Nannochloropsis oceanica as a Source of Bioactive Compounds: Mapping the Effects of Cultivation Conditions on Biomass Productivity and Composition Using Response Surface Methodology
Abstract
1. Introduction
2. Results
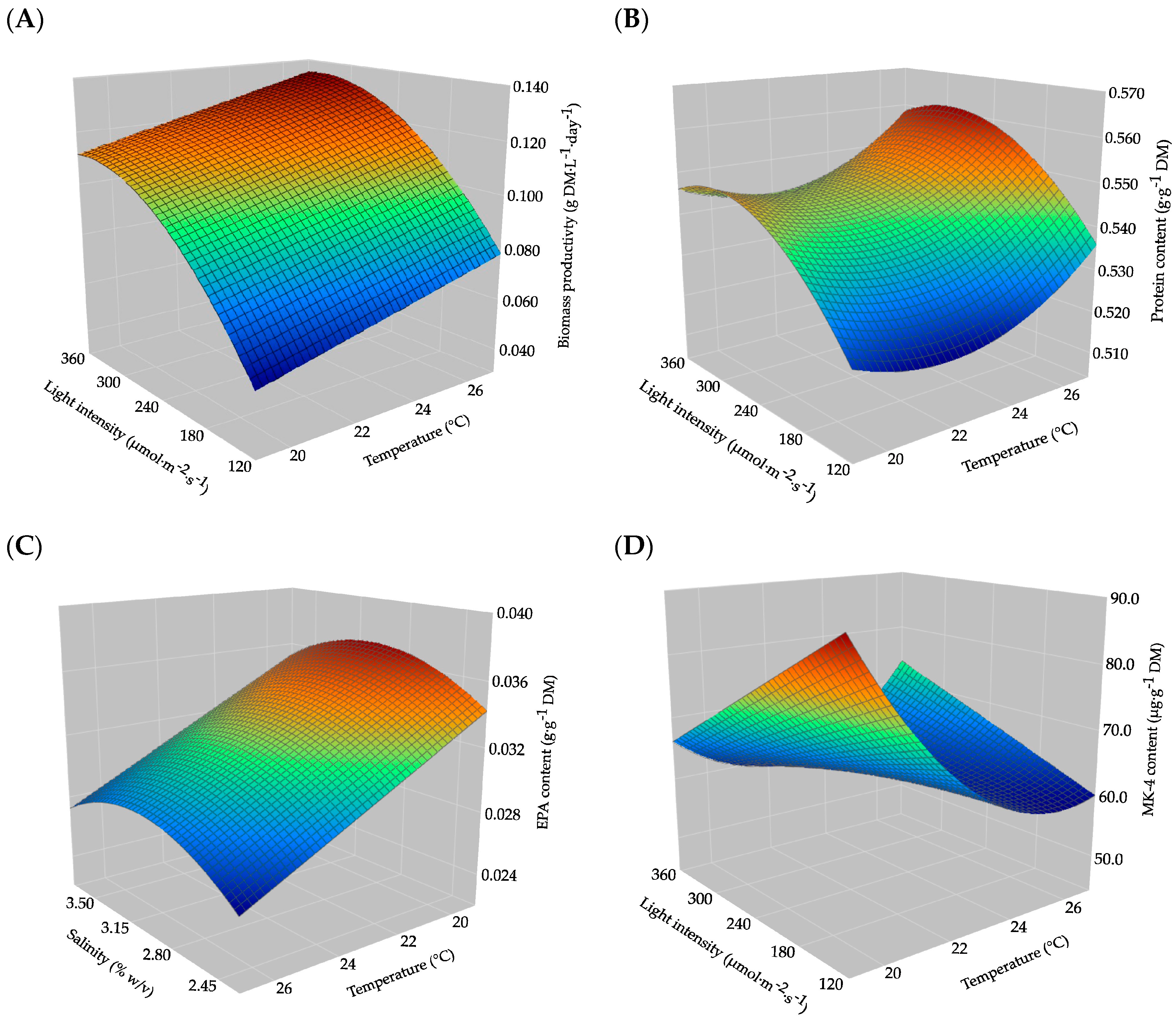
2.1. Modelling Biomass Productivity
2.2. Modelling Protein Content
2.3. Modelling EPA Content
2.4. Modelling Content of MK-4
2.5. Evaluation of Model Precision
3. Discussion
3.1. Biomass Productivity Is Dominated by Light Intensity
3.2. Protein Content Remained Stable Across the Design Space
3.3. EPA Content Enhanced by Lower Cultivation Temperature
3.4. MK-4 Increased Under Low Temperature and Low Light Intensity
3.5. Balancing Biomass Production and Nutritional Quality
4. Materials and Methods
4.1. Microalgae Strain, General Maintenance & Precultures
4.2. Design of Experiment (Box–Behnken Setup)
4.3. Cultivation and Monitoring of Experimental Units
4.4. Culture Dry Matter and Biomass Productivity
4.5. Harvesting and Drying of Biomass
4.6. Powder Moisture Content
4.7. Analysis of Protein
4.8. Fatty Acid Analysis (Direct-FAME)
4.9. Vitamin K Analysis
4.10. Modelling in JMP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Source | DF | Sum of Squares | Mean Square | F Ratio | p-Value | R2/R2 Adj. |
|---|---|---|---|---|---|---|
| Biomass productivity | ||||||
| Model | 3 | 0.009 | 0.003 | 33.91 | <0.0001 | |
| Error | 12 | 0.001 | 0.000 | |||
| C. total | 15 | 0.010 | ||||
| Lack of Fit | 5 | 0.0005 | 0.0001 | 1.32 | 0.356 | |
| Pure Error | 7 | 0.0005 | 0.00008 | |||
| Total Error | 12 | 0.0010 | ||||
| 0.894/0.868 | ||||||
| Protein content | ||||||
| Model | 4 | 0.0023 | 0.0006 | 8.26 | 0.0025 | |
| Error | 11 | 0.0008 | 0.00007 | |||
| C. total | 15 | 0.0030 | ||||
| Lack of Fit | 4 | 0.0002 | 0.00005 | 0.71 | 0.610 | |
| Pure Error | 7 | 0.0005 | 0.00008 | |||
| Total Error | 11 | 0.0008 | ||||
| 0.750/0.660 | ||||||
| EPA content | ||||||
| Model | 3 | 0.0002 | 0.00005 | 11.22 | 0.0008 | |
| Error | 12 | 0.000005 | 0.000005 | |||
| C. total | 15 | 0.0002 | ||||
| Lack of Fit | 5 | 0.00004 | 0.000008 | 4.57 | 0.036 | |
| Pure Error | 7 | 0.00001 | 0.000002 | |||
| Total Error | 12 | 0.00005 | ||||
| 0.737/0.62 | ||||||
| MK-4 content | ||||||
| Model | 4 | 942.34 | 235.6 | 5.88 | 0.0107 | |
| Error | 10 | 400.83 | 40.1 | |||
| C. total | 14 | 1343.17 | ||||
| Lack of Fit | 4 | 279.73 | 69.93 | 3.46 | 0.086 | |
| Pure Error | 6 | 121.10 | 20.18 | |||
| Total Error | 10 | 400.83 | ||||
| R2/R2 Adj. | 0.702/0.585 | |||||
Appendix B
| Fatty Acid | Unit 1 (Low EPA) | Unit 13 (High EPA) |
|---|---|---|
| 10:0 | - | - |
| 12:0 | - | - |
| 13:0 | - | - |
| 14:0 | 0.012 | 0.008 |
| 14:1 | 0.000 | 0.000 |
| 15:0 | 0.001 | 0.000 |
| 16:0 | 0.033 | 0.026 |
| 16:1 (n-7) | 0.041 | 0.037 |
| 16:2 (n-4) | 0.000 | 0.001 |
| 16:3 (n-4) | 0.000 | 0.000 |
| 17:0 | 0.001 | 0.001 |
| 17:1 | - | - |
| 16:4 (n-3) | 0.000 | 0.000 |
| 18:0 | 0.001 | 0.000 |
| 18:1 (n-9) | 0.004 | 0.004 |
| 18:1 (n-7) | 0.001 | 0.000 |
| 18:2 (n-6) | 0.009 | 0.007 |
| 18:2 (n-4) | 0.000 | 0.000 |
| 18:3 (n-6) | - | - |
| 18:3 (n-4) | 0.000 | 0.000 |
| 18:3 (n-3) | - | - |
| 18:4 (n-3) | 0.000 | 0.000 |
| 18:5 (n-3) | - | - |
| 20:0 | 0.000 | 0.000 |
| 20:1 (n-9, n-11) | - | - |
| 20:1 (n-7) | - | - |
| 20:2 (n-6) | 0.000 | 0.000 |
| 20:3 (n-6) | 0.000 | 0.000 |
| 20:4 (n-6) | 0.005 | 0.006 |
| 20:3 (n-3) | - | - |
| 20:4 (n-3) | 0.000 | 0.000 |
| 20:5 (n-3) | 0.025 | 0.038 |
| 22:1 (n-11) | - | - |
| 22:1 (n-9) | - | - |
| 21:5 (n-3) | 0.000 | 0.001 |
| 22:2 | - | - |
| 22:3 | - | - |
| 22:4 | - | - |
| 22:5 (n-3) | - | - |
| 22:6 (n-3) | - | - |
| 24:1 (n-9) | - | - |
| 22:0 | 0.000 | 0.000 |
| 24:0 | - | - |
| SFAs | 0.048 | 0.036 |
| MUFAs | 0.046 | 0.042 |
| PUFAs | 0.044 | 0.054 |
| FA total | 0.137 | 0.132 |
Appendix C
| Unit | Code | Temperature (°C) | Light Intensity (µmol·m−2·s−1) | Salinity (% w/v) | Cultivation Time (days) | DM at Harvest (g DM·L−1) |
|---|---|---|---|---|---|---|
| 1 | ++0 | 27 | 360 | 3.0 | 6.27 | 0.750 |
| 2 | +-0 | 27 | 120 | 3.0 | 8.86 | 0.720 |
| 3 | +0+ | 27 | 240 | 3.7 | 6.85 | 0.840 |
| 4 | +0- | 27 | 240 | 2.3 | 6.27 | 0.760 |
| 5 | 000 | 23 | 240 | 3.0 | 6.85 | 0.780 |
| 6 | 000 | 23 | 240 | 3.0 | 6.85 | 0.790 |
| 7 | 000 | 23 | 240 | 3.0 | 5.96 | 0.710 |
| 8 | 000 | 23 | 240 | 3.0 | 5.96 | 0.660 |
| 9 | 0++ | 23 | 360 | 3.7 | 5.02 | 0.710 |
| 10 | 0-+ | 23 | 120 | 3.7 | 9.96 | 0.735 |
| 11 | 0+- | 23 | 360 | 2.3 | 6.85 | 0.800 |
| 12 | 0-- | 23 | 120 | 2.3 | 8.89 | 0.520 |
| 13 | -+0 | 19 | 360 | 3.0 | 6.23 | 0.720 |
| 14 | --0 | 19 | 120 | 3.0 | 12.78 | 0.640 |
| 15 | -0+ | 19 | 240 | 3.7 | 7.93 | 0.675 |
| 16 | -0- | 19 | 240 | 2.3 | 6.23 | 0.615 |
| v1 | 27 | 350 | 3.0 | 6.24 | 0.767 | |
| v2 | 27 | 350 | 3.0 | 5.80 | 0.725 | |
| v3 | 27 | 350 | 3.0 | 5.80 | 0.715 |
References
- Diaz, C.J.; Douglas, K.J.; Kang, K.; Kolarik, A.L.; Malinovski, R.; Torres-Tiji, Y.; Molino, J.V.; Badary, A.; Mayfield, S.P. Developing algae as a sustainable food source. Front. Nutr. 2023, 9, 1029841. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, K.; Beuckels, A.; Depraetere, O.; Foubert, I.; Markou, G.; Vandamme, D. Wastewater as a Source of Nutrients for Microalgae Biomass Production. In Biomass and Biofuels from Microalgae; Springer: Berlin/Heidelberg, Germany, 2015; pp. 75–94. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Suda, S. The Biology of Nannochloropsis oceanica (a Microalga). 2019. Available online: https://www.ogtr.gov.au/resources/publications/biology-nannochloropsis-oceanica-suda-miyashita-microalga (accessed on 26 September 2024).
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of Omega-3 fatty acids. Neuromol. Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Monitoring of eicosapentaenoic acid (EPA) production in the microalgae Nannochloropsis oceanica. Algal Res. 2020, 45, 101766. [Google Scholar] [CrossRef]
- Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef]
- Skrede, A.; Mydland, L.; Ahlstrøm, Ø.; Reitan, K.; Gislerød, H.; Øverland, M. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. J. Anim. Feed. Sci. 2011, 20, 131–142. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a microalgal food intervention in diet-induced metabolic syndrome in rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Newman, P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, A.; Thulesen, E.T.; Jacobsen, C.; Jakobsen, J. UVB exposure stimulates production of vitamin D3 in selected microalgae. Algal Res. 2021, 59, 102472. [Google Scholar] [CrossRef]
- Jensen, M.B.; Švarc, P.L.; Jakobsen, J. Vitamin K (phylloquinone and menaquinones) in foods—Optimisation of extraction, clean-up and LC–ESI-MS/MS method for quantification. Food Chem. 2021, 345, 128835. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, J.; Yao, X.; Sun, Z.; Feng, Y.; Cui, Q. Simultaneous accumulation of neutral lipids and biomass in Nannochloropsis oceanica IMET1 under high light intensity and nitrogen replete conditions. Algal Res. 2015, 11, 55–62. [Google Scholar] [CrossRef]
- Ferrer-Ledo, N.; Stegemüller, L.; Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Growth and fatty acid distribution over lipid classes in Nannochloropsis oceanica acclimated to different temperatures. Front. Plant Sci. 2023, 14, 1078998. [Google Scholar] [CrossRef]
- Carneiro, M.; Cicchi, B.; Maia, I.; Pereira, H.; Zittelli, G.C.; Varela, J.; Malcata, F.X.; Torzillo, G. Effect of temperature on growth, photosynthesis and biochemical composition of Nannochloropsis oceanica, grown outdoors in tubular photobioreactors. Algal Res. 2020, 49, 101923. [Google Scholar] [CrossRef]
- Khatoon, H.; Rahman, N.A.; Banerjee, S.; Harun, N.; Suleiman, S.S.; Zakaria, N.H.; Lananan, F.; Hamid, S.H.A.; Endut, A. Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural condition. Int. Biodeterior. Biodegrad. 2014, 95, 11–18. [Google Scholar] [CrossRef]
- Wang, B.; Jia, J. Photoprotection mechanisms of Nannochloropsis oceanica in response to light stress. Algal Res. 2020, 46, 101784. [Google Scholar] [CrossRef]
- Sandnes, J.M.; Källqvist, T.; Wenner, D.; Gislerød, H.R. Combined influence of light and temperature on growth rates of Nannochloropsis oceanica: Linking cellular responses to large-scale biomass production. J. Appl. Phycol. 2005, 17, 515–525. [Google Scholar] [CrossRef]
- Kumaran, J.; Poulose, S.; Joseph, V.; Singh, I.S.B. Enhanced biomass production and proximate composition of marine microalga Nannochloropsis oceanica by optimization of medium composition and culture conditions using response surface methodology. Anim. Feed. Sci. Technol. 2021, 271, 114761. [Google Scholar] [CrossRef]
- Vonshak, A.; Novoplansky, N.; Benavides, A.M.S.; Torzillo, G.; Beardall, J.; Palacios, Y.M. Photosynthetic characterization of two Nannochloropsis species and its relevance to outdoor cultivation. J. Appl. Phycol. 2020, 32, 909–922. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Solovchenko, O.; Didi-Cohen, S.; Boussiba, S.; Khozin-Goldberg, I. Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur. J. Lipid Sci. Technol. 2014, 116, 635–644. [Google Scholar] [CrossRef]
- Guo, L.; Liang, S.; Zhang, Z.; Liu, H.; Wang, S.; Yang, G. Domestication of marine microalga Nannochloropsis oceanica to freshwater medium and the physiological responses. J. Oceanol. Limnol. 2019, 37, 1353–1362. [Google Scholar] [CrossRef]
- Jørgensen, L.; Olsen, M. Status og Perspektiver for Mikroalgeproduktion i Danmark. 2021. Available online: https://www.foodbiocluster.dk/Files/Files/FBCD/Viden/Status%20og%20perspektiver%20for%20mikroalgeproduktion%20i%20Danmark.pdf (accessed on 13 September 2024).
- Hulatt, C.J.; Wijffels, R.H.; Bolla, S.; Kiron, V. Production of fatty acids and protein by Nannochloropsis in flat-plate photobioreactors. PLoS ONE 2017, 12, e0170440. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Cerón-García, M.; Fernández-Sevilla, J.; Molina-Grima, E. The influence of culture conditions on biomass and high value product generation by Nannochloropsis gaditana in aquaculture. Algal Res. 2015, 11, 63–73. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Feng, P.; Gao, M.; Burgher, A.; Zhou, T.H.; Pramuk, K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr. Res. 2016, 60, 31042. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Huang, C.-C.; Lee, W.-L.; Chang, J.-S. Engineering strategies for enhancing the production of eicosapentaenoic acid (EPA) from an isolated microalga Nannochloropsis oceanica CY2. Bioresour. Technol. 2013, 147, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Patidar, S.K.; George, B.; Shah, F.; Mishra, S. A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res. 2015, 8, 161–167. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Wang, H.; Cao, X.; Xue, S.; Yang, Q.; Wang, W. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef]
- Gu, N.; Lin, Q.; Li, G.; Tan, Y.; Huang, L.; Lin, J. Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng. Life Sci. 2012, 12, 631–637. [Google Scholar] [CrossRef]
- Ljubic, A.; Holdt, S.L.; Jakobsen, J.; Bysted, A.; Jacobsen, C. Fatty acids, carotenoids, and tocopherols from microalgae: Targeting the accumulation by manipulating the light during growth. J. Appl. Phycol. 2021, 33, 2783–2793. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Tarento, T.D.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Basset, G.J.; Latimer, S.; Fatihi, A.; Soubeyrand, E.; Block, A. Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions. Mini-Reviews Med. Chem. 2017, 17, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Mimuro, M.; Tsuchiya, T.; Inoue, H.; Sakuragi, Y.; Itoh, Y.; Gotoh, T.; Miyashita, H.; Bryant, D.A.; Kobayashi, M. The secondary electron acceptor of photosystem I in Gloeobacter violaceus PCC 7421 is menaquinone-4 that is synthesized by a unique but unknown pathway. FEBS Lett. 2005, 579, 3493–3496. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Nakamura, A.; Watanabe, T. Reversed-phase HPLC Determination of Chlorophyll a′ and Naphthoquinones in Photosystem I of Red Algae: Existence of Two Menaquinone-4 Molecules in Photosystem I of Cyanidium caldarium. Anal. Sci. 2003, 19, 1001–1005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Öquist, G. Effects of low temperature on photosynthesis. Plant Cell Environ. 1983, 6, 281–300. [Google Scholar] [CrossRef]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N. Growth at Low Temperature Mimics High-Light Acclimation in Chlorella vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary reference values for vitamin K. EFSA J. 2017, 15, e04780. [Google Scholar] [CrossRef]
- Fábregas, J.; Otero, A.; Maseda, A.; Domínguez, A. Two-stage cultures for the production of Astaxanthin from Haematococcus plu6ialis. J. Biotechnol. 2001, 89, 65–71. [Google Scholar] [CrossRef]
- Prieto, A.; Cañavate, J.P.; García-González, M. Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J. Biotechnol. 2011, 151, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ge, H.; Zhou, X.; Zhang, D.; Hu, C. Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15. Bioresour. Technol. 2013, 144, 261–267. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; Mishra, S. Integrated process of two stage cultivation of Nannochloropsis sp. for nutraceutically valuable eicosapentaenoic acid along with biodiesel. Bioresour. Technol. 2015, 193, 363–369. [Google Scholar] [CrossRef]
- Mustapa, M.; Sallehudin, N.J.; Mohamed, M.S.; Noor, N.M.; Raus, R.A. Decontamination of Chlorella sp. culture using antibiotics and antifungal cocktail treatment. ARPN J. Eng. Appl. Sci. 2016, 11, 104–109. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Ljubic, A.; Møller, P.; Jacobsen, C. Two-Step Direct Transesterification as a Rapid Method for the Analysis of Fatty Acids in Microalgae Biomass. Eur. J. Lipid Sci. Technol. 2019, 121, 1700409. [Google Scholar] [CrossRef]
| Unit | Code | Temperature (°C) | Light Intensity (µmol·m−2·s−1) | Salinity (% w/v) | Actual Values | Predicted Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass Prod. (g DM·L−1·day−1) | Protein (g·g−1 DM) | EPA (g·g−1 DM) | MK-4 (µg·g−1 DM) | Biomass Prod. (g DM·L−1·day−1) | Protein (g·g−1 DM) | EPA (g·g−1 DM) | MK-4 (µg·g−1 DM) | |||||
| 1 | ++0 | 27 | 360 | 3.0 | 0.120 | 0.566 | 0.025 | 83.0 | 0.135 | 0.558 | 0.029 | 73.8 |
| 2 | +-0 | 27 | 120 | 3.0 | 0.081 | 0.534 | 0.030 | 62.4 | 0.078 | 0.536 | 0.029 | 59.9 |
| 3 | +0+ | 27 | 240 | 3.7 | 0.123 | 0.555 | 0.025 | 59.3 | 0.123 | 0.559 | 0.027 | 66.9 |
| 4 | +0- | 27 | 240 | 2.3 | 0.121 | 0.557 | 0.027 | 62.8 | 0.123 | 0.559 | 0.026 | 66.9 |
| 5 | 000 | 23 | 240 | 3.0 | 0.114 | 0.527 | 0.033 | 61.2 | 0.111 | 0.543 | 0.033 | 61.7 |
| 6 | 000 | 23 | 240 | 3.0 | 0.115 | 0.552 | 0.033 | 60.8 | 0.111 | 0.543 | 0.033 | 61.7 |
| 7 | 000 | 23 | 240 | 3.0 | 0.119 | 0.554 | 0.033 | 52.5 | 0.111 | 0.543 | 0.033 | 61.7 |
| 8 | 000 | 23 | 240 | 3.0 | 0.111 | 0.541 | 0.034 | 67.0 | 0.111 | 0.543 | 0.033 | 61.7 |
| 9 | 0++ | 23 | 360 | 3.7 | 0.141 | 0.546 | 0.033 | 58.0 | 0.124 | 0.542 | 0.031 | 59.4 |
| 10 | 0-+ | 23 | 120 | 3.7 | 0.074 | 0.513 | 0.035 | 66.8 | 0.066 | 0.520 | 0.031 | 64.1 |
| 11 | 0+- | 23 | 360 | 2.3 | 0.117 | 0.540 | 0.030 | 56.9 | 0.124 | 0.542 | 0.030 | 59.4 |
| 12 | 0-- | 23 | 120 | 2.3 | 0.059 | 0.522 | 0.032 | 70.7 | 0.066 | 0.520 | 0.030 | 64.1 |
| 13 | -+0 | 19 | 360 | 3.0 | 0.116 | 0.537 | 0.038 | 72.0 | 0.112 | 0.546 | 0.037 | 66.1 |
| 14 | --0 | 19 | 120 | 3.0 | 0.050 | 0.530 | 0.038 | 88.5 | 0.054 | 0.523 | 0.037 | 89.3 |
| 15 | -0+ | 19 | 240 | 3.7 | 0.085 | 0.550 | 0.032 | 49.6 | 0.099 | 0.546 | 0.035 | 77.7 |
| 16 | -0- | 19 | 240 | 2.3 | 0.099 | 0.545 | 0.032 | 72.5 | 0.099 | 0.546 | 0.034 | 77.7 |
| Model | Predicted Mean [95% CI] | Actual Mean ± Std | Difference in Means (%) |
|---|---|---|---|
| Biomass productivity (g DM·L−1·day−1) | 0.135 [0.124–0.147] | 0.124 ± 0.002 | 8.5 |
| Protein (g·g−1 DM) | 0.559 [0.548–0.571] | 0.520 ± 0.007 | 7.2 |
| EPA1 (g·g−1 DM) | 0.029 [0.027–0.031] | 0.027 ± 0.004 | 7.1 |
| MK-4 2 (µg·g−1 DM) | 73.0 [62.6–83.3] | 63.5 ± 12.1 | 13.9 |
| Factor | - | 0 | + |
|---|---|---|---|
| Temperature (°C) | 19 | 23 | 27 |
| Light intensity (µmol·m−2·s−1) | 120 | 240 | 360 |
| Salinity (% w/v) | 2.3 | 3.0 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundersen, E.; Jakobsen, J.; Holdt, S.L.; Jacobsen, C. Nannochloropsis oceanica as a Source of Bioactive Compounds: Mapping the Effects of Cultivation Conditions on Biomass Productivity and Composition Using Response Surface Methodology. Mar. Drugs 2024, 22, 505. https://doi.org/10.3390/md22110505
Gundersen E, Jakobsen J, Holdt SL, Jacobsen C. Nannochloropsis oceanica as a Source of Bioactive Compounds: Mapping the Effects of Cultivation Conditions on Biomass Productivity and Composition Using Response Surface Methodology. Marine Drugs. 2024; 22(11):505. https://doi.org/10.3390/md22110505
Chicago/Turabian StyleGundersen, Emil, Jette Jakobsen, Susan Løvstad Holdt, and Charlotte Jacobsen. 2024. "Nannochloropsis oceanica as a Source of Bioactive Compounds: Mapping the Effects of Cultivation Conditions on Biomass Productivity and Composition Using Response Surface Methodology" Marine Drugs 22, no. 11: 505. https://doi.org/10.3390/md22110505
APA StyleGundersen, E., Jakobsen, J., Holdt, S. L., & Jacobsen, C. (2024). Nannochloropsis oceanica as a Source of Bioactive Compounds: Mapping the Effects of Cultivation Conditions on Biomass Productivity and Composition Using Response Surface Methodology. Marine Drugs, 22(11), 505. https://doi.org/10.3390/md22110505









