Optimization of Collagen Extraction from Fish Scales Using Tris-Glycine Buffer: A Taguchi Methodological Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessing the Effective of Optimized Parameters on Collagen Yield
2.1.1. Effect of Acetic Acid Concentration on Collagen Extraction
2.1.2. Effect of Volume of Acetic Acid on Collagen Extraction
2.1.3. Effect of Time of Soaking on Collagen Extraction
2.1.4. Effect of Buffer Concentration on Collagen Extraction
2.2. Experimental Factors Affecting the Extraction of Collagen
2.3. ANOVA Model for Collagen Extraction Optimization
2.4. Responses Modelling
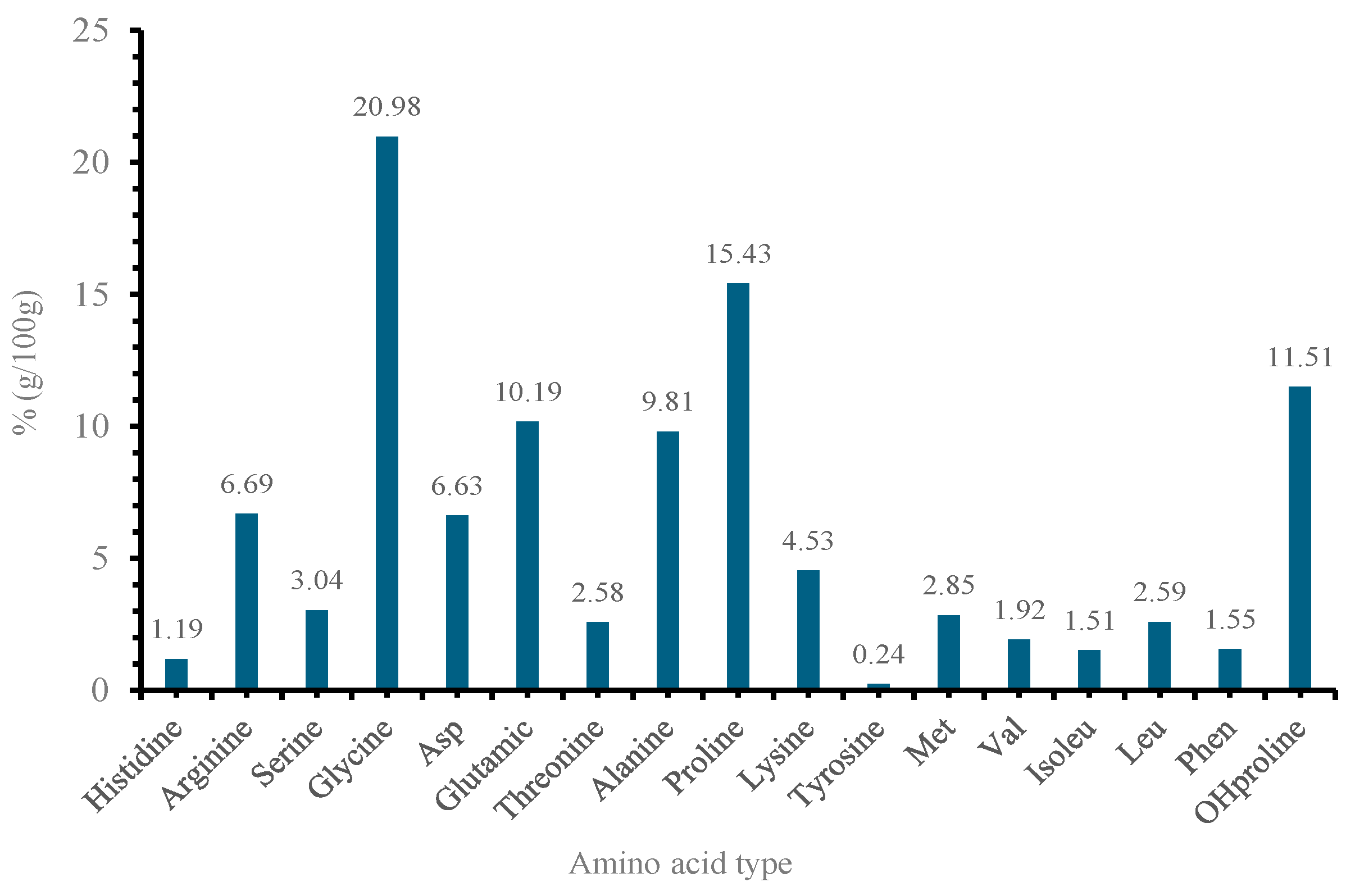
2.5. Amino Acid Quantification and Characterization
3. Materials and Methods
3.1. Preparation and Extraction of Fish Scale Collagen (FSC) from Fish Scales
3.2. Optimization of the Extraction Parameters
3.3. SDS-PAGE Analysis
3.4. Protein Estimation
3.5. Fourier Transform Infrared Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Kumar, S.; Singh, H.; Khan, M.A.; Barai, A.; Tewari, A.; Rana, R.S.; Bera, S.; Sen, S.; Sahni, A. Chemical evidence of preserved collagen in 54-million-year-old fish vertebrae. Palaeontology 2020, 63, 195–202. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913. [Google Scholar]
- San Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P. Collagen structure-function mapping informs applications for regenerative medicine. Bioengineering 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Song, C.; Yao, L.; Xiao, J. Recent progresses of collagen dressings for chronic skin wound healing. Collagen Leather 2023, 5, 31. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of collagen-based hydrogel in skin wound healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The use of collagen-based materials in bone tissue engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-based biomaterials for tissue engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, D.; Onwubu, S.C.; Mokhothu, T.H.; Mdluli, P.S.; Makgobole, M.U.; Mishra, A.K. Effectiveness of Fish Scale-Derived Collagen as an Alternative Filler Material in the Fabrication of Polyurethane Foam Composites. Adv. Polym. Technol. 2024, 2024, 1723927. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Naidoo, D.; Obiechefu, Z.; Mokhothu, T.H.; Mdluli, P.S.; Mishra, A.K. Enhancing Mechanical and Thermal Properties of Epoxy Composites with Fish Scale-Derived Collagen Reinforcement. Adv. Polym. Technol. 2024, 2024, 8890654. [Google Scholar]
- Jadach, B.; Mielcarek, Z.; Osmałek, T. Use of Collagen in Cosmetic Products. Curr. Issues Mol. Biol. 2024, 46, 2043–2070. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Pesterau, A.M.; Prasacu, I.; Ionescu, A.M.; Pascale, C.; Dragan, A.M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen-structure, properties and application. Eng. Biomater. 2020, 23. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Subhan, F.; Kang, H.Y.; Lim, Y.; Ikram, M.; Baek, S.Y.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S. Fish scale collagen peptides protect against CoCl2/TNF-α-induced cytotoxicity and inflammation via inhibition of ROS, MAPK, and NF-κB pathways in HaCaT cells. Oxidative Med. Cell. Longev. 2017, 2017, 9703609. [Google Scholar] [CrossRef]
- Dang, Q.; Liu, K.; Zhang, Z.; Liu, C.; Liu, X.; Xin, Y.; Cheng, X.; Xu, T.; Cha, D.; Fan, B. Fabrication and evaluation of thermosensitive chitosan/collagen/α, β-glycerophosphate hydrogels for tissue regeneration. Carbohydr. Polym. 2017, 167, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Septiani, R.A.; Aisyah, D.; Cahyati, S.A.; Gunarti, N.S. Pemanfaatan Kolagen dari Hewan. J. Buana Farma 2023, 3, 24–32. [Google Scholar]
- Smith, I.P.; Domingos, M.; Richardson, S.M.; Bella, J. Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo. Mar. Drugs 2023, 21, 59. [Google Scholar] [CrossRef]
- Browne, S.; Zeugolis, D.I.; Pandit, A. Collagen: Finding a solution for the source. Tissue Eng. Part A 2013, 19, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen derived from fish industry waste: Progresses and challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Kikionis, S.; Karkatzoulis, L.; Bethanis, K.; Roussis, V.; Ioannou, E. Valorization of fish waste: Isolation and characterization of acid-and pepsin-soluble collagen from the scales of mediterranean fish and fabrication of collagen-based nanofibrous scaffolds. Mar. Drugs 2022, 20, 664. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zeng, Y.; Yuan, X.; Wang, J.K.; Tay, C.Y. Waste-to-resource: Extraction and transformation of aquatic biomaterials for regenerative medicine. Biomater. Adv. 2024, 166, 214023. [Google Scholar] [CrossRef] [PubMed]
- Kıyak, B.D.; Çınkır, N.İ.; Çelebi, Y.; Malçok, S.D.; Koç, G.Ç.; Adal, S.; Yüksel, A.N.; Süfer, Ö.; Karabacak, A.Ö.; Ramniwas, S.; et al. Advanced technologies for the collagen extraction from food waste–A review on recent progress. Microchem. J. 2024, 201, 110404. [Google Scholar] [CrossRef]
- Lai, B.-C.; Wu, J.-G.; Luo, S.-C. Revisiting background signals and the electrochemical windows of Au, Pt, and GC electrodes in biological buffers. ACS Appl. Energy Mater. 2019, 2, 6808–6816. [Google Scholar] [CrossRef]
- Menezes, M.D.L.L.R.; Ribeiro, H.L.; Flávia de Oliveira, M.; de Andrade Feitosa, J.P. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids Surf. B-Biointerfaces 2020, 189, 110852. [Google Scholar] [CrossRef]
- Meena, N.K.; Swarnkar, A.; Yang, J.; Gupta, N.; Niazi, K.R. Modified taguchi-based approach for optimal distributed generation mix in distribution networks. IEEE Access 2019, 7, 135689–135702. [Google Scholar] [CrossRef]
- Hamzaçebi, C.; Li, P.; Pereira, P.A.R.; Navas, H. Taguchi method as a robust design tool. In Quality Control-Intelligent Manufacturing, Robust Design and Charts; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar]
- Mokrejš, P.; Gál, R.; Pavlačková, J. Enzyme conditioning of chicken collagen and taguchi design of experiments enhancing the yield and quality of prepared gelatins. Int. J. Mol. Sci. 2023, 24, 3654. [Google Scholar] [CrossRef] [PubMed]
- Aidat, O.; Belkacemi, L.; Belalia, M.; Zainol, M.K. Optimisation of gelatine extraction from chicken feet-heads blend using Taguchi design and response surface methodology. Int. Food Res. J. 2023, 30, 1201–1211. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. The stability of dimeric D-amino acid oxidase from porcine kidney strongly depends on the buffer nature and concentration. Catalysts 2022, 12, 1009. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Kiew, P.L.; Don, M.M. The influence of acetic acid concentration on the extractability of collagen from the skin of hybrid Clarias sp. and its physicochemical properties: A preliminary study. Focus. Mod. Food Ind. 2013, 2, 123–128. [Google Scholar]
- Kim, H.K.; Kim, Y.H.; Park, H.J.; Lee, N.H. Application of ultrasonic treatment to extraction of collagen from the skins of sea bass Lateolabrax japonicus. Fish. Sci. 2013, 79, 849–856. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Du, X.; Yang, Y.; Liu, J. Optimization of conditions for extraction of acid-soluble collagen from grass carp (Ctenopharyngodon idella) by response surface methodology. Innov. Food Sci. Emerg. Technol. 2008, 9, 604–607. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Inanc, S.; Keles, D.; Oktay, G. An improved collagen zymography approach for evaluating the collagenases MMP-1, MMP-8, and MMP-13. Biotechniques 2017, 63, 174–180. [Google Scholar] [CrossRef]
- Martin-Morales, L.; Manzano, S.; Rodrigo-Faus, M.; Vicente-Barrueco, A.; Lorca, V.; Núñez-Moreno, G.; Bragado, P.; Porras, A.; Caldes, T.; Garre, P.; et al. Germline gain-of-function MMP11 variant results in an aggressive form of colorectal cancer. Int. J. Cancer 2023, 152, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al-Hawary, I.I.; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef]
- Wahyuningsih, R.; Nurliyani, R.; Pertiwiningrum, A.; Rohman, A.; Fitriyanto, N.A.; Erwanto, Y. Optimization of acid soluble collagen extraction from Indonesian local “Kacang” goat skin and physico-chemical properties characterization. Chem. Eng. Trans. 2018, 63, 703–708. [Google Scholar]
- Dixit, A.; Kumar, K. Optimization of mechanical properties of silica gel reinforced aluminium MMC by using Taguchi method. Mater. Today Proc. 2015, 2, 2359–2366. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Jafari, M.; Edrissi, M.; Javadian, H. Taguchi L8 (27) orthogonal array design method for the optimization of synthesis conditions of manganese phosphate (Mn3(PO4)2) nanoparticles using water-in-oil microemulsion method. J. Mol. Liq. 2016, 219, 1131–1136. [Google Scholar] [CrossRef]
- Meena, K.; Kumar, A.; Pandya, S.N. Optimization of friction stir processing parameters for 60/40 brass using Taguchi method. Mater. Today Proc. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 2017, 4, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Hurych, J. Methods of hydroxyproline determination. Kozarstvi 1962, 12, 317–323. [Google Scholar]




| Level | Concentration of Acid | Volume of Acidic Acid (mL) | Time of Soaking (Minutes) | Buffer (mL) |
|---|---|---|---|---|
| 1 | 18.9100 | 8.1615 | 5.1312 | 1.5647 |
| 2 | 4.0194 | 0.0000 | 6.3310 | 4.5850 |
| 3 | 1.1995 | 0.0000 | 0.3480 | 13.7750 |
| Delta | 17.7105 | 8.1615 | 5.9830 | 12.2103 |
| Rank | 1 | 3 | 4 | 2 |
| Source | Df | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 4 | 1750.99 | 437.747 | 21.87 | 0.000 |
| Concentration of acetic acid | 1 | 250.47 | 250.471 | 12.52 | 0.001 |
| Volume of acetic acid (mL) | 1 | 92.54 | 92.545 | 4.62 | 0.039 |
| Time of soaking (min) | 1 | 4.78 | 4.778 | 0.24 | 0.629 |
| Buffer (mL) | 1 | 243.41 | 243.410 | 12.16 | 0.001 |
| Error | 31 | 620.42 | 20.014 | ||
| Lack-of-fit | 18 | 429.93 | 23.885 | 1.63 | 0.187 |
| Pure Error | 13 | 190.49 | 14.653 | ||
| Total | 35 | 2371.41 |
| Response | The Obtain Model | Factors | Coef | SE Coef | R2 | T-Value | p-Value | VIF |
|---|---|---|---|---|---|---|---|---|
| Yield (%) | 6.17 − 1.504 Concentration of acetic acid − 0.01330 Volume of acetic acid (mL) + 0.0127 Time of soaking fish + 1.152 Buffer (mL) | Constant | 6.17 | 3.88 | 73.84 | 1.59 | 0.122 | |
| Concentration of acetic acids | −1.504 | 0.425 | −3.54 | 0.001 | 1.41 | |||
| Volume of acetic acid | −0.01330 | 0.00619 | −2.15 | 0.039 | 1.61 | |||
| Time of soaking | 0.0127 | 0.0260 | 0.49 | 0.629 | 1.18 | |||
| Buffer | 1.152 | 0.330 | 3.49 | 0.001 | 2.22 |
| Factors | Symbols | Coded Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Concentration of acetic acid | A | 0.5 | 1 | 5 |
| Volume of acetic acid (mL) | B | 100 | 300 | 500 |
| Time of soaking fish | C | 30 | 60 | 120 |
| Buffer (mL) | D | 2.5 | 5 | 10 |
| Run | Concentration of Acetic Acid | Volume of Acetic Acid (mL) | Time of Soaking Fish | Buffer (mL) | Yield |
|---|---|---|---|---|---|
| 1 | 0.5 | 100 | 30 | 2.5 | 0 |
| 2 | 0.5 | 100 | 60 | 2.5 | 0 |
| 3 | 0.5 | 100 | 120 | 2.5 | 0 |
| 4 | 0.5 | 300 | 30 | 2.5 | 0 |
| 5 | 0.5 | 300 | 60 | 2.5 | 0 |
| 6 | 0.5 | 300 | 120 | 2.5 | 0 |
| 7 | 0.5 | 500 | 30 | 2.5 | 0 |
| 8 | 0.5 | 500 | 60 | 2.5 | 0 |
| 9 | 0.5 | 500 | 120 | 2.5 | 0 |
| 10 | 5 | 100 | 30 | 2.5 | 0 |
| 11 | 5 | 100 | 60 | 2.5 | 21.73 |
| 12 | 5 | 100 | 120 | 2.5 | 3.48 |
| 13 | 5 | 300 | 30 | 2.5 | 0 |
| 14 | 1 | 300 | 60 | 2.5 | 0 |
| 15 | 1 | 300 | 120 | 2.5 | 0 |
| 16 | 1 | 500 | 30 | 2.5 | 0 |
| 17 | 5 | 500 | 60 | 2.5 | 0 |
| 18 | 5 | 500 | 120 | 2.5 | 0 |
| 19 | 0.5 | 100 | 30 | 5 | 0 |
| 20 | 0.5 | 100 | 30 | 5 | 0 |
| 21 | 0.5 | 100 | 60 | 5 | 4.53 |
| 22 | 5 | 100 | 60 | 5 | 0 |
| 23 | 5 | 100 | 60 | 5 | 11.94 |
| 24 | 5 | 100 | 60 | 5 | 11.04 |
| 25 | 0.5 | 100 | 30 | 10 | 11.64 |
| 26 | 0.5 | 100 | 60 | 10 | 11.85 |
| 27 | 0.5 | 100 | 60 | 10 | 10.89 |
| 28 | 5 | 100 | 30 | 10 | 11.56 |
| 29 | 5 | 100 | 30 | 10 | 14.81 |
| 30 | 5 | 100 | 60 | 10 | 11.5 |
| 31 | 0.5 | 100 | 30 | 10 | 9.19 |
| 32 | 0.5 | 100 | 30 | 10 | 20.9 |
| 33 | 0.5 | 100 | 60 | 10 | 22.28 |
| 34 | 5 | 100 | 30 | 10 | 20.13 |
| 35 | 5 | 100 | 30 | 10 | 22.03 |
| 36 | 5 | 100 | 60 | 10 | 20.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makgobole, M.U.; Onwubu, S.C.; Baruwa, A.; Mpofana, N.; Obiechefu, Z.; Naidoo, D.; Khathi, A.; Mkhwanazi, B. Optimization of Collagen Extraction from Fish Scales Using Tris-Glycine Buffer: A Taguchi Methodological Approach. Mar. Drugs 2024, 22, 562. https://doi.org/10.3390/md22120562
Makgobole MU, Onwubu SC, Baruwa A, Mpofana N, Obiechefu Z, Naidoo D, Khathi A, Mkhwanazi B. Optimization of Collagen Extraction from Fish Scales Using Tris-Glycine Buffer: A Taguchi Methodological Approach. Marine Drugs. 2024; 22(12):562. https://doi.org/10.3390/md22120562
Chicago/Turabian StyleMakgobole, Mokgadi Ursula, Stanley Chibuzor Onwubu, Abayomi Baruwa, Nomakhosi Mpofana, Zodidi Obiechefu, Deneshree Naidoo, Andile Khathi, and Blessing Mkhwanazi. 2024. "Optimization of Collagen Extraction from Fish Scales Using Tris-Glycine Buffer: A Taguchi Methodological Approach" Marine Drugs 22, no. 12: 562. https://doi.org/10.3390/md22120562
APA StyleMakgobole, M. U., Onwubu, S. C., Baruwa, A., Mpofana, N., Obiechefu, Z., Naidoo, D., Khathi, A., & Mkhwanazi, B. (2024). Optimization of Collagen Extraction from Fish Scales Using Tris-Glycine Buffer: A Taguchi Methodological Approach. Marine Drugs, 22(12), 562. https://doi.org/10.3390/md22120562





