Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. Effects of Temperature, Time, and Biomass-to-Water Ratio on the Extraction Yield of Polysaccharides
2.2. Statistical Analysis and Model Fitting Based on RSM
2.3. Interaction Among Extraction Conditions
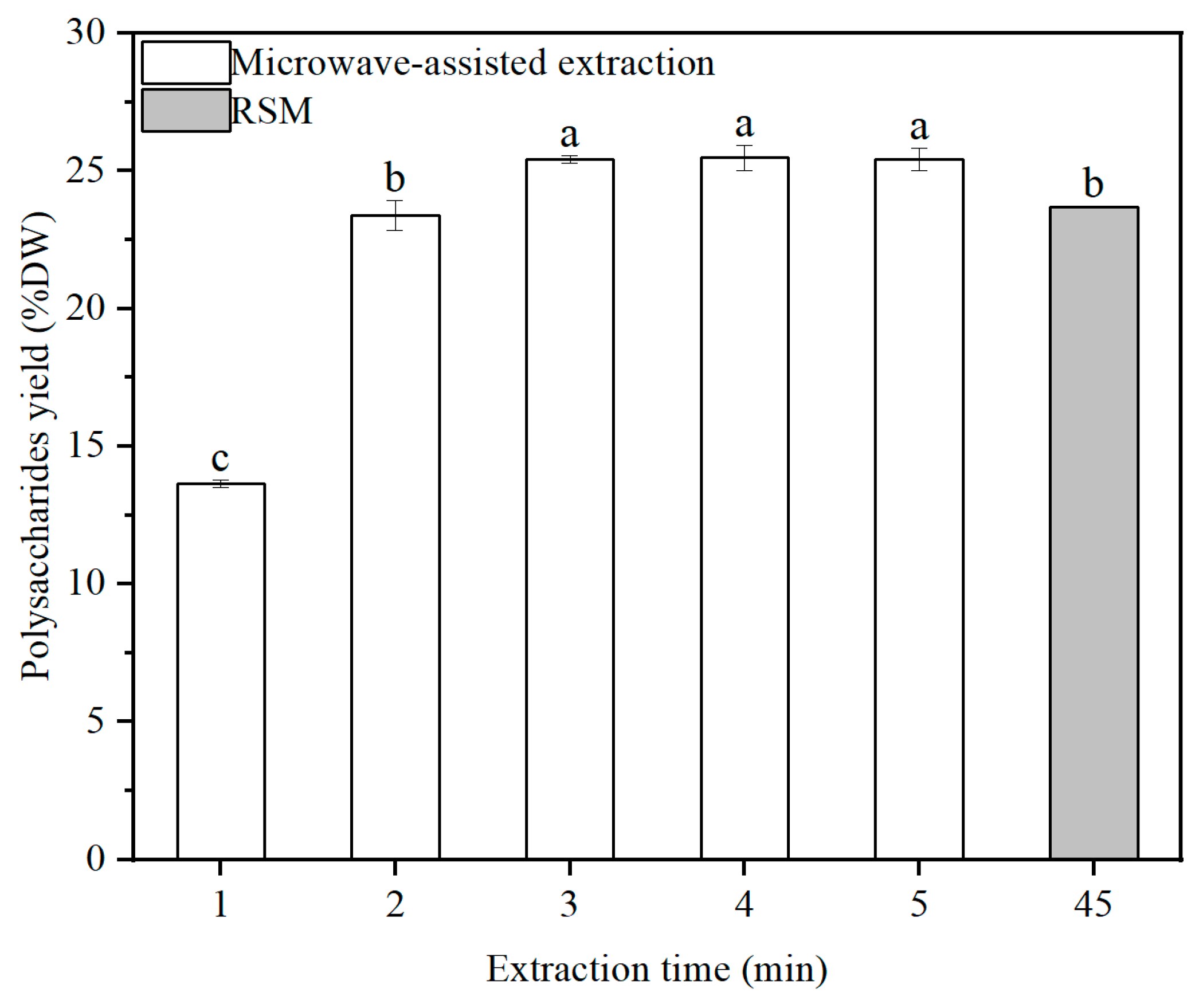
2.4. Effect of Microwave-Assisted Extraction on the Yield of Polysaccharides
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
4.2. Determination of the Content of Polysaccharides and Moisture of Wet Biomass
4.3. Single-Factor Experiment
4.4. RSM Experiment
4.5. Microwave-Assisted Extraction
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from Cyanobacteria and Microalgae and their Commercial Application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.H.; Ko, J.Y.; Jang, J.H.; Kim, G.H.; Lee, J.S.; Nah, J.W.; Jeon, Y.J. Rapid Preparation of Functional Polysaccharides from Pyropia yezoensis by Microwave-assistant Rapid Enzyme Digest System. Carbohydr. Polym. 2016, 153, 512–517. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, An Inhibitor of Enveloped Virus Replication, from A Blue-green Alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the Production of Bioactive Substances from Marine Unicellular Microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Fernando, S.; Kim, K.N.; Kim, D.; You, J.J. Algal Polysaccharides: Potential Bioactive Substances for Cosmeceutical Applications. Crit. Rev. Biotechnol. 2018, 39, 99–113. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of Different Molecular Weight Polysaccharides from Porphyridium cruentum and their Antioxidant Activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. Polysaccharide against Herpes Simplex Viruses in Vitro and in Vivo. J. Biochem. Biophys. Methods 2002, 48, 189–200. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Huleihel, M.; Hamias, R.; Wolak, T.; Paran, E. An Anti-inflammatory Effect of Red Microalga Polysaccharides in Coronary Artery Endothelial Cells. Atherosclerosis 2017, 264, 11–18. [Google Scholar] [CrossRef]
- Ben, H.H.; Farhat, A.; Akermi, S.; Khemakhem, B.; Ben, H.Y.; Michaud, P.; Fendri, I.; Abdelkafi, S. In Silico Evidence of Antiviral Activity against SARS-CoV-2 main Protease of Oligosaccharides from Porphyridium sp. Sci. Total Environ. 2022, 836, 155580. [Google Scholar]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and Antitumor Activities of Different-molecular-weight Polysaccharides from Porphyridium cruentum. Carbohyd. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, H.; Jiang, P.; Chen, Z.; Xiang, W. Growth and Biochemical Composition of Porphyridium purpureum SCS-02 under Different Nitrogen Concentrations. Mar. Drugs 2019, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Bayu, A.; Noerdjito, D.R.; Rahmawati, S.I.; Putra, M.Y.; Karnjanakom, S. Biological and Technical Aspects on Valorization of Red Microalgae Genera Porphyridium. Biomass Convers. Biorefinery 2022, 13, 12395–12411. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A Novel Three-step Extraction Strategy for High-value Products from Red Algae Porphyridium purpureum. Foods 2021, 10, 2164. [Google Scholar] [CrossRef]
- Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-destructive extraction. Mar. Drugs 2022, 20, 102. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Ascophyllum nodosum and its Antioxidant Activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.; Jhurry, D.; Couprie, J. Ultrasound-assisted Extraction and Structural Characterization by NMR of Alginates and Carrageenans from Seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Enzyme-assistant Extraction (EAE) of Bioactive Components: A Useful Approach for Recovery of Industrially Important Metabolites from Seaweeds: A Review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Ritta, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of Different Non-conventional Extraction Methods on the Antibacterial and Antiviral Activity of Fucoidans Extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Sujin Jeba Kumar, T.; Moses Packiaraj, R.; Veeraraj, A.; Prakash, S. Anti-oxidant Activity of Polysaccharides Extracted from Isocrysis galbana using RSM Optimized Conditions. Int. J. Biol. Macromol. 2013, 60, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tesvichian, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Buakeaw, A.; Puthong, S.; Thitiprasert, S.; Mekboonsonglarp, W.; Liangsakul, J.; Sopon, A.; et al. Sulfated Polysaccharides from Caulerpa lentillifera: Optimizing the Process of Extraction, Structural Characteristics, Antioxidant Capabilities, and Anti-glycation Properties. Heliyon 2024, 10, e24444. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from Green Algae Ulva intestinalis: Optimization of Ultrasound-assisted Extraction and Antioxidant Activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Birgersson, P.S.; Oftebro, M.; Strand, W.I.; Aarstad, O.A.; Sætrom, G.I.; Sletta, H.; Arlov, Ø.; Aachmann, F.L. Sequential Extraction and Fractionation of Four Polysaccharides from Cultivated Brown Algae Saccharina latissima and Alaria esculenta. Algal Res. 2023, 69, 102928. [Google Scholar] [CrossRef]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from An Arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef]
- Kurd, F.; Samavati, V. Water Soluble Polysaccharides from Spirulina platensis: Extraction and in Vitro Anti-cancer Activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response Surface Optimization of Ultrasound-assisted Polysaccharides Extraction from Pomegranate Peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, Characterization and Biological Activity of Sulfated Polysaccharides from Seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of Ultrasound and/or Heating on the Extraction of Pectin from Grapefruit Peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Z.; Ma, S.; Rasool, M.A.; Wang, L.; Zhang, J. Optimization of Ultrasonic-assisted Extraction, Refinement and Characterization of Water-soluble Polysaccharide from Dictyosphaerium sp. and Evaluation of Antioxidant Activity in Vitro. J. Food Meas. Charact. 2020, 14, 963–977. [Google Scholar] [CrossRef]
- Ge, Y.; Ni, Y.; Yan, H.; Chen, Y.; Cai, T. Optimization of the Supercritical Fluid Extraction of Natural Vitamin E from Wheat Germ Using Response Surface Methodology. J. Food Sci. 2002, 67, 239–243. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of Phycobiliprotein Pigments Extraction from Red Algae Gracilaria gracilis for Substitution of Synthetic Food Colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dai, L.; Chen, Z.; Li, T.; Wu, J.; Wu, H.; Wu, H.; Xiang, W. Extraction Optimization, Physicochemical Characterization, and Antioxidant Activity of Polysaccharides from Rhodosorus sp. SCSIO-45730. J. Appl. Phycol. 2022, 34, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Torabi, P.; Hamdami, N.; Keramat, J. Optimization and Kinetics of Microwave-assisted Extraction of Sulfated Fucose-rich Polysaccharides from Nizamuddinia zanardinii. Biomass Convers. Biorefinery 2022, 14, 14707–14723. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]

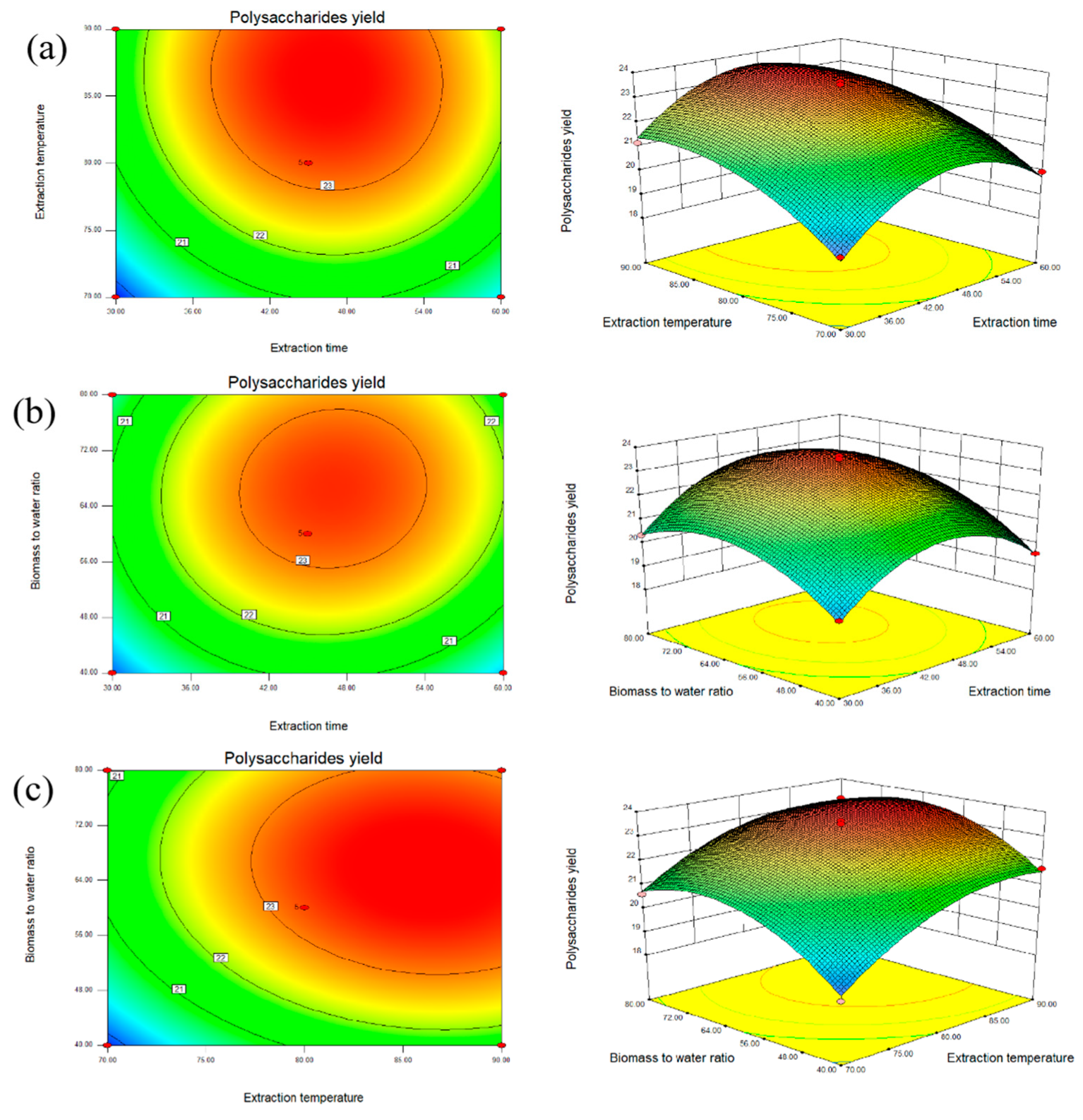
| Run | (A) Extraction Time (min) | (B) Extraction Temperature (°C) | (C) Biomass to Water Ratio (g mL−1) | Extraction Yield (%DW) |
|---|---|---|---|---|
| 1 | 45 | 90 | 40 | 21.69 |
| 2 | 60 | 70 | 60 | 19.96 |
| 3 | 30 | 90 | 60 | 21.15 |
| 4 | 45 | 80 | 60 | 23.64 |
| 5 | 60 | 80 | 40 | 19.56 |
| 6 | 45 | 70 | 40 | 18.55 |
| 7 | 45 | 80 | 60 | 23.15 |
| 8 | 30 | 80 | 80 | 20.35 |
| 9 | 60 | 90 | 60 | 21.84 |
| 10 | 30 | 70 | 60 | 18.84 |
| 11 | 45 | 80 | 60 | 23.11 |
| 12 | 60 | 80 | 80 | 21.45 |
| 13 | 45 | 70 | 80 | 20.60 |
| 14 | 45 | 80 | 60 | 23.52 |
| 15 | 45 | 90 | 80 | 23.12 |
| 16 | 45 | 80 | 60 | 22.88 |
| 17 | 30 | 80 | 40 | 19.12 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 47.28 | 9 | 5.25 | 53.58 | <0.0001 ** |
| A | 1.4 | 1 | 1.4 | 14.31 | 0.0069 ** |
| B | 12.13 | 1 | 12.13 | 123.68 | <0.0001 ** |
| C | 5.45 | 1 | 5.45 | 55.53 | 0.0001 ** |
| AB | 0.046 | 1 | 0.046 | 0.47 | 0.5144 |
| AC | 0.11 | 1 | 0.11 | 1.11 | 0.3270 |
| BC | 0.096 | 1 | 0.096 | 0.98 | 0.3552 |
| A2 | 14.27 | 1 | 14.27 | 145.57 | <0.0001 ** |
| B2 | 3.97 | 1 | 3.97 | 40.5 | 0.0004 ** |
| C2 | 7.1 | 1 | 7.1 | 72.43 | <0.0001 ** |
| Residual | 0.69 | 7 | 0.098 | ||
| Lack of fit | 0.3 | 3 | 0.098 | 1.01 | 0.4764 |
| Pure error | 0.39 | 4 | 0.098 | ||
| Cor total | 47.97 | 16 | |||
| R2 | 0.9857 | C.V.% | 1.47 | ||
| R2Adj | 0.9673 | Adeq. precision | 19.028 |
| Independent Variables | Factor Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A (Extraction time, min) | 30 | 45 | 60 |
| B (Extraction temperature, °C) | 70 | 80 | 90 |
| C (Biomass-to-water ratio, g mL−1) | 40 | 60 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, Q.; Xu, B.; Xiang, W.; Li, A.; Li, T. Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Mar. Drugs 2024, 22, 498. https://doi.org/10.3390/md22110498
Chen Y, Li Q, Xu B, Xiang W, Li A, Li T. Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Marine Drugs. 2024; 22(11):498. https://doi.org/10.3390/md22110498
Chicago/Turabian StyleChen, Yi, Qianmei Li, Bingqi Xu, Wenzhou Xiang, Aifen Li, and Tao Li. 2024. "Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology" Marine Drugs 22, no. 11: 498. https://doi.org/10.3390/md22110498
APA StyleChen, Y., Li, Q., Xu, B., Xiang, W., Li, A., & Li, T. (2024). Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Marine Drugs, 22(11), 498. https://doi.org/10.3390/md22110498









