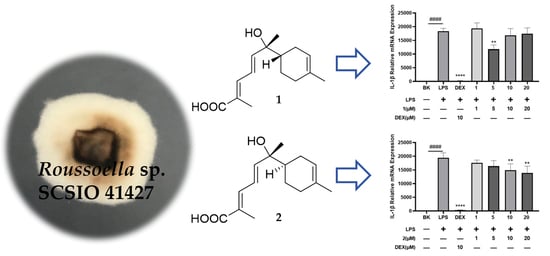
Two New Sesquiterpenoids and a New Shikimic Acid Metabolite from Mangrove Sediment-Derived Fungus Roussoella sp. SCSIO 41427
Abstract
:1. Introduction
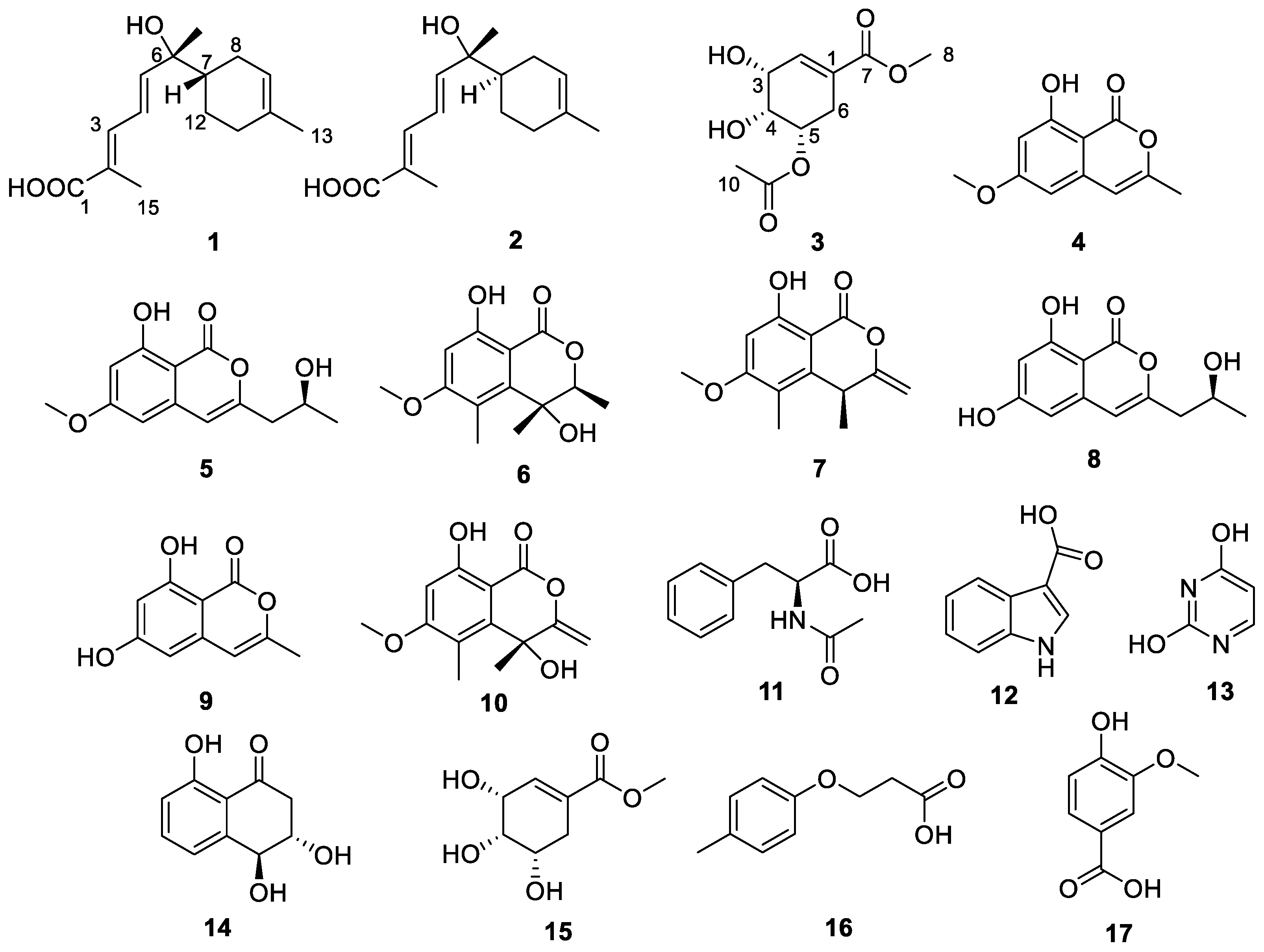
2. Results and Discussion
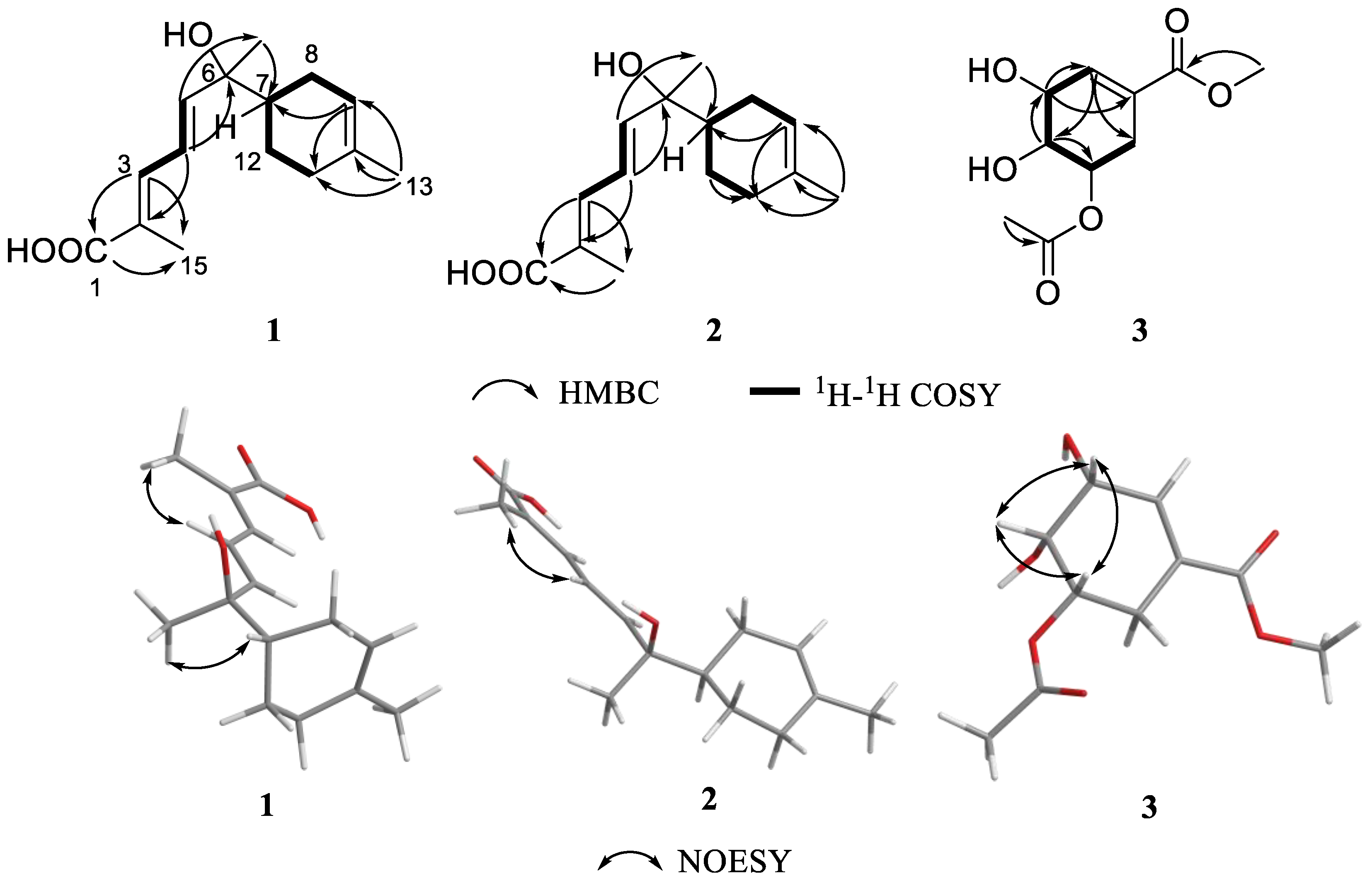
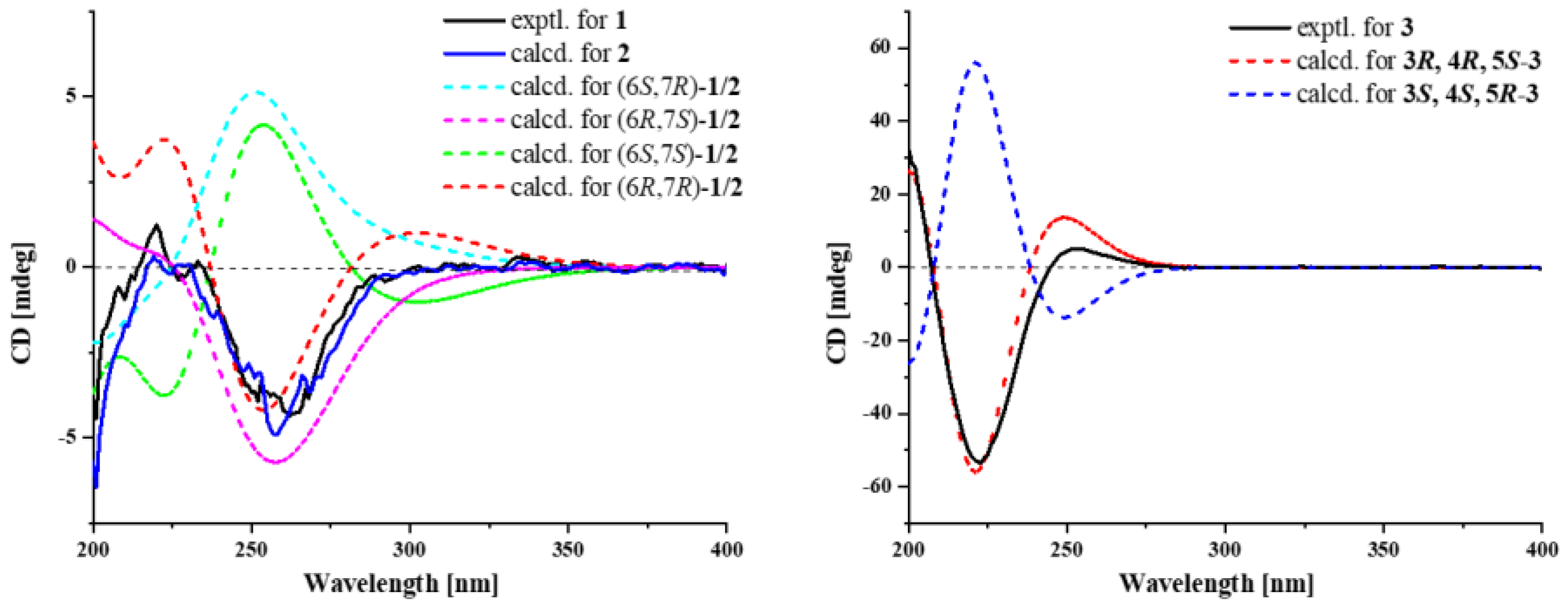
2.1. Structural Determination
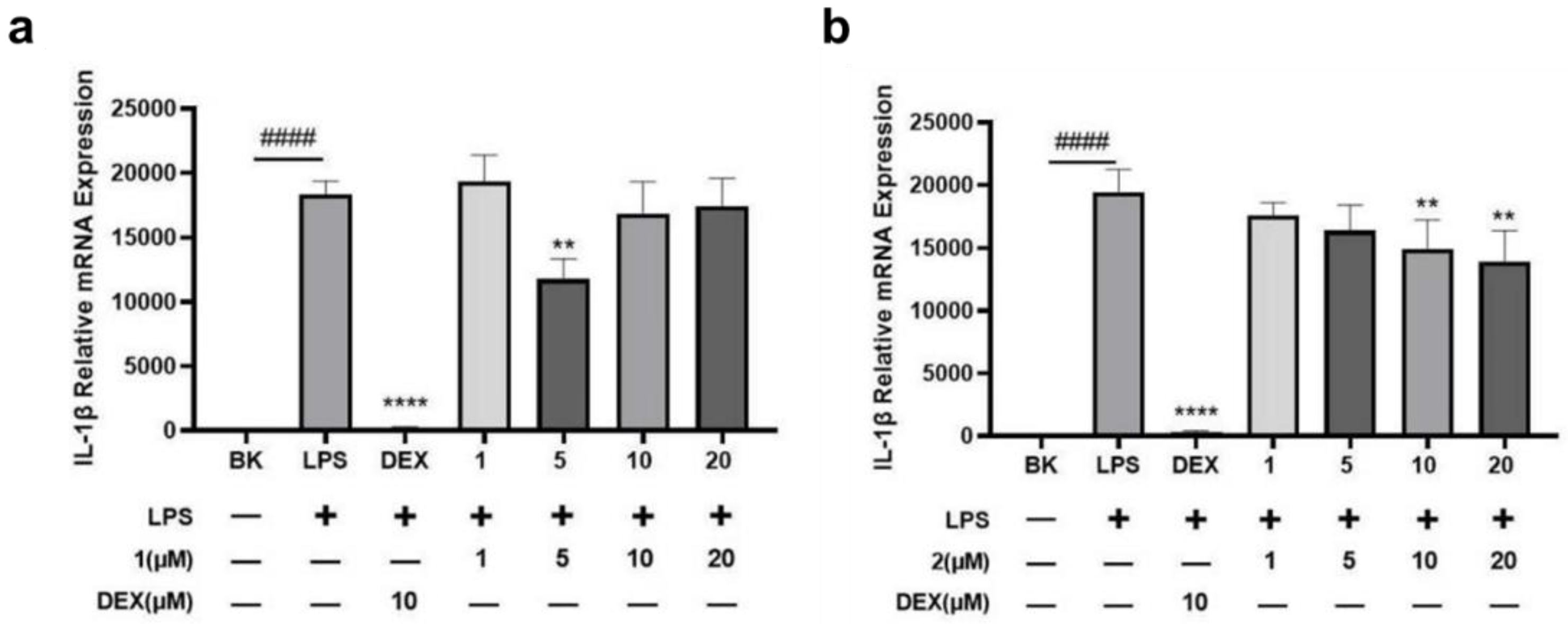
2.2. Bioactive Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Source and Strain Identification
3.3. Fungal Cultivation and Fermentation
3.4. Extraction and Isolation
3.5. Spectroscopic Data of 1–3
3.6. ECD Calculation of 1–3
3.7. Anti-Inflammatory Assay
3.8. Cytotoxicity Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The Emerging Role of Ferroptosis in Inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and Upper Gastrointestinal Effects of Non-Steroidal Anti-Inflammatory Drugs: Meta-Analyses of Individual Participant Data from Randomised Trials. Lancet 2013, 382, 769–779. [Google Scholar] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural Products from Mangrove Sediments-Derived Microbes: Structural Diversity, Bioactivities, Biosynthesis, and Total Synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef] [PubMed]
- Muria-Gonzalez, M.J.; Chooi, Y.-H.; Breen, S.; Solomon, P.S. The Past, Present and Future of Secondary Metabolite Research in the Dothideomycetes. Mol. Plant Pathol. 2015, 16, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, H.; Tanaka, K.; Fukushi, E.; Matsuo, K.; Nehira, T.; Hashimoto, M. Roussoellols A and B, Tetracyclic Fusicoccanes from Roussoella Hysterioides. J. Nat. Prod. 2013, 76, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Phukhamsakda, C.; Macabeo, A.P.G.; Yuyama, K.T.; Hyde, K.D.; Stadler, M. Biofilm Inhibitory Abscisic Acid Derivatives from the Plant-Associated Dothideomycete Fungus, Roussoella Sp. Molecules 2018, 23, 2190. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ren, X.; Tao, H.; Cai, W.; Chen, Y.; Luo, X.; Guo, P.; Liu, Y. Anti-Inflammatory Polyketides from an Alga-Derived Fungus Aspergillus ochraceopetaliformis SCSIO 41020. Mar. Drugs 2022, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Cai, J.; Xiao, Z.; Liu, M.; Li, X.; Yang, B.; Fang, W.; Huang, Y.; Chen, C.; Zhou, X.; et al. Bioactive Polyketides and Benzene Derivatives from Two Mangrove Sediment-Derived Fungi in the Beibu Gulf. Mar. Drugs 2023, 21, 327. [Google Scholar] [CrossRef]
- Ye, Y.; Liang, J.; She, J.; Lin, X.; Wang, J.; Liu, Y.; Yang, D.; Tan, Y.; Luo, X.; Zhou, X. Two New Alkaloids and a New Butenolide Derivative from the Beibu Gulf Sponge-Derived Fungus Penicillium sp. SCSIO 41413. Mar. Drugs 2023, 21, 27. [Google Scholar] [CrossRef]
- Armesto, N.; Fernández, S.; Ferrero, M.; Gotor, V. Influence of Intramolecular Hydrogen Bonds in the Enzyme-Catalyzed Regioselective Acylation of Quinic and Shikimic Acid Derivatives. Tetrahedron 2006, 62, 5401–5410. [Google Scholar] [CrossRef]
- Cheng, T.; Chepkirui, C.; Decock, C.; Matasyoh, J.C.; Stadler, M. Sesquiterpenes from an Eastern African Medicinal Mushroom Belonging to the Genus Sanghuangporus. J. Nat. Prod. 2019, 82, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Gremaud, G.; Tabacchi, R. Isocoumarins of the Fungus Ceratocystis Fimbriata coffea. Nat. Prod. Lett. 1994, 5, 95–103. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Li, A.; Yan, B.; Chen, R.; Li, X.; Du, X.; Sun, H.; Pu, J. Polyketides from the Endophytic Fungus Phomopsis sp. Sh917 by Using the One Strain/Many Compounds Strategy. Tetrahedron 2017, 73, 3577–3584. [Google Scholar] [CrossRef]
- Boonyaketgoson, S.; Trisuwan, K.; Bussaban, B.; Rukachaisirikul, V.; Phongpaichit, S. Isochromanone Derivatives from the Endophytic Fungus Fusarium sp. PDB51F5. Tetrahedron Lett. 2015, 56, 5076–5078. [Google Scholar] [CrossRef]
- Tayone, W.C.; Kanamaru, S.; Honma, M.; Tanaka, K.; Nehira, T.; Hashimoto, M. Absolute Stereochemistry of Novel Isochromanone Derivatives from Leptosphaeria sp. KTC 727. Biosci. Biotech. Bioch. 2011, 75, 2390–2393. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, Y.; Chen, S.; Liu, Z.; Liu, H.; Zhang, W.; Yan, H. Study on the Secondary Metabolites from the Deep-Sea-Derived Fungus Neoroussoella sp. and Their Biological Activities. Nat. Prod. Res. Dev. 2021, 33, 1165. [Google Scholar]
- Singh, B.; Parshad, R.; Khajuria, R.K.; Guru, S.K.; Pathania, A.S.; Sharma, R.; Chib, R.; Aravinda, S.; Gupta, V.K.; Khan, I.A.; et al. Saccharonol B, a New Cytotoxic Methylated Isocoumarin from Saccharomonospora azurea. Tetrahedron Lett. 2013, 54, 6695–6699. [Google Scholar] [CrossRef]
- Koshti, N.; Naik, S.; Parab, B. Polymer-Bound Cationic Rh(I) Phosphine Catalyst for Homogeneous Asymmetric Hydrogenation. Indian J. Chem. B 2005, 44B, 2555–2559. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Cui, C.; Wang, B. Chemical Constituents of Endophytic Fungus Hypocreales sp. Derived from the Red Alga Symphyocladia Latiuscula. Haiyang Kexue. 2008, 32, 51–55. [Google Scholar]
- Lee, Y.S.; Lee, H.S.; Shin, K.H.; Kim, B.-K.; Lee, S. Constituents of the Halophyte Salicornia Herbacea. Arch. Pharmacal Res. 2004, 27, 1034–1036. [Google Scholar] [CrossRef]
- Cui, H.; Li, X.; Li, M.; Lu, F.; Wang, Y.; Liu, D.; Kang, J. Study on Anti-HBV Secondary Metabolites from Sponge-associated Fungus Penicillium janthinellum LZDX-32-1. Chin. J. Mar. Drugs 2017, 36, 42–46. [Google Scholar]
- Zou, M.; Wang, R.; Yin, Q.; Liu, L. Bioassay-Guided Isolation and Identification of Anti-Alzheimer’s Active Compounds from Spiranthes Sinensis (Pers.) Ames. Med. Chem. Res. 2021, 30, 1849–1855. [Google Scholar] [CrossRef]
- Cao, L.; Tian, H.; Wang, Y.; Zhou, X.; Jiang, R.; Liu, Y. Chemical Constituents in the Fruits of Mangrove Plant Sonneratia Apetala Buch. Ham. J. Trop. Oceanogr. 2015, 34, 77–82. [Google Scholar]
- Dinarello, C.A. Clinical Perspective of IL-1β as the Gatekeeper of Inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Dong, X.; Wei, W.; Li, X.; Li, X.; Liu, J.; Feng, T. Bisabolane and Drimane Sesquiterpenes from the Fungus Coprinellus sp. Phytochem. Lett. 2023, 55, 30–33. [Google Scholar] [CrossRef]
- De Cássia Da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. Sesquiterpenes from Essential Oils and Anti-Inflammatory Activity. Nat. Prod. Commun. 2015, 10, 1767–1774. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, Cytotoxic Chloroazaphilones from the Marine Mangrove Endophytic Fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
| Pos. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | 173.5, C | 173.1, C | 129.2, C | |||
| 2 | 126.1, C | 126.0, C | 140.5, CH | 6.79, brs | ||
| 3 | 140.1, CH | 7.29, d, (11.4) | 140.1, CH | 7.30, d, (11.4) | 69.2, CH | 4.42, m |
| 4 | 123.5, CH | 6.59, dd, (15.1, 11.4) | 123.3, CH | 6.61, dd, (15.2, 11.4) | 69.7, CH | 4.08, m |
| 5 | 148.0, CH | 6.17, d, (15.1) | 148.8, CH | 6.17, d, (15.2) | 72.4, CH | 5.05, m |
| 6 | 75.3, C | 75.5, C | 26.5, CH2 | 2.63, m | ||
| 7 | 44.3, CH | 1.63, m | 44.2, CH | 1.63, m | 168.1, C | |
| 8 | 26.9, CH2 | 1.81, m; 2.03, m | 25.8, CH2 | 1.81, m; 2.03, m | 52.4, CH3 | 3.76, s |
| 9 | 120.3, CH | 5.36, m | 120.4, CH | 5.36, m | 172.3, C | |
| 10 | 134.2, C | 134.2, C | 21.0, CH3 | 2.11, s | ||
| 11 | 30.8, CH2 | 1.99, m | 30.9, CH2 | 1.99, m | ||
| 12 | 23.6, CH2 | 1.24, m; 1.90, m | 24.2, CH2 | 1.24, m; 1.90, m | ||
| 13 | 23.5, CH3 | 1.64, s | 23.5, CH3 | 1.64, s | ||
| 14 | 26.1, CH3 | 1.33, s | 26.3, CH3 | 1.32, s | ||
| 15 | 12.6, CH3 | 1.96, s | 12.6, CH3 | 1.97, s | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Cai, J.; Chen, T.; Wang, Y.; Chen, Y.; Zhu, Y.; Chen, C.; Yang, B.; Zhou, X.; Tao, H. Two New Sesquiterpenoids and a New Shikimic Acid Metabolite from Mangrove Sediment-Derived Fungus Roussoella sp. SCSIO 41427. Mar. Drugs 2024, 22, 103. https://doi.org/10.3390/md22030103
Xiao Z, Cai J, Chen T, Wang Y, Chen Y, Zhu Y, Chen C, Yang B, Zhou X, Tao H. Two New Sesquiterpenoids and a New Shikimic Acid Metabolite from Mangrove Sediment-Derived Fungus Roussoella sp. SCSIO 41427. Marine Drugs. 2024; 22(3):103. https://doi.org/10.3390/md22030103
Chicago/Turabian StyleXiao, Zimin, Jian Cai, Ting Chen, Yilin Wang, Yixin Chen, Yongyan Zhu, Chunmei Chen, Bin Yang, Xuefeng Zhou, and Huaming Tao. 2024. "Two New Sesquiterpenoids and a New Shikimic Acid Metabolite from Mangrove Sediment-Derived Fungus Roussoella sp. SCSIO 41427" Marine Drugs 22, no. 3: 103. https://doi.org/10.3390/md22030103
APA StyleXiao, Z., Cai, J., Chen, T., Wang, Y., Chen, Y., Zhu, Y., Chen, C., Yang, B., Zhou, X., & Tao, H. (2024). Two New Sesquiterpenoids and a New Shikimic Acid Metabolite from Mangrove Sediment-Derived Fungus Roussoella sp. SCSIO 41427. Marine Drugs, 22(3), 103. https://doi.org/10.3390/md22030103