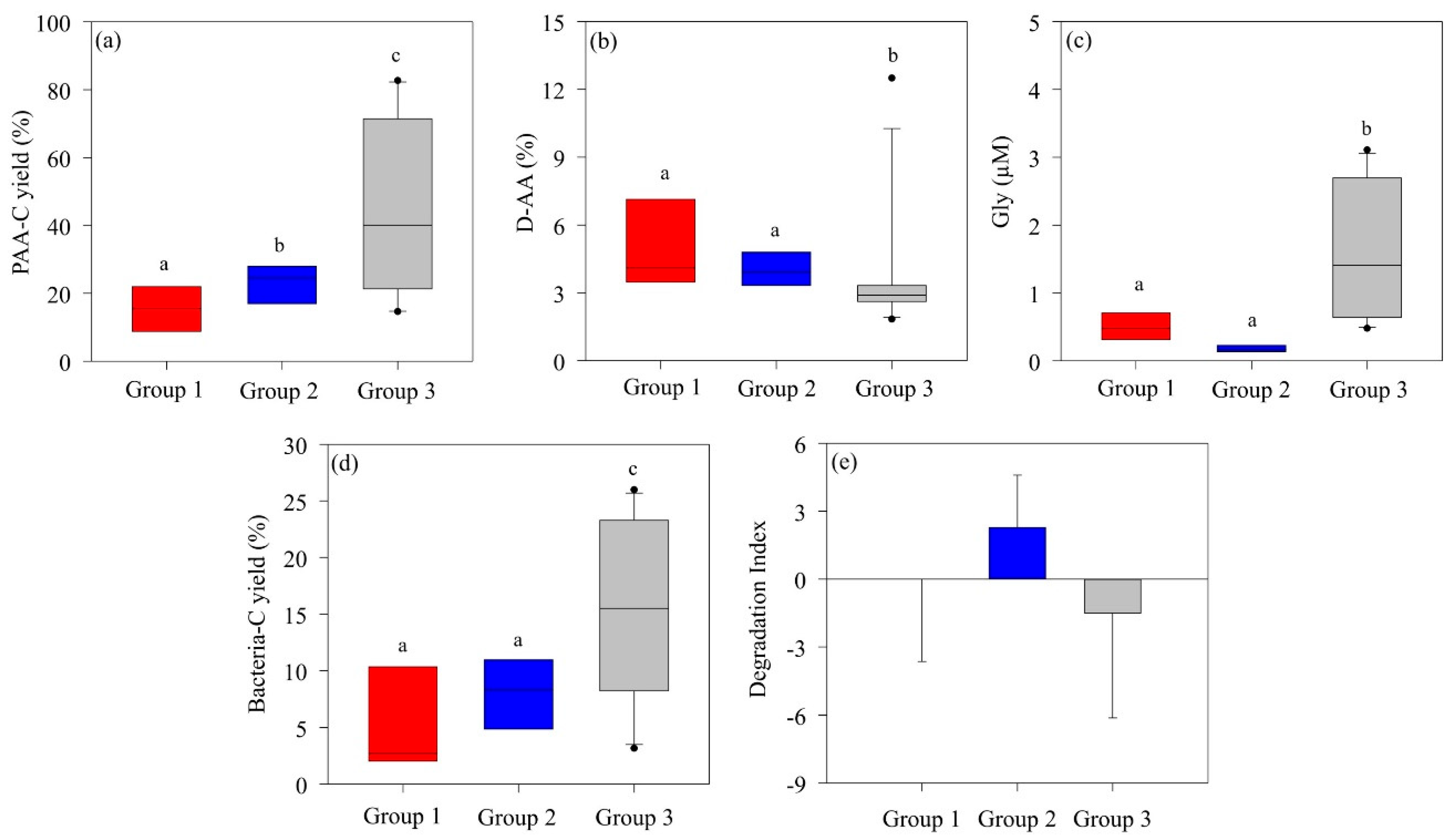
3.2. Amino Acid Composition by Phytoplankton Community
3.3. Tracing of DOM Sources Using Carbon Stable Isotopes
3.4. Influence of Phytoplankton Community Shifts on the Biological Carbon Pump
4. Materials and Methods
4.1. Study Area
4.2. Physico-Chemical Parameters
4.3. Stable Carbon Isotope Analysis
R = 13C/12C
4.4. Hydrolysis Particulate Amino Acid
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.; Kaiser, K.; Benner, R. Amino Acid and Amino Sugar Yields and Compositions as Indicators of Dissolved Organic Matter Diagenesis. Org. Geochem. 2009, 40, 343–352. [Google Scholar] [CrossRef]
- Gaye, B.; Lahajnar, N.; Harms, N.; Paul, S.A.L.; Rixen, T.; Emeis, K.C. What Can We Learn from Amino Acids about Oceanic Organic Matter Cycling and Degradation? Biogeosciences 2022, 19, 807–830. [Google Scholar] [CrossRef]
- Cowie, G.L.; Hedges, J.I. Sources and Reactivities of Amino Acids in a Coastal Marine Environment. Limnol. Oceanogr. 1992, 37, 703–724. [Google Scholar] [CrossRef]
- Dauwe, B.; Middelburg, J.J.; Herman, P.M.J.; Heip, C.H.R. Linking Diagenetic Alteration of Amino Acids and Bulk Organic Matter Reactivity. Limnol. Oceanogr. 1999, 44, 1809–1814. [Google Scholar] [CrossRef]
- Kaiser, K.; Benner, R. Erratum: Major Bacterial Contribution to the Ocean Reservoir of Detrital Organic Carbon and Nitrogen. Limnol. Oceanogr. 2008, 53, 1192. [Google Scholar] [CrossRef]
- Tremblay, L.; Benner, R. Organic Matter Diagenesis and Bacterial Contributions to Detrital Carbon and Nitrogen in the Amazon River System. Limnol. Oceanogr. 2009, 54, 681–691. [Google Scholar] [CrossRef]
- Bourgoin, L.H.; Tremblay, L. Bacterial Reworking of Terrigenous and Marine Organic Matter in Estuarine Water Columns and Sediments. Geochim. Cosmochim. Acta 2010, 74, 5593–5609. [Google Scholar] [CrossRef]
- Lehmann, M.F.; Carstens, D.; Deek, A.; McCarthy, M.; Schubert, C.J.; Zopfi, J. Amino Acid and Amino Sugar Compositional Changes During In Vitro Degradation of Algal Organic Matter Indicate Rapid Bacterial Re-Synthesis. Geochim. Cosmochim. Acta 2020, 283, 67–84. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A Review of Coastal Palaeoclimate and Relative Sea-Level Reconstructions Using δ13C and C/N Ratios in Organic Material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable Isotopes in Ecosystem Studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Barber, A.; Sirois, M.; Chaillou, G.; Gélinas, Y. Stable Isotope Analysis of Dissolved Organic Carbon in Canada’s Eastern Coastal Waters. Limnol. Oceanogr. 2017, 62, S71–S84. [Google Scholar] [CrossRef]
- Lobbes, J.M.; Fitznar, H.P.; Kattner, G. Biogeochemical Characteristics of Dissolved and Particulate Organic Matter in Russian Rivers Entering the Arctic Ocean. Geochim. Cosmochim. Acta 2000, 64, 2973–2983. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Scofield, O.; Vernet, M.; Stammerjohn, S.; Erickson, M. Multiscale Control of Bacterial Production by Phytoplankton Dynamics and Sea Ice along the Western Antarctic Peninsula: A Regional and Decadal Investigation. J. Mar. Syst. 2012, 98–99, 26–39. [Google Scholar] [CrossRef]
- Williams, C.M.; Dupont, A.M.; Loevenich, J.; Post, A.F.; Dinasquet, J.; Yager, P.L. Pelagic Microbial Heterotrophy in Response to a Highly Productive Bloom of Phaeocystis antarctica in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anthr. 2016, 4, 000102. [Google Scholar] [CrossRef]
- Fang, L.; Kim, M. Radiocarbon Constraints on Carbon Release from the Antarctic Ice Sheet into the Amundsen Sea Embayment. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007053. [Google Scholar] [CrossRef]
- Hood, E.; Battin, T.J.; Fellman, J.; O’neel, S.; Spencer, R.G.M. Storage and Release of Organic Carbon from Glaciers and Ice Sheets. Nat. Geosci. 2015, 8, 91–96. [Google Scholar] [CrossRef]
- Wadham, J.L.; Hawkings, J.R.; Tarasov, L.; Gregoire, L.J.; Spencer, R.G.M.; Gutjahr, M.; Ridgwell, A.; Kohfeld, K.E. Ice Sheets Matter for the Global Carbon Cycle. Nat. Commun. 2019, 10, 3567. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.; Long, M. Coastal Southern Ocean: A Strong Anthropogenic CO2 Sink. Geophys. Res. Lett. 2008, 35, L21602. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Wanninkhof, R.; Sweeney, C.; Feely, R.A.; Chipman, D.W.; Hales, B.; Friederich, G.; Chavez, F.; Sabine, C.; et al. Climatological Mean and Decadal Change in Surface Ocean pCO2, and Net Sea–Air CO2 Flux over the Global Oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 554–577. [Google Scholar] [CrossRef]
- Smith, W.O.; Barber, D.G. Polynyas and Climate Change: A View to the Future. In Elsevier Oceanography Series; Smith, W.O., Barber, D.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 74, pp. 411–419. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Phytoplankton Dynamics within 37 Antarctic Coastal Polynya Systems. J. Geophys. Res. Ocean. 2003, 108, 3271. [Google Scholar] [CrossRef]
- Fragoso, G.M.; Smith, W.O. Influence of Hydrography on Phytoplankton Distribution in the Amundsen and Ross Seas, Antarctica. J. Mar. Syst. 2012, 89, 19–29. [Google Scholar] [CrossRef]
- Smith, W.O.; Shields, A.R.; Peloquin, J.A.; Catalano, G.; Tozzi, S.; Dinniman, M.S.; Asper, V.A. Interannual Variations in Nutrients, Net Community Production, and Biogeochemical Cycles in the Ross Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 815–833. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Buma, A.G.J.; Van Rijssel, M. The Carbohydrates of Phaeocystis and Their Degradation in the Microbial Food Web. Biogeochemistry 2007, 83, 99–118. [Google Scholar] [CrossRef]
- Delmont, T.O.; Hammar, K.M.; Ducklow, H.W.; Yager, P.L.; Post, A.F. Phaeocystis antarctica Blooms Strongly Influence Bacterial Community Structures in the Amundsen Sea Polynya. Front. Microbiol. 2014, 5, 646. [Google Scholar] [CrossRef]
- Rignot, E.; Mouginot, J.; Scheuchl, B.; Van Den Broeke, M.; Van Wessem, M.J.; Morlighem, M. Four Decades of Antarctic Ice Sheet Mass Balance from 1979–2017. Proc. Natl. Acad. Sci. USA 2019, 116, 1095–1103. [Google Scholar] [CrossRef]
- Jenkins, A.; Dutrieux, P.; Jacobs, S.S.; McPhail, S.D.; Perrett, J.R.; Webb, A.T.; White, D. Observations beneath Pine Island Glacier in West-Antarctica and Implications for Its Retreat. Nat. Geosci. 2010, 3, 468–472. [Google Scholar] [CrossRef]
- Wåhlin, A.K.; Graham, A.G.C.; Hogan, K.A.; Queste, B.Y.; Boehme, L.; Larter, R.D.; Pettit, E.C.; Wellner, J.; Heywood, K.J. Pathways and Modification of Warm Water Flowing beneath Thwaites Ice Shelf, West Antarctica. Sci. Adv. 2021, 7, eabd7254. [Google Scholar] [CrossRef]
- Cape, M.R.; Straneo, F.; Beaird, N.; Bundy, R.M.; Charette, M.A. Nutrient Release to Oceans from Buoyancy-Driven Upwelling at Greenland Tidewater Glaciers. Nat. Geosci. 2019, 12, 34–39. [Google Scholar] [CrossRef]
- Death, R.; Wadham, J.L.; Monteiro, F.; Le Brocq, A.M.; Tranter, M.; Ridgwell, A.; Dutkiewicz, S.; Raiswell, R. Antarctic Ice Sheet Fertilises the Southern Ocean. Biogeosciences 2014, 11, 2635–2643. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.H.; Joo, H.T.; Song, H.J.; Yang, E.J.; Lee, S.H.; Lee, S.H. Macromolecular Compositions of Phytoplankton in the Amundsen Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 123, 42–49. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.H.; Ha, S.Y.; Jung, J.; Kim, T.W.; Yang, E.J.; Jo, N.; Lim, Y.J.; Park, J.; Lee, S.H. Vertical Distributions of Macromolecular Composition of Particulate Organic Matter in the Water Column of the Amundsen Sea Polynya during the Summer in 2014. J. Geophys. Res. Ocean. 2018, 123, 1393–1405. [Google Scholar] [CrossRef]
- Chen, M.; Jung, J.; Lee, Y.K.; Kim, T.W.; Hur, J. Production of Tyrosine-Like Fluorescence and Labile Chromophoric Dissolved Organic Matter (DOM) and Low Surface Accumulation of Low Molecular Weight-Dominated DOM in a Productive Antarctic Sea. Mar. Chem. 2019, 213, 40–48. [Google Scholar] [CrossRef]
- Son, J.; Jung, J.; Lee, Y.; Kim, T.W.; Park, J.; Jeon, M.H.; Park, M.O. Contrasting Optical Properties of Dissolved Organic Matter between Oceanic Regions near the Getz and Dotson Ice Shelves in the Amundsen Sea, West Antarctica. Mar. Chem. 2024, 258, 104335. [Google Scholar] [CrossRef]
- Jeon, M.H.; Jung, J.; Park, M.O.; Aoki, S.; Kim, T.W.; Kim, S.K. Tracing Circumpolar Deep Water and Glacial Meltwater Using Humic-Like Fluorescent Dissolved Organic Matter in the Amundsen Sea, Antarctica. Mar. Chem. 2021, 235, 104008. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Jenkins, A.; Giulivi, C.F.; Dutrieux, P. Stronger Ocean Circulation and Increased Melting under Pine Island Glacier Ice Shelf. Nat. Geosci. 2011, 4, 519–523. [Google Scholar] [CrossRef]
- Randall-Goodwin, E.; Meredith, M.P.; Jenkins, A.; Yager, P.L.; Sherrell, R.M.; Abrahamsen, E.P.; Guerrero, R.; Yuan, X.; Mortlock, R.A.; Gavahan, K.; et al. Freshwater Distributions and Water Mass Structure in the Amundsen Sea Polynya Region, Antarctica. Elem. Sci. Anthr. 2015, 3, 000065. [Google Scholar] [CrossRef]
- Mankoff, K.D.; Jacobs, S.S.; Tulaczyk, S.M.; Stammerjohn, S.E. The Role of Pine Island Glacier Ice Shelf Basal Channels in Deep-Water Upwelling, Polynyas, and Ocean Circulation in Pine Island Bay, Antarctica. Ann. Glaciol. 2012, 53, 123–128. [Google Scholar] [CrossRef]
- Lee, Y.; Yang, E.J.; Park, J.; Jung, J.; Kim, T.W.; Lee, S.H. Physical-Biological Coupling in the Amundsen Sea, Antarctica: Influence of Physical Factors on Phytoplankton Community Structure and Biomass. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 117, 51–60. [Google Scholar] [CrossRef]
- Fang, L.; Lee, S.; Lee, S.A.; Hahm, D.; Kim, G.; Druffel, E.R.M.; Hwang, J. Removal of Refractory Dissolved Organic Carbon in the Amundsen Sea, Antarctica. Sci. Rep. 2020, 10, 1213. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.; Kim, T.W.; Yang, E.J.; Park, J. Phytoplankton Growth Rates in the Amundsen Sea (Antarctica) during Summer: The Role of Light. Environ. Res. 2022, 207, 112165. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Robinson, D.H.; Worthen, D.L.; Dunbar, R.B.; DiTullio, G.R.; VanWoert, M.; Lizotte, M.P. Phytoplankton Community Structure and the Drawdown of Nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Park, M.O.; Jung, J.; Yang, E.J.; Lee, S.H. Taxonomic Variability of Phytoplankton and Relationship with Production of CDOM in the Polynya of the Amundsen Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 123, 30–41. [Google Scholar] [CrossRef]
- Fabiano, M.; Povero, P.; Danovaro, R. Distribution and Composition of Particulate Organic Matter in the Ross Sea (Antarctica). Polar Biol. 1993, 13, 525–533. [Google Scholar] [CrossRef]
- Yager, P.; Sherrell, R.; Stammerjohn, S.; Ducklow, H.; Schofield, O.; Ingall, E.; Wilson, S.; Lowry, K.; Williams, C.; Riemann, L.; et al. A Carbon Budget for the Amundsen Sea Polynya, Antarctica: Estimating Net Community Production and Export in a Highly Productive Polar Ecosystem. Elem. Sci. Anthr. 2016, 4, 000140. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M.; Quay, P.D.; Richey, J.E.; Hedges, J.I. Loss of Organic Matter from Riverine Particles in Deltas. Geochim. Cosmochim. Acta 1997, 61, 1507–1511. [Google Scholar] [CrossRef]
- Megens, L.; Van Der Plicht, J.; De Leeuw, J.W.; Smedes, F. Stable Carbon and Radiocarbon Isotope Compositions of Particle Size Fractions to Determine Origins of Sedimentary Organic Matter in an Estuary. Org. Geochem. 2002, 33, 945–952. [Google Scholar] [CrossRef][Green Version]
- Schouten, S.; Klein Breteler, W.C.M.; Blokker, P.; Schogt, N.; Rijpstra, W.I.C.; Grice, K.; Baas, M.; Sinninghe Damsté, J.S. Biosynthetic Effects on the Stable Carbon Isotopic Compositions of Algal Lipids: Implications for Deciphering the Carbon Isotopic Biomarker Record. Geochim. Cosmochim. Acta 1998, 62, 1397–1406. [Google Scholar] [CrossRef]
- Laane, R.W.P.M.; Turkstra, E.; Mook, W.G. Stable Carbon Isotope Composition of Pelagic and Benthic Organic Matter in the North Sea and Adjacent Estuaries. In Facets of Modern Biogeochemistry; Springer: Dordrecht, The Netherlands, 1990; pp. 214–224. [Google Scholar]
- Benner, R.; Fogel, M.; Sprague, E.; Hodson, R. Depletion of 13C in Lignin and Its Implications for Stable Carbon Isotope Studies. Nature 1987, 329, 708–710. [Google Scholar] [CrossRef]
- Van Dongen, B.E.; Schouten, S.; Sinninghe Damsté, J.S. Carbon Isotope Variability in Monosaccharides and Lipids of Aquatic Algae and Terrestrial Plants. Mar. Ecol. Prog. Ser. 2002, 232, 83–92. [Google Scholar] [CrossRef]
- Guo, J.; Achterberg, E.P.; Shen, Y.; Yuan, H.; Song, J.; Liu, J.; Li, X.; Duan, L. Stable Carbon Isotopic Composition of Amino Sugars in Heterotrophic Bacteria and Phytoplankton: Implications for Assessment of Marine Organic Matter Degradation. Limnol. Oceanogr. 2023, 68, 2814–2825. [Google Scholar] [CrossRef]
- Jo, N.; La, H.S.; Kim, J.H.; Kim, K.; Kim, B.K.; Kim, M.J.; Son, W.; Lee, S.H. Different Biochemical Compositions of Particulate Organic Matter Driven by Major Phytoplankton Communities in the Northwestern Ross Sea. Front. Microbiol. 2021, 12, 623600. [Google Scholar] [CrossRef] [PubMed]
- Hubberten, U.; Lara, R.J.; Kattner, G. Refractory Organic Compounds in Polar Waters: Relationship between Humic Substances and Amino Acids in the Arctic and Antarctic. J. Mar. Res. 1995, 53, 137–149. [Google Scholar] [CrossRef]
- Tremblay, L.; Caparros, J.; Leblanc, K.; Obernosterer, I. Origin and Fate of Particulate and Dissolved Organic Matter in a Naturally Iron-Fertilized Region of the Southern Ocean. Biogeosciences 2015, 12, 607–621. [Google Scholar] [CrossRef]
- Kaiser, K.; Benner, R. Biochemical Composition and Size Distribution of Organic Matter at the Pacific and Atlantic Time-Series Stations. Mar. Chem. 2009, 113, 63–77. [Google Scholar] [CrossRef]
- Wei, W.; Blankenship, D.D.; Greenbaum, J.S.; Gourmelen, N.; Dow, C.F.; Richter, T.G.; Greene, C.A.; Young, D.A.; Lee, S.H.; Kim, T.W.; et al. Getz Ice Shelf Melt Enhanced by Freshwater Discharge from beneath the West Antarctic Ice Sheet. Cryosphere 2020, 14, 1399–1408. [Google Scholar] [CrossRef]
- Brogi, S.R.; Ha, S.Y.; Kim, K.; Derrien, M.; Lee, Y.K.; Hur, J. Optical and Molecular Characterization of Dissolved Organic Matter (DOM) in the Arctic Ice Core and the Underlying Seawater (Cambridge Bay, Canada): Implication for Increased Autochthonous DOM during Ice Melting. Sci. Total Environ. 2018, 627, 802–811. [Google Scholar] [CrossRef]
- Thomas, D.N.; Lara, R.J.; Eicken, H.; Kattner, G.; Skoog, A. Dissolved Organic Matter in Arctic Multi-Year Sea Ice during Winter: Major Components and Relationship to Ice Characteristics. Polar Biol. 1995, 15, 477–483. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Le Quéré, C.; Rödenbeck, C.; Buitenhuis, E.T.; Conway, T.J.; Langenfelds, R.; Gomez, A.; Labuschagne, C.; Ramonet, M.; Nakazawa, T.; Metzl, N.; et al. Saturation of the Southern Ocean CO2 Sink due to Recent Climate Change. Science 2007, 316, 1735–1738. [Google Scholar] [CrossRef]
- Nissen, C.; Vogt, M. Factors Controlling the Competition between Phaeocystis and Diatoms in the Southern Ocean and Implications for Carbon Export Fluxes. Biogeosciences 2021, 18, 251–283. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Wilson, S.E.; Post, A.F.; Stammerjohn, S.E.; Erickson, M.; Lee, S.H.; Lowry, K.E.; Sherrell, R.M.; Yager, P.L. Particle Flux on the Continental Shelf in the Amundsen Sea Polynya and Western Antarctic Peninsula. Elem. Sci. Anthr. 2015, 3, 46. [Google Scholar] [CrossRef]
- DeJong, H.B.; Dunbar, R.B.; Koweek, D.A.; Mucciarone, D.A.; Bercovici, S.K.; Hansell, D.A. Net Community Production and Carbon Export during the Late Summer in the Ross Sea, Antarctica. Glob. Biogeochem. Cycles 2017, 31, 473–491. [Google Scholar] [CrossRef]
- Brainerd, K.E.; Gregg, M.C. Surface Mixed and Mixing Layer Depths. Deep Sea Res. Part I Oceanogr. Res. Pap. 1995, 42, 1521–1543. [Google Scholar] [CrossRef]
- Gordon, L.I.; Jennings, J.C., Jr.; Ross, A.A.; Krest, J.M. A Suggested Protocol for Continuous Flow Automated Analysis of Seawater Nutrients (Phosphate, Nitrate, Nitrite, and Silicic Acid) in the WOCE Hydrographic Program Office. Methods Man. WHPO 1993, 68, 1–52. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Kim, M.S.; Lee, W.S.; Suresh Kumar, K.; Shin, K.H.; Robarge, W.; Kim, M.; Lee, S.R. Effects of HCl Pretreatment, Drying, and Storage on the Stable Isotope Ratios of Soil and Sediment Samples. Rapid Commun. Mass Spectrom. 2016, 30, 1567–1575. [Google Scholar] [CrossRef]
- Fitznar, H.P.; Lobbes, J.M.; Kattner, G. Determination of Enantiomeric Amino Acids with High-Performance Liquid Chromatography and Pre-Column Derivatisation with O-Phthaldialdehyde and N-Isobutyrylcysteine in Seawater and Fossil Samples (Mollusks). J. Chromatogr. A 1999, 832, 123–132. [Google Scholar] [CrossRef]
- Kaiser, K.; Benner, R. Hydrolysis-Induced Racemization of Amino Acids. Limnol. Oceanogr. Methods 2005, 3, 318–325. [Google Scholar] [CrossRef]
- Kumagai, H. Automated Precolumn Derivatization for the Enantioseparation of Amino Acids Using the Agilent 1290 Infinity II LC; Application Note 5991-8329EN; Agilent Technologies Japan Ltd.: Tokyo, Japan, 2017; Available online: https://www.agilent.com/chem (accessed on 14 June 2023).
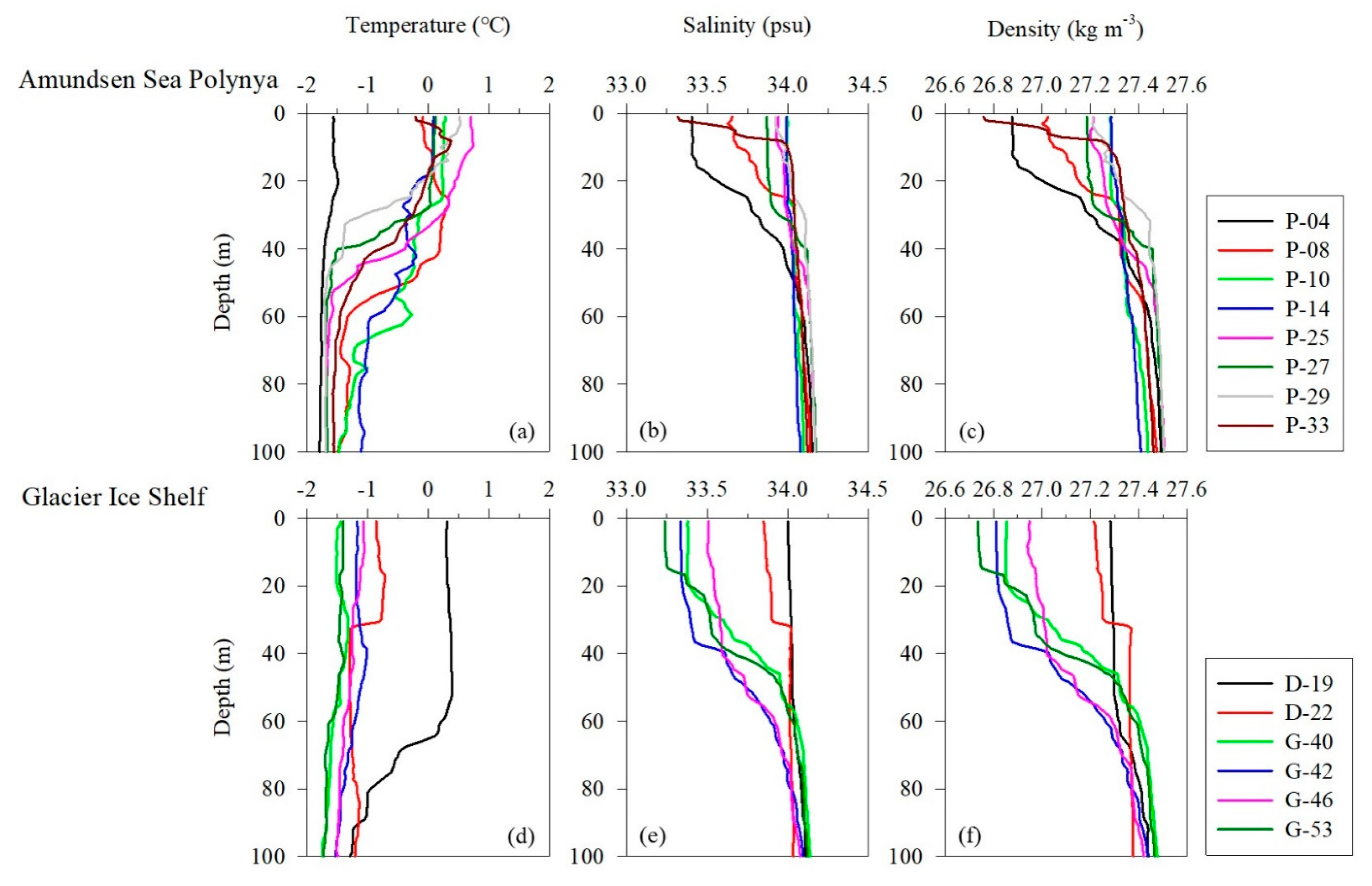

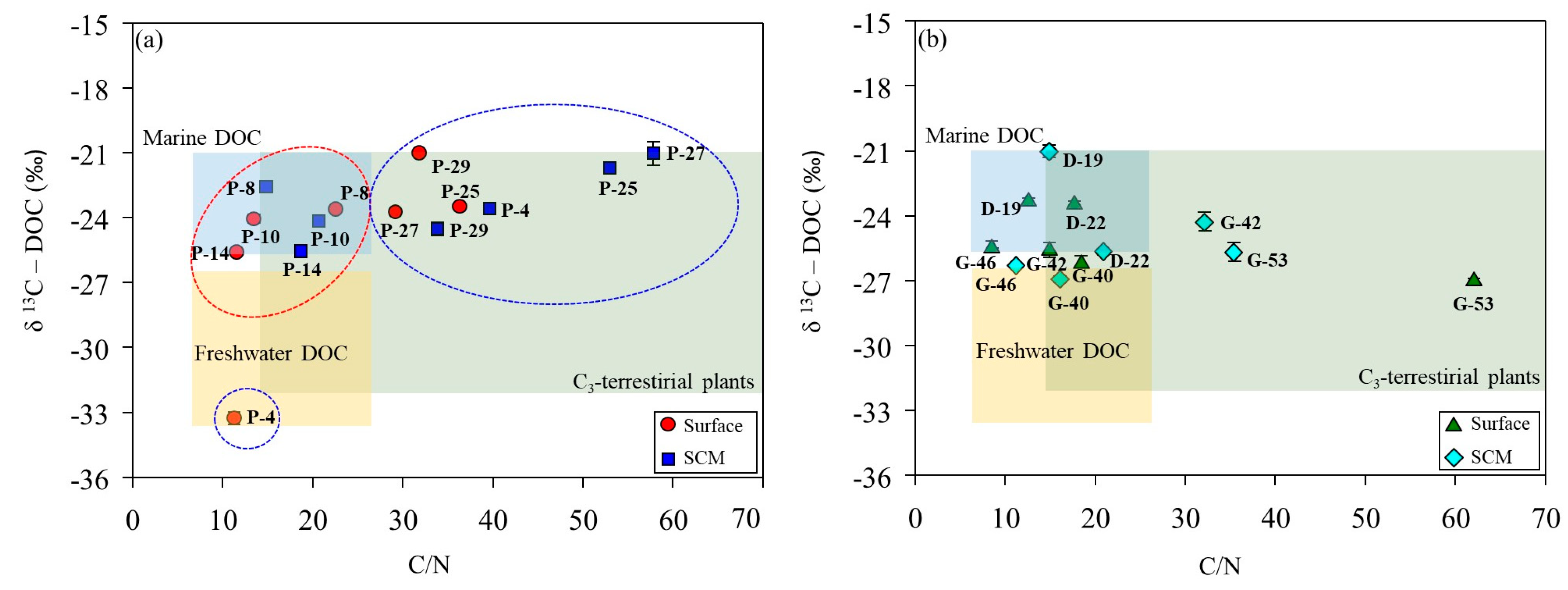
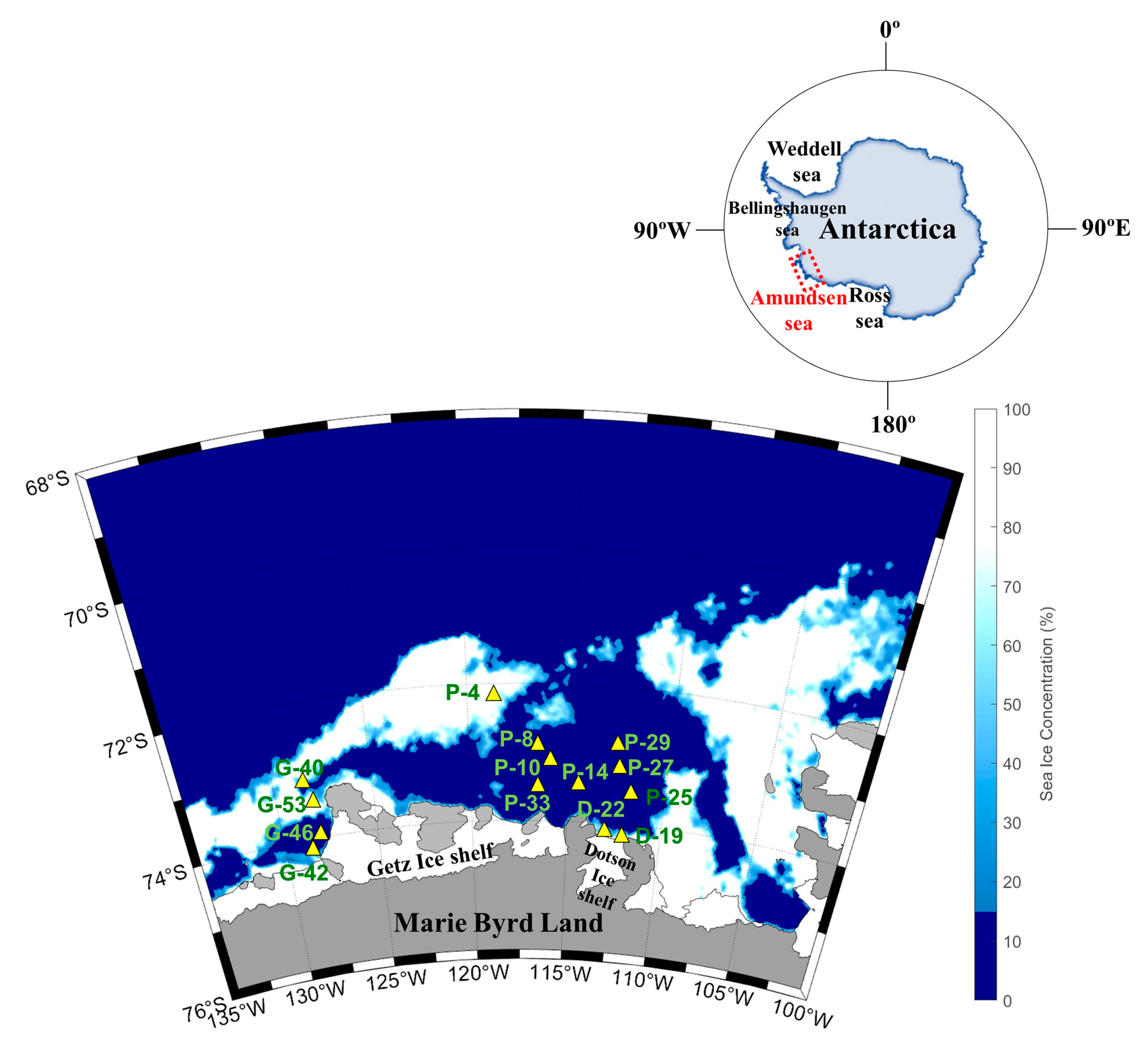
| Oceanography Setting | Station | Latitude | Longitude | Water Depth | Sampling Depth | T | S | D | MLD | NO3 | PO4 | Chl-a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (s) | (w) | (m) | (m) | (°C) | (psu) | (kg m−3) | (m) | (µM) | (µM) | (µg L−1) | ||
| Amundsen Sea Polynya | P-04 | −72.098 | −118.884 | 745 | 0 | −1.57 | 33.4 | 26.9 | 17 | 17.2 | 1.27 | 4.24 |
| 15 | −1.55 | 33.4 | 26.9 | 19.6 | 1.52 | 5.31 | ||||||
| P-08 | −72.800 | −116.501 | 627 | 0 | −0.11 | 33.6 | 27.0 | 17 | 12.9 | 0.84 | 3.40 | |
| 20 | 0.11 | 33.8 | 27.2 | 16.5 | 1.06 | 3.86 | ||||||
| P-10 | −73.040 | −115.725 | 710 | 0 | 0.25 | 34.0 | 27.3 | 27 | 19.8 | 1.50 | 2.07 | |
| 20 | 0.24 | 34.0 | 27.3 | 20.3 | 1.52 | 3.56 | ||||||
| P-14 | −73.499 | −113.999 | 710 | 0 | 0.09 | 34.0 | 27.3 | 44 | 22.0 | 1.54 | 1.57 | |
| 20 | −0.18 | 34.0 | 27.3 | 22.4 | 1.52 | 2.23 | ||||||
| P-25 | −73.699 | −111.597 | 398 | 0 | 0.71 | 33.9 | 27.2 | 26 | 5.60 | 0.79 | 9.80 | |
| 10 | 0.73 | 33.9 | 27.2 | 10.1 | 1.05 | 11.9 | ||||||
| P-27 | −73.283 | −112.166 | 443 | 0 | 1.01 | 34.0 | 27.2 | 27 | 10.3 | 0.91 | 3.83 | |
| 15 | 0.65 | 34.0 | 27.3 | 14.6 | 1.28 | 10.5 | ||||||
| P-29 | −72.846 | −112.481 | 448 | 0 | 0.52 | 33.9 | 27.2 | 20 | 16.8 | 1.29 | 3.24 | |
| 20 | 0.52 | 33.9 | 27.2 | 16.8 | 1.29 | 4.58 | ||||||
| P-33 | −73.499 | −116.499 | 374 | 0 | 0.02 | 33.5 | 26.9 | 22 | 9.30 | 0.65 | 1.64 | |
| 10 | 0.34 | 34.0 | 27.3 | 13.2 | 0.91 | 1.86 | ||||||
| Average | 0.10 | 33.9 | 27.2 | 25 | 15.5 | 1.18 | 4.60 | |||||
| (SD ± 1) | (0.71) | (0.22) | (0.15) | (9) | (4.91) | (0.30) | (3.25) | |||||
| Dotson Ice Shelf | D-19 | −74.171 | −112.528 | 1034 | 0 | 0.30 | 34.0 | 27.3 | 65 | 17.3 | 1.38 | 2.51 |
| 15 | 0.31 | 34.0 | 27.3 | 18.0 | 1.42 | 5.26 | ||||||
| D-22 | −74.171 | −113.328 | 634 | 0 | −0.85 | 33.9 | 27.2 | 30 | 22.6 | 1.89 | 3.17 | |
| 20 | −0.74 | 33.9 | 27.2 | 23.7 | 1.92 | 3.91 | ||||||
| Average | −0.25 | 34.0 | 27.3 | 48 | 20.4 | 1.65 | 3.71 | |||||
| (SD ± 1) | (0.64) | (0.06) | (0.06) | (25) | (3.22) | (0.29) | (1.18) | |||||
| Getz Ice Shelf | G-40 | −73.077 | −128.181 | 560 | 0 | −1.50 | 33.4 | 26.9 | 23 | 14.9 | 1.18 | 3.85 |
| 30 | −1.32 | 33.6 | 27.0 | 16.1 | 1.42 | 6.15 | ||||||
| G-42 | −74.138 | −128.213 | 596 | 0 | −1.17 | 33.3 | 26.8 | 32 | 12.2 | 0.94 | 0.70 | |
| 45 | −1.05 | 33.6 | 27.1 | 19.2 | 1.64 | 4.25 | ||||||
| G-46 | −73.940 | −127.799 | 780 | 0 | −1.07 | 33.5 | 26.9 | 25 | 11.8 | 1.01 | 3.83 | |
| 38 | −1.26 | 33.6 | 27.0 | 15.3 | 1.29 | 4.65 | ||||||
| G-53 | −73.350 | −128.050 | 614 | 0 | −1.41 | 33.2 | 26.7 | 17 | 11.8 | 1.01 | 3.82 | |
| 25 | −1.43 | 33.5 | 26.9 | 14.3 | 1.27 | 5.07 | ||||||
| Average | −0.34 | 33.8 | 27.1 | 32 | 14.5 | 1.22 | 4.04 | |||||
| (SD ± 1) | (0.86) | (0.26) | (0.18) | (17) | (2.55) | (0.24) | (1.57) |
| Oceanography Setting | Station | Sampling Depth | POC | PON | POC/ PON | δ13C- POC | DOC | DON | DOC/ DON | δ13C- DOC | PAA | PAA-C Yield | D-AA | Bacterial-C Yield | DI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | (µM) | (µM) | - | (‰) | (µM) | (µM) | - | (‰) | (µM) | (%) | (%) | (%) | - | ||
| Amundsen Sea Polynya | P-04 | 0 | 57 | 8 | 7 | −25.4 | 47 | 4 | 11 | −33.3 | 3.64 | 28.1 | 7.69 | 10.3 | 1.79 |
| 15 | 54 | 7 | 7 | −26.2 | 46 | 1 | 40 | −23.6 | 3.39 | 27.7 | 4.20 | 10.3 | 2.69 | ||
| P-08 | 0 | 67 | 9 | 7 | −27.3 | 45 | 2 | 23 | −23.6 | 1.21 | 7.68 | 3.92 | 3.31 | 3.58 | |
| 20 | 62 | 8 | 7 | −27.6 | 51 | 1 | 36 | −22.6 | 0.83 | 5.65 | 3.25 | 2.11 | 4.33 | ||
| P-10 | 0 | 70 | 9 | 7 | −26.4 | 45 | 3 | 13 | −24.1 | 3.43 | 22.7 | 4.30 | 12.1 | 1.47 | |
| 20 | 57 | 8 | 7 | −25.0 | 44 | 2 | 21 | −24.2 | 4.22 | 34.6 | 4.57 | 15.4 | −0.86 | ||
| P-14 | 0 | 56 | 7 | 7 | −25.1 | 49 | 4 | 12 | −22.8 | 2.52 | 20.2 | 3.36 | 5.24 | 1.46 | |
| 20 | 48 | 6 | 8 | −24.3 | 46 | 2 | 19 | −24.2 | 1.26 | 11.5 | 3.75 | 2.05 | 0.54 | ||
| P-25 | 0 | 117 | 8 | 14 | −24.1 | 49 | 3 | 15 | −25.6 | 7.00 | 28.0 | 2.97 | 11.2 | 0.31 | |
| 10 | 73 | 15 | 5 | −25.1 | 45 | 1 | 53 | −25.1 | 6.83 | 45.3 | 3.20 | 13.5 | −0.53 | ||
| P-27 | 0 | 69 | 9 | 8 | −26.5 | 38 | 1 | 32 | −26.5 | 3.02 | 21.3 | 3.67 | 6.24 | −0.17 | |
| 15 | 72 | 9 | 8 | −27.1 | 40 | 1 | 58 | −22.3 | 2.54 | 16.1 | 3.63 | 4.87 | 4.42 | ||
| P-29 | 0 | 75 | 9 | 8 | −27.6 | 36 | 1 | 29 | −21.0 | 1.68 | 10.9 | 4.62 | 3.10 | 4.43 | |
| 20 | 72 | 8 | 9 | −26.2 | 36 | 1 | 34 | −21.0 | 2.99 | 18.9 | 4.87 | 4.79 | 5.39 | ||
| P-33 | 0 | 67 | 11 | 6 | −23.9 | 50 | 3 | 17 | − | 1.78 | 13.4 | 8.01 | 1.48 | −4.96 | |
| 10 | 50 | 7 | 7 | −24.5 | 49 | 2 | 25 | − | 1.80 | 17.3 | 8.70 | 1.97 | −5.60 | ||
| Average | 67 | 9 | 8 | −25.8 | 45 | 2 | 27 | −24.3 | 3.01 | 20.6 | 4.67 | 6.75 | 1.14 | ||
| (SD ± 1) | (16) | (2) | (2) | (1.26) | (5) | (1) | (14) | (3.04) | (1.81) | (10.4) | (1.81) | (4.65) | (3.17) | ||
| Dotson Ice Shelf | D-19 | 0 | 76 | 10 | 7 | −26.5 | 45 | 4 | 13 | −23.3 | 8.24 | 51.1 | 3.35 | 12.0 | 4.55 |
| 15 | 70 | 11 | 7 | −26.5 | 44 | 3 | 15 | −21.0 | 9.31 | 62.8 | 3.16 | 16.4 | 4.75 | ||
| D-22 | 0 | 57 | 5 | 11 | −28.7 | 45 | 3 | 18 | −23.5 | 10.2 | 81.3 | 2.87 | 22.6 | 5.00 | |
| 20 | 45 | 3 | 15 | −27.6 | 40 | 2 | 21 | −25.7 | 8.41 | 82.7 | 2.57 | 14.1 | 4.34 | ||
| Average | 62 | 7 | 10 | −27.3 | 44 | 3 | 17 | −23.4 | 9.05 | 69.5 | 2.99 | 16.3 | 4.66 | ||
| (SD ± 1) | (14) | (4) | (4) | (1.05) | (2) | (1) | (4) | (1.89) | (0.92) | (15.2) | (0.34) | (4.57) | (0.28) | ||
| Getz Ice Shelf | G-40 | 0 | 71 | 10 | 7 | −25.5 | 46 | 3 | 18 | −26.1 | 2.03 | 15.0 | 2.13 | 26.0 | −5.09 |
| 30 | 70 | 11 | 7 | −23.1 | 43 | 3 | 16 | −26.9 | 3.41 | 21.0 | 3.33 | 3.16 | −4.19 | ||
| G-42 | 0 | 52 | 5 | 11 | −24.1 | 48 | 3 | 15 | −25.6 | 1.62 | 14.6 | 2.87 | 4.31 | −4.54 | |
| 45 | 57 | 3 | 16 | −24.3 | 46 | 1 | 32 | −24.2 | 2.15 | 21.9 | 5.05 | 25.0 | −5.91 | ||
| G-46 | 0 | 71 | 8 | 9 | −25.5 | 45 | 5 | 8 | −25.3 | 3.72 | 29.0 | 2.93 | 14.5 | −4.41 | |
| 38 | 60 | 8 | 9 | −26.1 | 47 | 4 | 11 | −26.2 | 8.06 | 73.8 | 1.84 | 6.87 | −4.76 | ||
| G-53 | 0 | 67 | 6 | 9 | −24.2 | 45 | 1 | 63 | −25.9 | 8.33 | 64.7 | 2.70 | 18.9 | −4.29 | |
| 25 | 69 | 6 | 9 | −23.1 | 38 | 1 | 36 | −27.1 | 4.58 | 26.8 | 12.5 | 23.6 | −3.74 | ||
| Average | 65 | 7 | 10 | −24.5 | 45 | 3 | 25 | −25.9 | 4.24 | 33.3 | 4.17 | 15.3 | −4.62 | ||
| (SD ± 1) | (7) | (3) | (3) | (1.12) | (3) | (2) | (18) | (0.93) | (2.63) | (22.8) | 3.50 | (9.48) | (0.66) |
| Amino Acid | Factor Coefficient (First Axis) | Factor Coefficient (Second Axis) | Mean | SD |
|---|---|---|---|---|
| Asx | 0.30 | 0.35 | 2.02 | 0.80 |
| Glx | −0.36 | 0.09 | 2.22 | 1.83 |
| Ser | −0.21 | 0.56 | 0.54 | 0.42 |
| His | −0.67 | 0.58 | 4.88 | 6.61 |
| Thr | 0.91 | 0.13 | 3.77 | 1.74 |
| Gly | 0.52 | 0.68 | 26.4 | 7.01 |
| Arg | 0.52 | −0.08 | 6.24 | 2.87 |
| Ala | 0.12 | −0.63 | 10.8 | 5.23 |
| Tyr | −0.40 | 0.66 | 2.86 | 2.83 |
| Cys | −0.70 | −0.05 | 3.40 | 3.26 |
| Val | −0.31 | −0.66 | 7.41 | 4.86 |
| IlU | 0.04 | −0.20 | 9.27 | 5.56 |
| Phe | 0.56 | 0.03 | 8.22 | 3.92 |
| Leu | −0.42 | −0.62 | 2.01 | 3.92 |
| Lys | 0.83 | −0.18 | 4.72 | 3.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.-O.; Kim, M.-S.; Lee, B.; Gal, J.-K.; Jung, J.; Kim, T.-W.; Park, J.; Ha, S.-Y. Characterization of Phytoplankton-Derived Amino Acids and Tracing the Source of Organic Carbon Using Stable Isotopes in the Amundsen Sea. Mar. Drugs 2024, 22, 476. https://doi.org/10.3390/md22100476
Min J-O, Kim M-S, Lee B, Gal J-K, Jung J, Kim T-W, Park J, Ha S-Y. Characterization of Phytoplankton-Derived Amino Acids and Tracing the Source of Organic Carbon Using Stable Isotopes in the Amundsen Sea. Marine Drugs. 2024; 22(10):476. https://doi.org/10.3390/md22100476
Chicago/Turabian StyleMin, Jun-Oh, Min-Seob Kim, Boyeon Lee, Jong-Ku Gal, Jinyoung Jung, Tae-Wan Kim, Jisoo Park, and Sun-Yong Ha. 2024. "Characterization of Phytoplankton-Derived Amino Acids and Tracing the Source of Organic Carbon Using Stable Isotopes in the Amundsen Sea" Marine Drugs 22, no. 10: 476. https://doi.org/10.3390/md22100476
APA StyleMin, J.-O., Kim, M.-S., Lee, B., Gal, J.-K., Jung, J., Kim, T.-W., Park, J., & Ha, S.-Y. (2024). Characterization of Phytoplankton-Derived Amino Acids and Tracing the Source of Organic Carbon Using Stable Isotopes in the Amundsen Sea. Marine Drugs, 22(10), 476. https://doi.org/10.3390/md22100476







