Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances
Abstract
1. Introduction
2. Results and Discussion
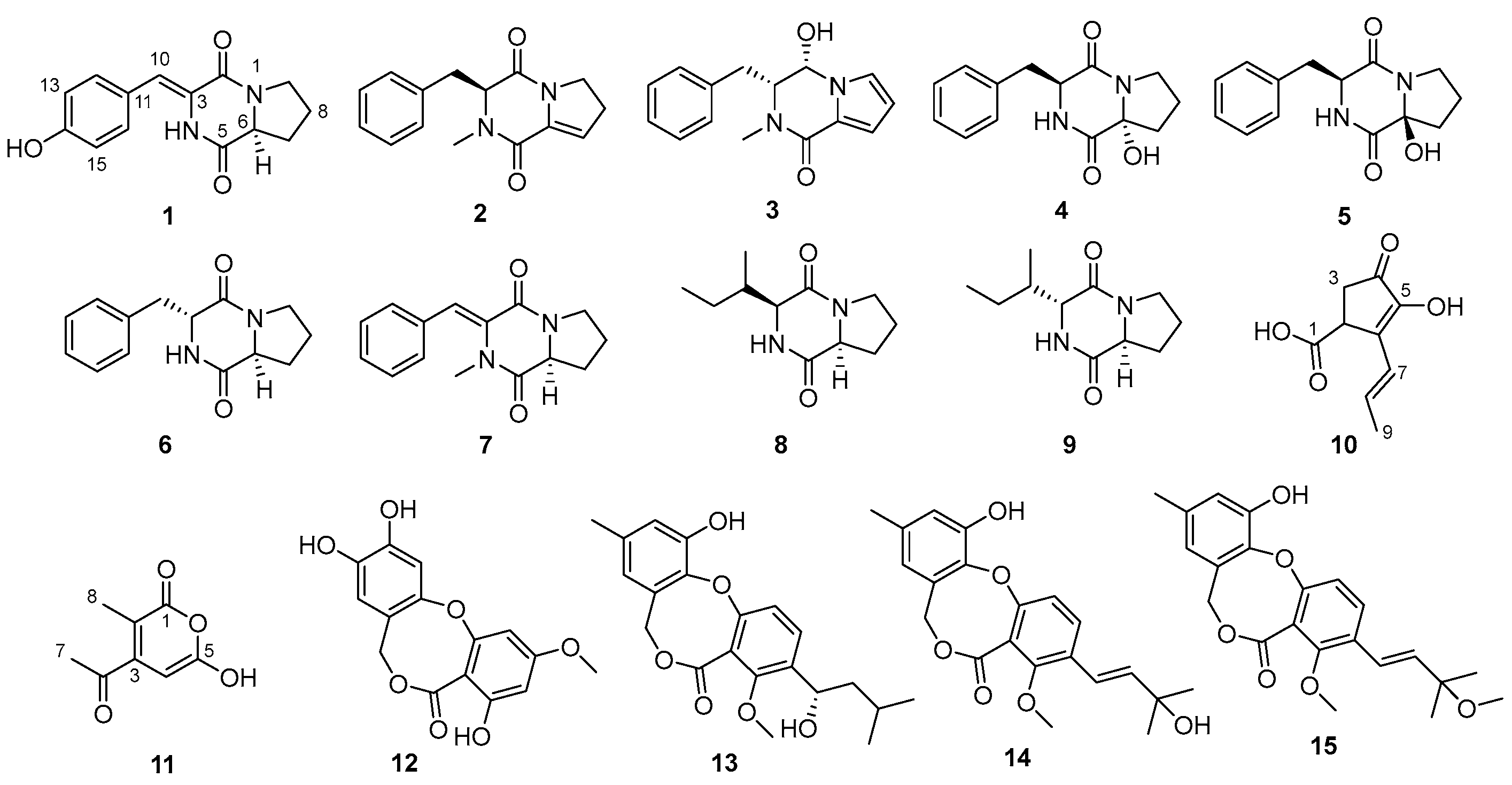
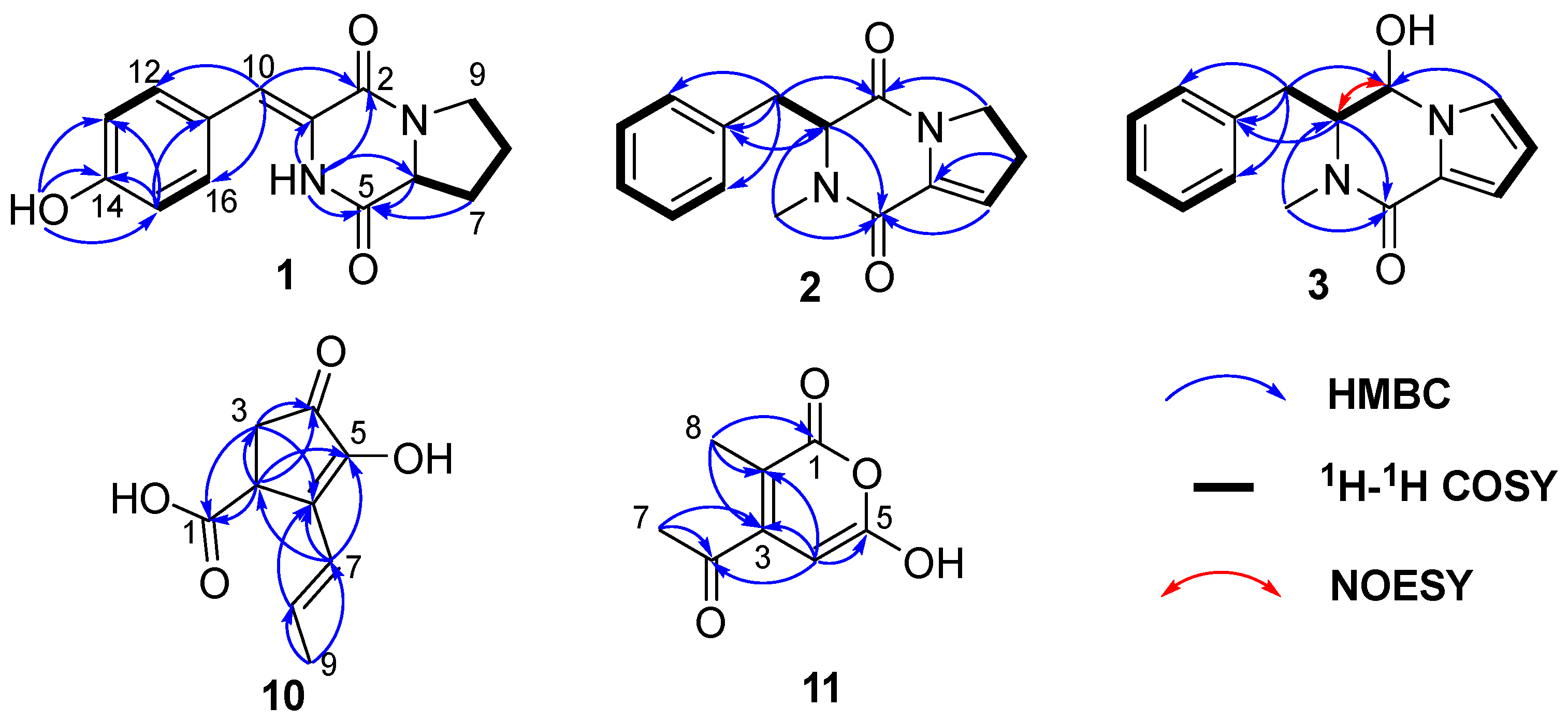
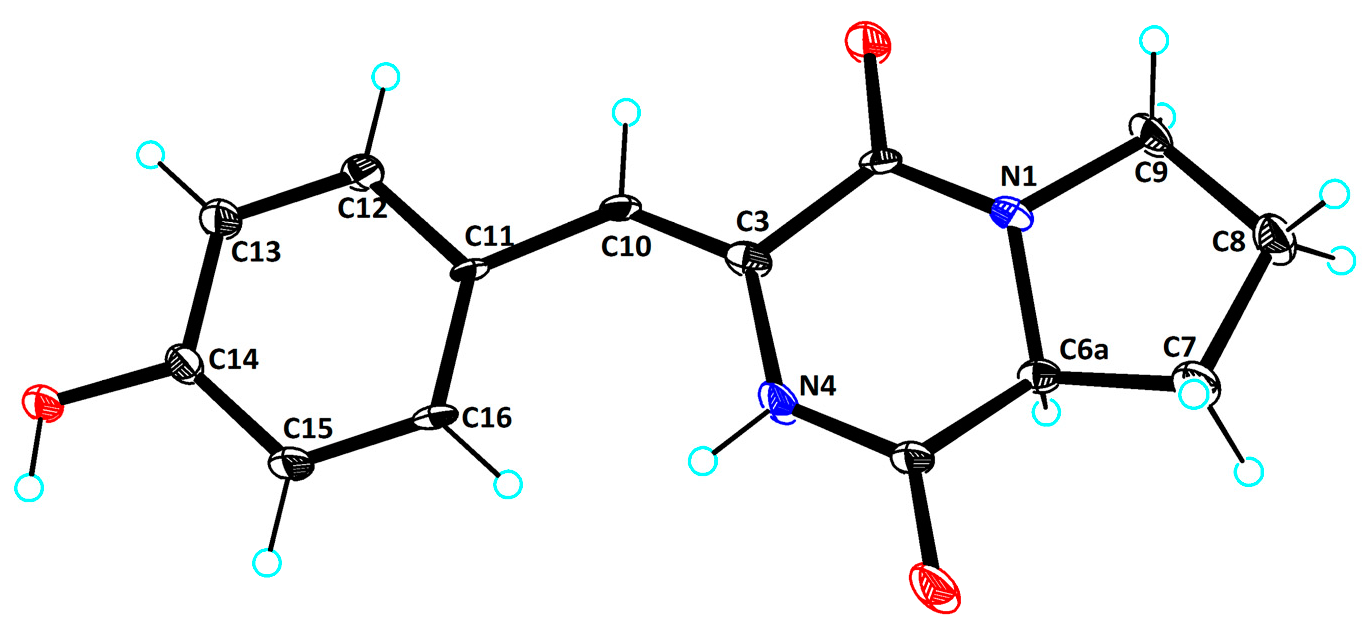
2.1. Structural Determination
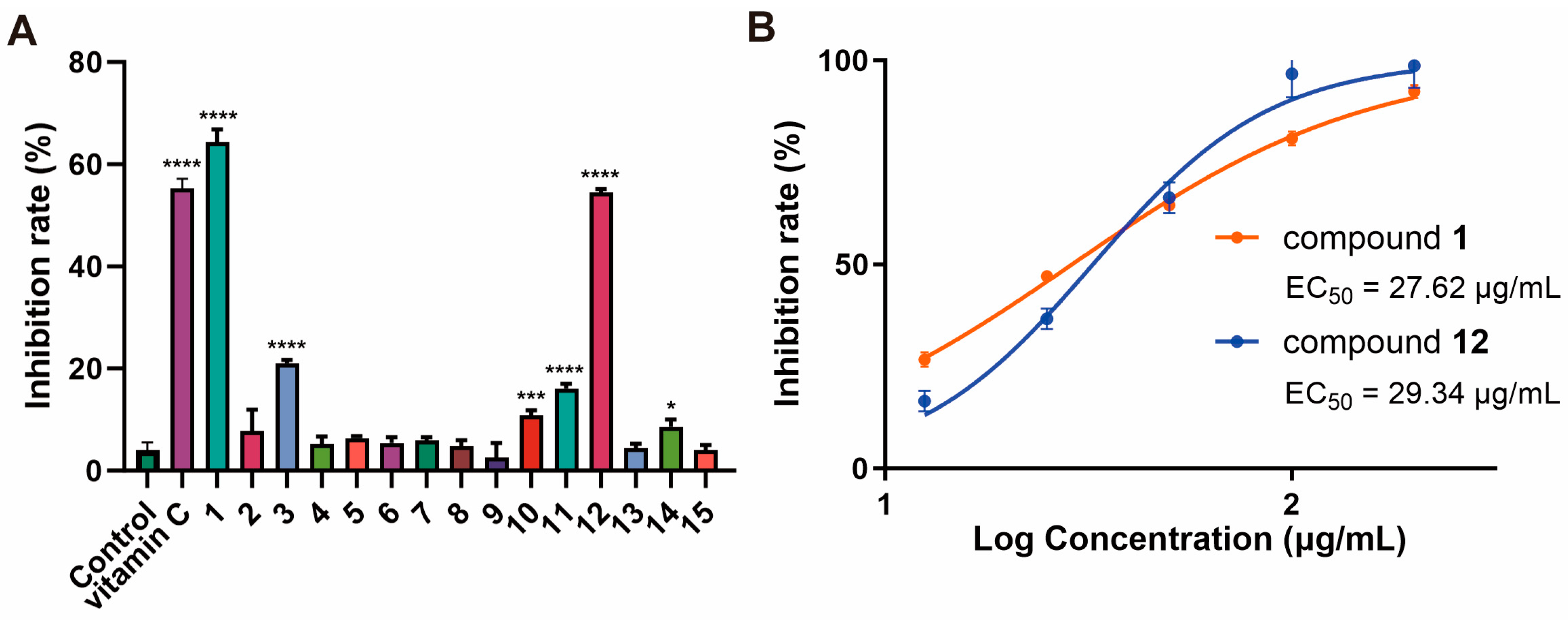
2.2. Bioactivity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation and Purification
3.5. Spectroscopic Data of Compounds
3.6. X-Ray Crystallographic Analysis
3.7. ECD Computation Section
3.8. Antioxidant Activity Assay
3.9. Antimicrobial Activity Assay
3.10. PDE4 and NF-κB Inhibitory Screening Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal Ecosystem-Based Management with Nonlinear Ecological Functions and Values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and Biotechnological Potential of Microorganisms from Mangrove Ecosystems: A Review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Xu, J. Bioactive Natural Products Derived from Mangrove-Associated Microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural Products from Mangrove Sediments-Derived Microbes: Structural Diversity, Bioactivities, Biosynthesis, and Total Synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Xiong, D.; Zhou, L.; Kong, F.; Wang, Q. New Secondary Metabolites of Mangrove-Associated Strains. Mar. Drugs 2024, 22, 372. [Google Scholar] [CrossRef]
- Liu, F.; Cai, X.-L.; Yang, H.; Xia, X.-K.; Guo, Z.-Y.; Yuan, J.; Li, M.-F.; She, Z.-G.; Lin, Y.-C. The Bioactive Metabolites of the Mangrove Endophytic Fungus Talaromyces sp. ZH-154 Isolated from Kandelia candel (L.) Druce. Planta Med. 2010, 76, 185–189. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with Immunosuppressive Activities from Mangrove Endophytic Fungus Penicillium sp. ZJ-SY2. Mar. Drugs 2016, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Fang, T.; Wu, L.; Liu, W.; Tang, J.; Long, Y. New Steroid and Isocoumarin from the Mangrove Endophytic Fungus Talaromyces sp. SCNU-F0041. Molecules 2022, 27, 5766. [Google Scholar] [CrossRef]
- Nicoletti, R.; Salvatore, M.; Andolfi, A. Secondary Metabolites of Mangrove-Associated Strains of Talaromyces. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic Norsesquiterpene Peroxides from the Endophytic Fungus Talaromyces flavus Isolated from the Mangrove Plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef]
- Cai, R.; Chen, S.; Long, Y.; Li, C.; Huang, X.; She, Z. Depsidones from Talaromyces stipitatus SK-4, an Endophytic Fungus of the Mangrove Plant Acanthus Ilicifolius. Phytochem. Lett. 2017, 20, 196–199. [Google Scholar] [CrossRef]
- Park, Y.C.; Gunasekera, S.P.; Lopez, J.V.; McCarthy, P.J.; Wright, A.E. Metabolites from the Marine-Derived Fungus Chromocleista sp. Isolated from a Deep-Water Sediment Sample Collected in the Gulf of Mexico. J. Nat. Prod. 2006, 69, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Du, G.; Deng, W.; Yang, H.; Li, R.; Xu, Q.; Guo, Q. Two Nematicidal Compounds from Lysinimonas M4 against the Pine Wood Nematode, Bursaphelenchus xylophilus. Forests 2022, 13, 1191. [Google Scholar] [CrossRef]
- Lv, M.; Tan, M.; Lu, L.; Bao, S.; Guo, Z.; Deng, Z.; Zhou, K. Secondary Metabolites from Endophytes of Elaeocarpus Decipiens Hemsl. with Co-Cultivation Method. J. China Three Gorges Univ. Sci. 2018, 5, 2758. [Google Scholar]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M.; Yang, M.Q. Bioactive Aromatic Metabolites from the Sea Urchin-Derived Actinomycete Streptomyces spectabilis Strain HDa1. Phytochem. Lett. 2018, 25, 132–135. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Cytotoxic Metabolites from the Fungal Endophyte Alternaria sp. and Their Subsequent Detection in Its Host Plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Shim, S.H. Chemical Constituents of the Endophyte Penicillium sp. Isolated from Artemisia princeps. Chem. Nat. Compd. 2020, 56, 122–124. [Google Scholar] [CrossRef]
- Xia, X.; Kim, S.; Liu, C.; Shim, S. Secondary Metabolites Produced by an Endophytic Fungus Pestalotiopsis sydowiana and Their 20S Proteasome Inhibitory Activities. Molecules 2016, 21, 944. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure-Activity Relationship of Antioxidant Dipeptides: Dominant Role of Tyr, Trp, Cys and Met Residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A-F, Cytotoxic Chloroazaphilones from the Marine Mangrove Endophytic Fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, J.; Xia, Z.; Chen, C.; Liu, Y.; Jayasinghe, L.; Wang, X.; Zhou, X. New Bioactive Polyketides from the Mangrove-Derived Fungus Penicillium sp. SCSIO 41411. Mar. Drugs 2024, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, X.; Yang, Z.; Tan, Y.; Peng, B.; Liu, Y.; Zhou, X. Thiodiketopiperazines and Alkane Derivatives Produced by the Mangrove Sediment-Derived Fungus Penicillium ludwigii SCSIO 41408. Front. Microbiol. 2022, 13, 857041. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhou, Q.; Qi, X.; Zhang, F.; Yang, J.; Chen, C.; Zhang, K.; Chen, Z.; Luo, H.-B.; Liu, Y.; et al. Discovery of Oxidized P-Terphenyls as Phosphodiesterase 4 Inhibitors from Marine-Derived Fungi. J. Nat. Prod. 2024, 87, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Gao, L.; Wang, Y.; Zheng, Y.; Lin, X.; Zhou, P.; Chen, C.; Liu, K.; Tang, L.; Liu, Y.; et al. Discovery of a Novel Anti-Osteoporotic Agent from Marine Fungus-Derived Structurally Diverse Sirenins. Eur. J. Med. Chem. 2024, 265, 116068. [Google Scholar] [CrossRef]

| Pos. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC Type | δH (J in Hz) | δC Type | δH (J in Hz) | δC Type | δH (J in Hz) | |
| 2 | 159.2, C | 77.0, CH | 5.68, d (3.7) | 165.5, C | ||
| 3 | 124.3, C | 63.7, CH | 3.92, dt (9.0, 3.7) | 62.8, CH | 4.37, t (4.4) | |
| 5 | 167.1, C | 158.1, C | 158.8, C | |||
| 6 | 58.2, CH | 4.34, m | 122.9, C | 127.0, C | ||
| 7 | 28.0, CH2 | 2.20, m; 1.88, overlapped | 120.3, CH | 6.60, dd (3.7, 1.6) | 114.4, CH | 5.40, t (7.7) |
| 8 | 21.7, CH2 | 1.88, overlapped | 108.9, CH | 6.15, dd (3.7, 2.7) | 28.6, CH2 | 2.04, m |
| 9 | 45.1, CH2 | 3.51, m | 112.2, CH | 6.99, dd (2.7, 1.6) | 59.8, CH2 | 3.23, m |
| 10 | 115.5, CH | 6.59, s | 33.3, CH2 | 2.56, m; 3.08, dd (13.6, 5.2) | 30.7, CH2 | 3.04, dd (13.8, 3.8); 3.15, dd (13.8, 5.1) |
| 11 | 126.1, C | 138.0, C | 134.8, C | |||
| 12 | 131.1, CH | 7.38, d (8.3) | 129.4, CH | 7.16, d (6.8) | 129.7, CH | 7.03, m |
| 13 | 115.5, CH | 6.78, d (8.4) | 128.3, CH | 7.28, d (7.4) | 128.1, CH | 7.21, overlapped |
| 14 | 157.6, C | 126.3, CH | 7.21, m | 126.9, CH | 7.21, overlapped | |
| 15 | 115.5, CH | 6.78, d (8.4) | 128.3, CH | 7.28, d (7.4) | 128.1, CH | 7.21, overlapped |
| 16 | 131.1, CH | 7.38, d (8.3) | 129.4, CH | 7.16, d (6.8) | 129.7, CH | 7.03, m |
| 17 | 32.9, CH3 | 2.54, s | 32.3, CH3 | 2.97, s | ||
| NH | 9.83, s | NH | 9.79, s | |||
| 14-OH | 9.76, s | |||||
| Pos. | 10 | 11 | ||
|---|---|---|---|---|
| δC Type | δH (J in Hz) | δC Type | δH (J in Hz) | |
| 1 | 175.0, C | 163.2, C | ||
| 2 | 40.1, CH | 3.64, d (7.0) | 104.0, C | |
| 3 | 36.4, CH2 | 2.62, dd (18.4, 7.0); 2.28, d (18.4) | 151.7, C | |
| 4 | 200.2, C | 104.8, CH | 6.85, s | |
| 5 | 149.3, C | 164.2, C | ||
| 6 | 137.5, C | 191.1, C | ||
| 7 | 123.8, CH | 6.49, d (15.9) | 25.6, CH3 | 2.14, s |
| 8 | 131.2, CH | 6.15, dq (15.9, 6.8) | 9.2, CH3 | 1.84, s |
| 9 | 19.0, CH3 | 1.84, d (6.8) | ||
| 5-OH | 9.78, s | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Cai, J.; Chen, Y.; Li, X.; Chen, C.; Liu, Y.; Jayasinghe, L.; Zhou, X. Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances. Mar. Drugs 2024, 22, 559. https://doi.org/10.3390/md22120559
Zeng Z, Cai J, Chen Y, Li X, Chen C, Liu Y, Jayasinghe L, Zhou X. Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances. Marine Drugs. 2024; 22(12):559. https://doi.org/10.3390/md22120559
Chicago/Turabian StyleZeng, Zhihao, Jian Cai, Yi Chen, Xinlong Li, Chunmei Chen, Yonghong Liu, Lalith Jayasinghe, and Xuefeng Zhou. 2024. "Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances" Marine Drugs 22, no. 12: 559. https://doi.org/10.3390/md22120559
APA StyleZeng, Z., Cai, J., Chen, Y., Li, X., Chen, C., Liu, Y., Jayasinghe, L., & Zhou, X. (2024). Three New Dipeptide and Two New Polyketide Derivatives from the Mangrove-Derived Fungus Talaromyces sp.: Antioxidant Activity of Two Isolated Substances. Marine Drugs, 22(12), 559. https://doi.org/10.3390/md22120559










