Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting
Abstract
1. Introduction
2. Overview of 3D Bioprinting Technologies
2.1. Jetting Bioprinting
2.1.1. Inkjet Bioprinting
2.1.2. Micro-Valve Bioprinting
2.1.3. Laser-Assisted Bioprinting
2.1.4. Acoustic Bioprinting
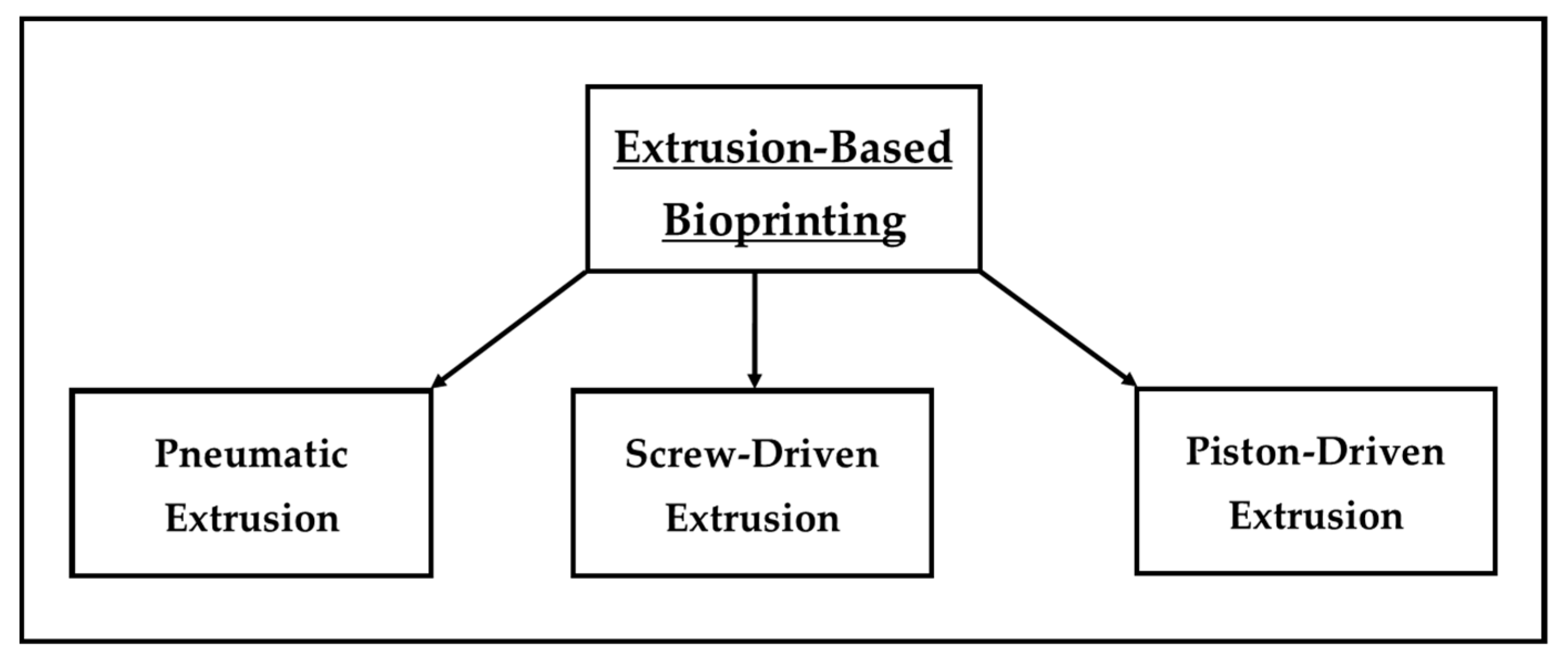
2.2. Extrusion Bioprinting
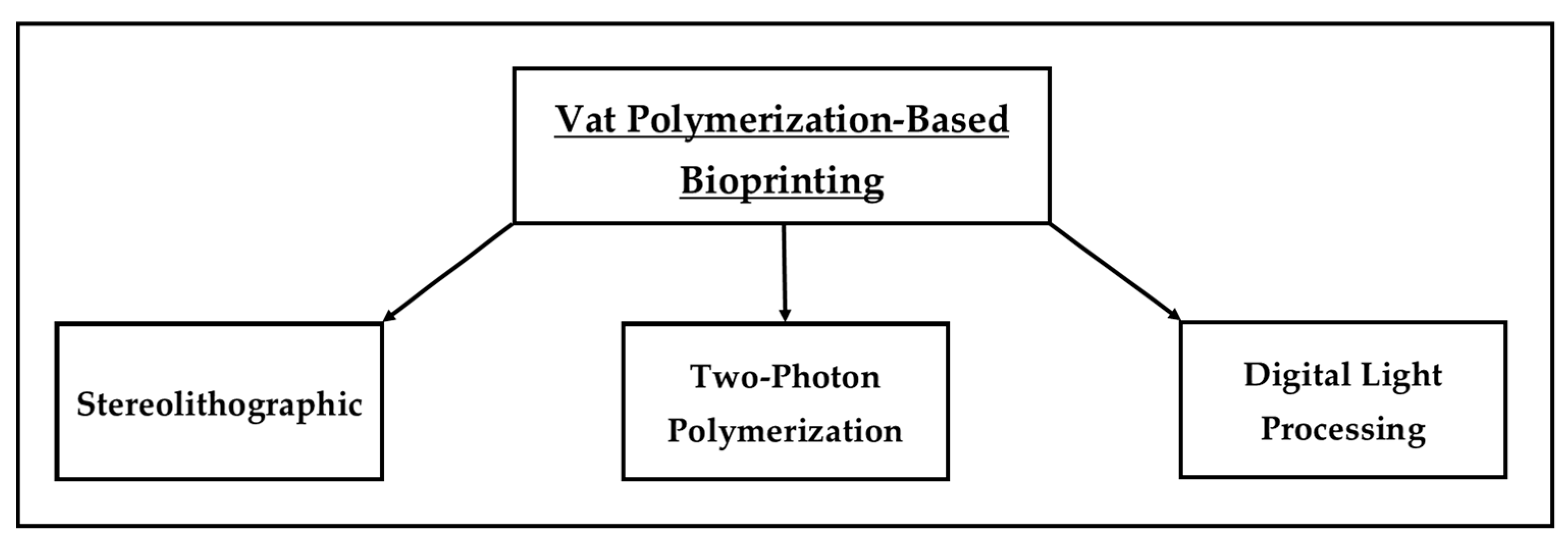
2.3. Vat Polymerization Bioprinting
2.3.1. Stereolithographic Bioprinting
2.3.2. Two-Photon Polymerization
2.3.3. Digital Light Processing
3. Marine-Derived Biomaterials for Bio-Ink Formulations
3.1. Polysaccharides-Based Marine-Derived Biomaterials
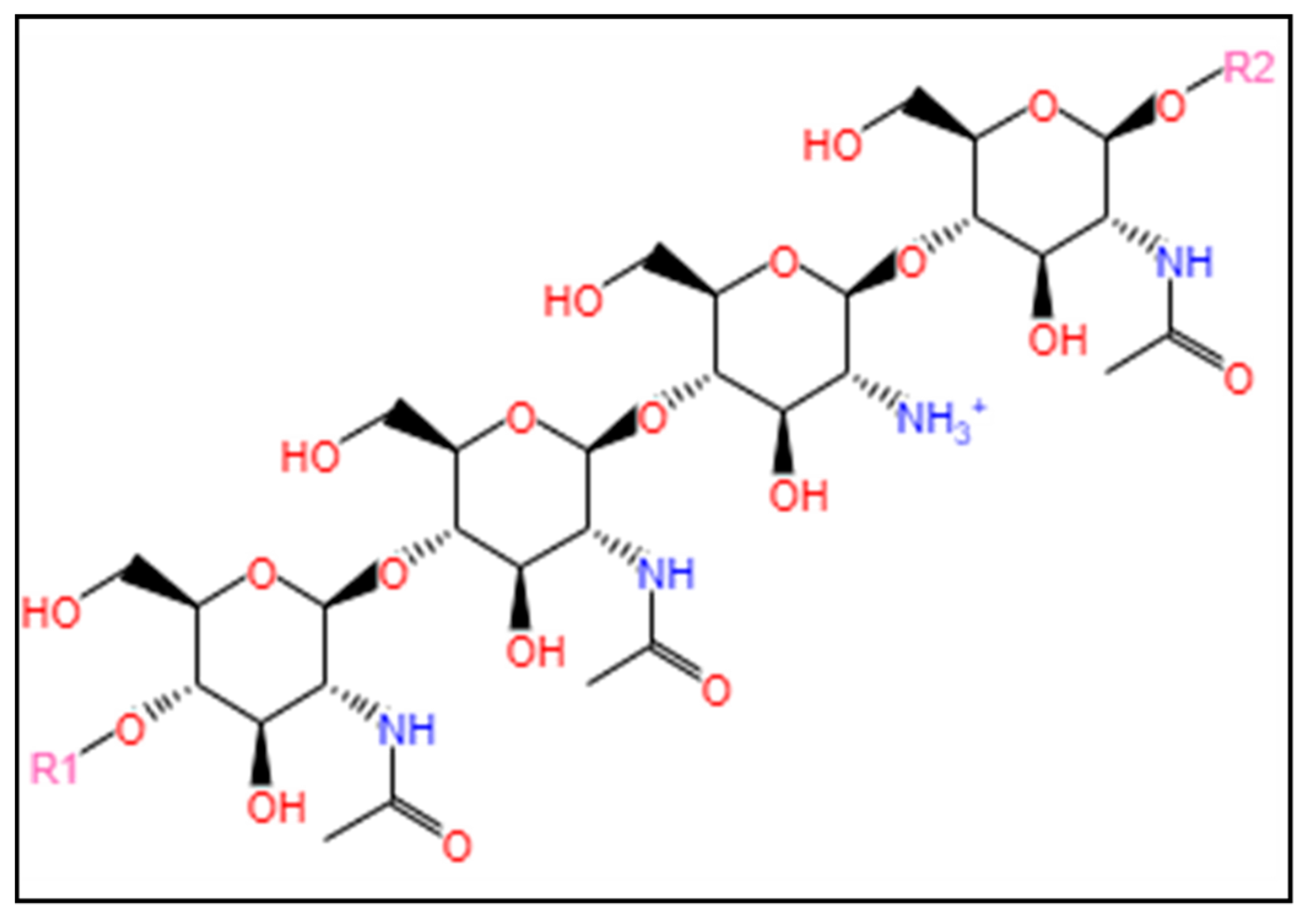
3.1.1. Chitosan
3.1.2. Glycosaminoglycans
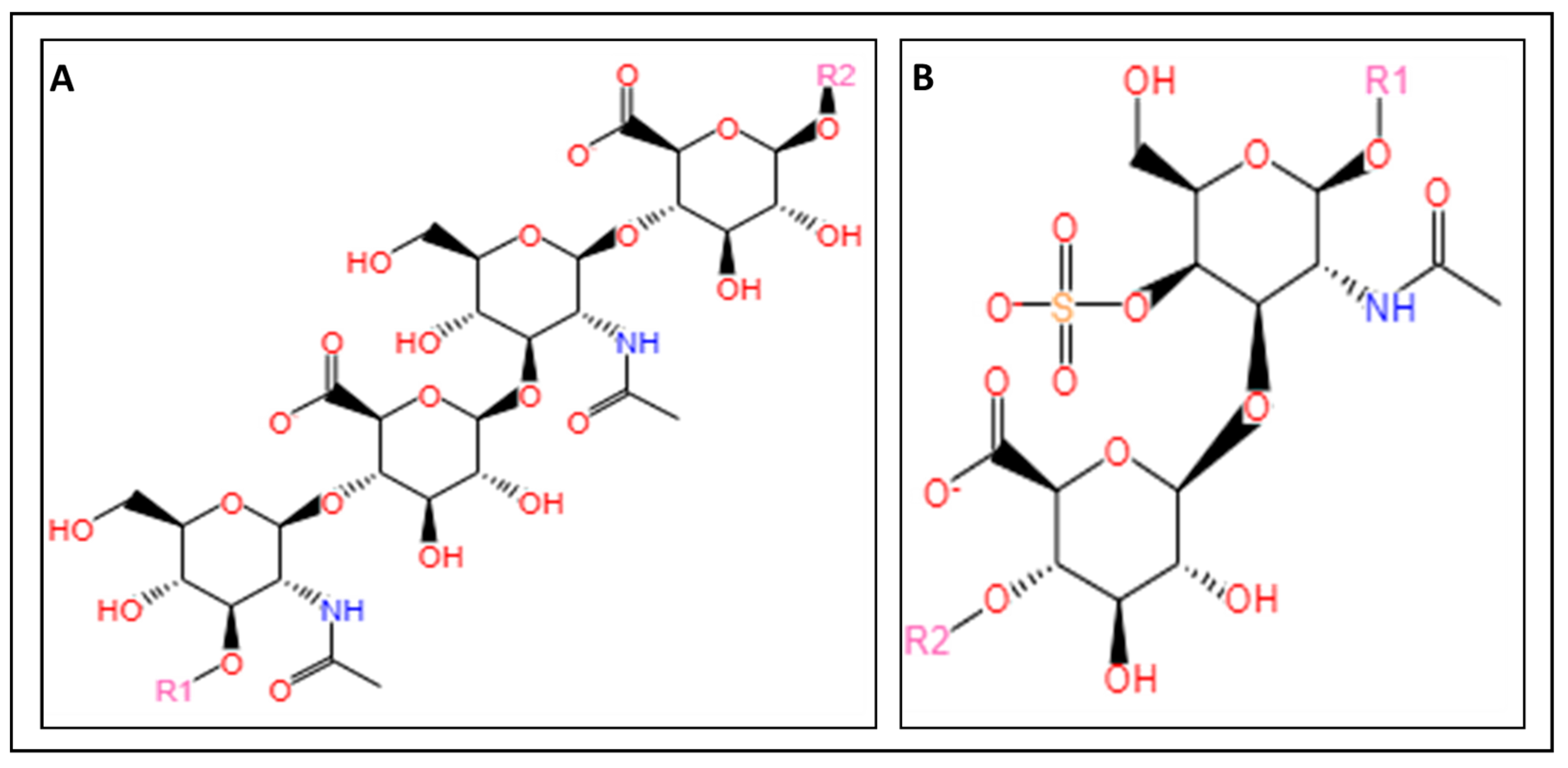
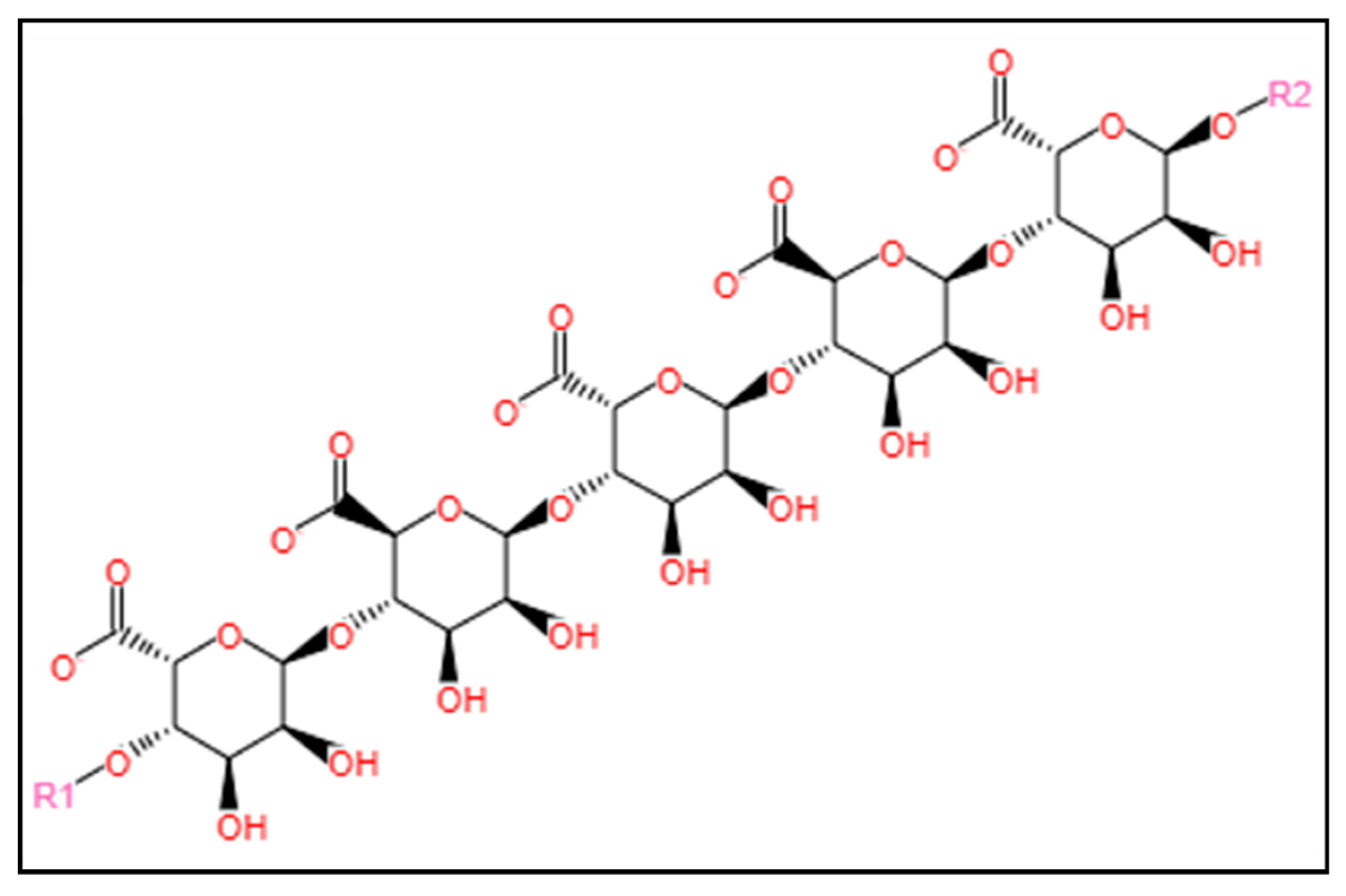
3.1.3. Alginate
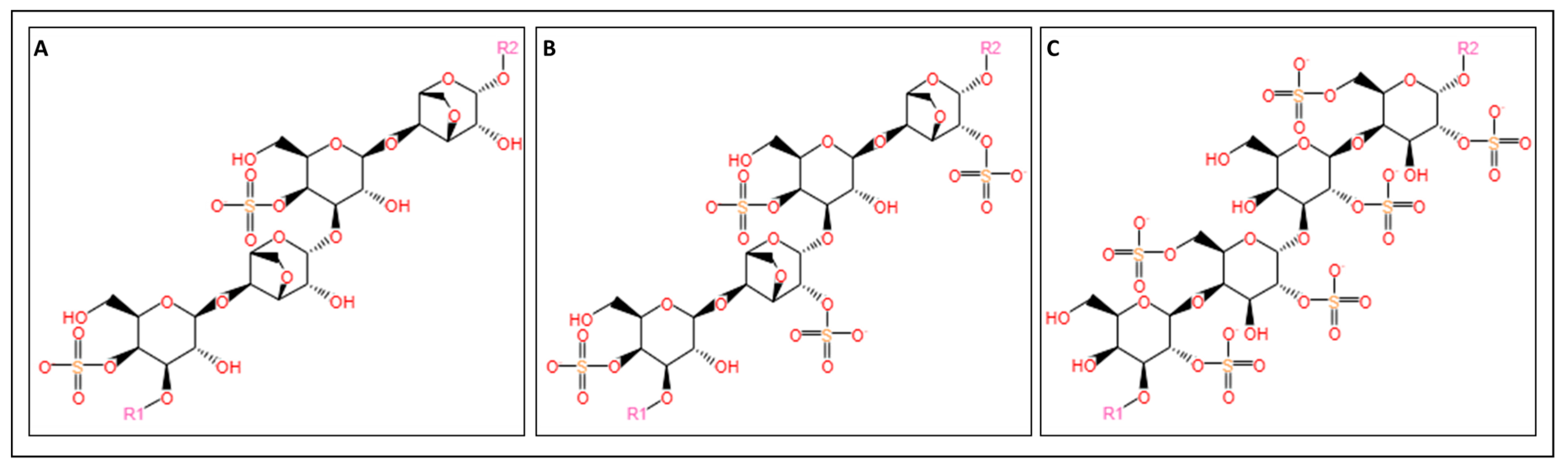
3.1.4. Carrageenan
3.2. Protein-Based Marine-Derived Biomaterials
3.2.1. Collagen
3.2.2. Gelatin
4. Current Developments and Challenges in the Application of Marine-Derived Biomaterials in Bio-Ink Formulations for 3D Bioprinting
| Biopolymers | Positive Aspects | Negative Aspects | Biomedical Applications |
|---|---|---|---|
| Alginate | Natural, non-toxic, biocompatible, and biodegradable | Low mechanical properties | Vascular tissues [123,124], bone [125,126], skin [127], retina [128], and cartilage [129,130,131] |
| Gelatin | Natural, non-toxic, biocompatible, and biodegradable | Low mechanical properties | Bone [143,144], vascularized tissues [145], and cartilage [146,147,148] |
| Collagen | Natural, non-toxic, biocompatible, and biodegradable | Low mechanical properties | Bone [138], skin [139,140], and cartilage [141,142] |
| Hyaluronic acid | Natural, non-toxic, biocompatible, and biodegradable Supports cell growth | Low mechanical properties and slow gelation | Bone [149,150] and cartilage [151] |
| Chitosan | Natural, non-toxic, biocompatible, and biodegradable High mechanical strength | Low thermoplastic characteristics Possible degradation at high temperature | Bone [152,153], skin [154,155], scaffolds suitable for repair of complex structures [156] and cartilage [157] |
| Chondroitin Sulfate and Dermatan Sulfate | Natural, non-toxic, biocompatible, and biodegradable Great stability Low immunogenicity | Possible degradation Low integration with cartilage | Cartilage [158] |
| Carrageenan | Natural, non-toxic, biocompatible, and biodegradable | Brittle and instable | Cartilage tissue engineering [168,169] |
5. Machine Learning in 3D Bioprinting
6. Conclusion and Future Prospects of Bio-Ink Formulations Using Marine-Derived Biomaterials for 3D Bioprinting
Funding
Acknowledgments
Conflicts of Interest
References
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. Trans. ASME 2014, 136, 061016. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent Advances in Waste-Recycled Nanomaterials for Biomedical Applications: Waste-to-Wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Koçak, E.; Yıldız, A.; Acartürk, F. Three Dimensional Bioprinting Technology: Applications in Pharmaceutical and Biomedical Area. Colloids Surf. B Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Udyawar, D. A Review on Current State of Art of Bioprinting. In 3D Printing and Additive Manufacturing Technologies; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D Bioprinting for Drug Discovery and Development in Pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Al Rashid, A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A Review of Processes, Materials and Applications. Bioprinting 2021, 23, e00148. [Google Scholar] [CrossRef]
- Ng, W.L.; Shkolnikov, V. Optimizing Cell Deposition for Inkjet-Based Bioprinting. Int. J. Bioprint 2024, 2135. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.X.A.; Yeong, W.Y.; Shen, Y.F. Vat Polymerization-Based Bioprinting–Process, Materials, Applications and Regulatory Challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials Based on Marine Resources for 3D Bioprinting Applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Bedell, M.L.; Guo, J.L.; Xie, V.Y.; Navara, A.M.; Mikos, A.G. Polymer Scaffold Fabrication. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–315. [Google Scholar]
- Roque, R.; Barbosa, G.F.; Guastaldi, A.C. Design and 3D Bioprinting of Interconnected Porous Scaffolds for Bone Regeneration. An Additive Manufacturing Approach. J. Manuf. Process 2021, 64, 655–663. [Google Scholar] [CrossRef]
- Adepu, S.; Dhiman, N.; Laha, A.; Sharma, C.S.; Ramakrishna, S.; Khandelwal, M. Three-Dimensional Bioprinting for Bone Tissue Regeneration. Curr. Opin. Biomed. Eng. 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a Three-Dimensional Bioprinter: Construction of Cell Supporting Structures Using Hydrogel and State-of-the-Art Inkjet Technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing of Viable Mammalian Cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.P.; Sadia, M.; Jaganathan, S.K.; Khudzari, A.Z.; Supriyanto, E.; Saidin, S.; Ramakrishna, S.; Ismail, A.F.; Faudzi, A.A.M. A Review on 3D Printing in Tissue Engineering Applications. J. Polym. Eng. 2022, 42, 243–265. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Mehrotra, D. 3D Bioprinting and Craniofacial Regeneration. J. Oral Biol. Craniofac. Res. 2020, 10, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef]
- Gómez-Blanco, J.C.; Galván-Chacón, V.; Patrocinio, D.; Matamoros, M.; Sánchez-Ortega, Á.J.; Marcos, A.C.; Duarte-León, M.; Marinaro, F.; Pagador, J.B.; Sánchez-Margallo, F.M. Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle. Materials 2021, 14, 3100. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J. Extrusion-Based 3D Printing for Pharmaceuticals: Contemporary Research and Applications. Curr. Pharm. Des. 2018, 24, 4991–5008. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Soman, P.; Chung, P.H.; Zhang, A.P.; Chen, S. Digital Microfabrication of User-Defined 3D Microstructures in Cell-Laden Hydrogels. Biotechnol. Bioeng. 2013, 110, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Lim, K.S.; Li, W.; Asua, A.U.; Peña, L.B.; Wang, M.; Falandt, M.; Bernal, P.N.; Gawlitta, D.; Zhang, Y.S.; et al. High-Resolution Lithographic Biofabrication of Hydrogels with Complex Microchannels from Low-Temperature-Soluble Gelatin Bioresins. Mater. Today Bio 2021, 12, 100162. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Bhaduri, B.; Mir, M.; Shkumatov, A.; Lee, M.K.; Popescu, G.; Kong, H.; Bashir, R. High-Resolution Projection Microstereolithography for Patterning of Neovasculature. Adv. Healthc. Mater. 2016, 5, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Wang, P.; Zheng, Y.; Liu, L.; Hu, J.; Liu, Y.; Gao, Q.; He, Y. Lightweight 3D Bioprinting with Point by Point Photocuring. Bioact. Mater. 2021, 6, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; Van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-Resin for High Resolution Lithography-Based Biofabrication of Complex Cell-Laden Constructs. Biofabrication 2018, 10, 034101. [Google Scholar] [CrossRef]
- Liang, R.; Gu, Y.; Wu, Y.; Bunpetch, V.; Zhang, S. Lithography-Based 3D Bioprinting and Bioinks for Bone Repair and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 806–816. [Google Scholar] [CrossRef]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D Bioprinting in Tissue Engineering: Advantages, Deficiencies, Improvements, and Future Perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef]
- Tudor, A.; Delaney, C.; Zhang, H.; Thompson, A.J.; Curto, V.F.; Yang, G.Z.; Higgins, M.J.; Diamond, D.; Florea, L. Fabrication of Soft, Stimulus-Responsive Structures with Sub-Micron Resolution via Two-Photon Polymerization of Poly(Ionic Liquid)s. Mater. Today 2018, 21, 807–816. [Google Scholar] [CrossRef]
- Fischer, J.; Mueller, J.B.; Kaschke, J.; Wolf, T.J.A.; Unterreiner, A.-N.; Wegener, M. Three-Dimensional Multi-Photon Direct Laser Writing with Variable Repetition Rate. Opt. Express 2013, 21, 26244–26260. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Popescu, R.C.; Mustaciosu, C.C.; Zamfirescu, M.; Calin, B.S.; Mihailescu, M.; Dinescu, M.; Popescu, A.; Chioibasu, D.; Soproniy, M.; et al. Laser-Direct Writing by Two-Photon Polymerization of 3D Honeycomb-like Structures for Bone Regeneration. Biofabrication 2018, 10, 025009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Larsen, N.B. Stereolithographic Hydrogel Printing of 3D Culture Chips with Biofunctionalized Complex 3D Perfusion Networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.; Hepburn, M.S.; Chen, J.; Aldana, A.A.; Allardyce, B.J.; Shafei, S.; Doyle, B.J.; Kennedy, B.F.; Dilley, R.J. Bioprinting Silk Fibroin Using Two-Photon Lithography Enables Control over the Physico-Chemical Material Properties and Cellular Response. Bioprinting 2022, 25, e00183. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Obata, K.; El-Tamer, A.; Koch, L.; Hinze, U.; Chichkov, B.N. High-Aspect 3D Two-Photon Polymerization Structuring with Widened Objective Working Range (WOW-2PP). Light Sci. Appl. 2013, 2, e116. [Google Scholar] [CrossRef]
- Perevoznik, D.; Nazir, R.; Kiyan, R.; Kurselis, K.; Koszarna, B.; Gryko, D.T.; Chichkov, B.N. High-Speed Two-Photon Polymerization 3D Printing with a Microchip Laser at Its Fundamental Wavelength. Opt. Express 2019, 27, 25119–25125. [Google Scholar] [CrossRef]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. High-Resolution Two-Photon Polymerization: The Most Versatile Technique for the Fabrication of Microneedle Arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Weisgrab, G.; Guillaume, O.; Guo, Z.; Heimel, P.; Slezak, P.; Poot, A.; Grijpma, D.; Ovsianikov, A. 3D Printing of Large-Scale and Highly Porous Biodegradable Tissue Engineering Scaffolds from Poly(Trimethylene-Carbonate) Using Two-Photon-Polymerization. Biofabrication 2020, 12, 045036. [Google Scholar] [CrossRef]
- Huh, J.T.; Moon, Y.W.; Park, J.; Atala, A.; Yoo, J.J.; Lee, S.J. Combinations of Photoinitiator and UV Absorber for Cell-Based Digital Light Processing (DLP) Bioprinting. Biofabrication 2021, 13, 034103. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.M.; Tuan, R.S. Application of Visible Light-Based Projection Stereolithography for Live Cell-Scaffold Fabrication with Designed Architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhao, L.; Jian, M.; Mao, Y.; Yu, M.; Guo, X. EHMP-DLP: Multi-Projector DLP with Energy Homogenization for Large-Size 3D Printing. Rapid Prototyp. J. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Bao, Y.; Paunović, N.; Leroux, J.C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, H.W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting Technology and Its Applications. Eur. J. Cardio-Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Suntornnond, R.; Yeong, W.Y. Polyvinylpyrrolidone-Based Bioink: Influence of Bioink Properties on Printing Performance and Cell Proliferation during Inkjet-Based Bioprinting. Biodes Manuf. 2023, 6, 676–690. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Huang, Y.; Ogale, A.; Fu, J.; Markwald, R.R. Study of Droplet Formation Process during Drop-on-Demand Inkjetting of Living Cell-Laden Bioink. Langmuir 2014, 30, 9130–9138. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting Is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling Droplet Impact Velocity and Droplet Volume: Key Factors to Achieving High Cell Viability in Sub-Nanoliter Droplet-Based Bioprinting. Int. J. Bioprint 2022, 8, 424. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.E.; Seshadri, V.; Burg, T.C.; Burg, K.J.L.; Groff, R.E. Characterizing the Effects of Cell Settling on Bioprinter Output. Biofabrication 2012, 4, 011001. [Google Scholar] [CrossRef] [PubMed]
- Morgan, F.L.C.; Moroni, L.; Baker, M.B. Dynamic Bioinks to Advance Bioprinting. Adv. Healthc. Mater. 2020, 9, e1901798. [Google Scholar] [CrossRef] [PubMed]
- Dudman, J.; Ferreira, A.M.; Gentile, P.; Wang, X.; Dalgarno, K. Microvalve Bioprinting of MSC-Chondrocyte Co-Cultures. Cells 2021, 10, 3329. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Szabó, G.; Kolozsvári, L.; Kafetzopoulos, D.; Fotakis, C.; Nógrádi, A. Femtosecond Laser Printing of Living Cells Using Absorbing Film-Assisted Laser-Induced Forward Transfer. Opt. Eng. 2012, 51, 014302. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin Tissue Generation by Laser Cell Printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, A.; Chattopadhyaya, S.; Ding, C.F. A Review on Critical Challenges in Additive Manufacturing via Laser-Induced Forward Transfer. Opt. Laser Technol. 2024, 168, 109893. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective Bioprinting Resolution in Tissue Model Fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Jentsch, S.; Nasehi, R.; Kuckelkorn, C.; Gundert, B.; Aveic, S.; Fischer, H. Multiscale 3D Bioprinting by Nozzle-Free Acoustic Droplet Ejection. Small Methods 2021, 5, 2000971. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A Brief Review of Extrusion-Based Tissue Scaffold Bio-Printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in Extrusion Bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, 2103691. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3d Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D.M.; Noorani, R.; McGrath, P. Additive Manufacturing: Vat Polymerization for Medical Applications. AM&P Tech. Artic. 2022, 180, 17–20. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, X.; Zhang, Y.; Hou, D. Recent Progress of the Vat Photopolymerization Technique in Tissue Engineering: A Brief Review of Mechanisms, Methods, Materials, and Applications. Polymers 2023, 15, 3940. [Google Scholar] [CrossRef]
- Panda, S.M.; Hosseinabadi, H.G.; Fattel, H.; Tripathy, U.; Miri, A.K. Ink Formulation and Selection for Biological Applications of Two-Photon Polymerization. ACS Appl. Opt. Mater. 2023, 1, 1501–1512. [Google Scholar] [CrossRef]
- Jing, X.; Fu, H.; Yu, B.; Sun, M.; Wang, L. Two-Photon Polymerization for 3D Biomedical Scaffolds: Overview and Updates. Front. Bioeng. Biotechnol. 2022, 10, 994355. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Zuazola, J.; Vijayavenkataraman, S. Bioprinting the Future Using Light: A Review on Photocrosslinking Reactions, Photoreactive Groups, and Photoinitiators. SLAS Technol. 2023, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Crivello, J.V.; Reichmanis, E. Photopolymer Materials and Processes for Advanced Technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, X.; Sun, Y.; Gao, Q.; Fu, J.; Cai, X.; He, Y. Printability during Projection-Based 3D Bioprinting. Bioact. Mater. 2022, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, W.; Hao, J.; Gonzales, A.; Zhao, Z.; Flores, R.S.; Kuang, X.; Mu, X.; Ching, T.; Tang, G.; et al. Molecularly Cleavable Bioinks Facilitate High-Performance Digital Light Processing-Based Bioprinting of Functional Volumetric Soft Tissues. Nat. Commun. 2022, 13, 3317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Kuang, G.; Yu, Y.; Zhao, Y. Photopolymerized 3D Printing Scaffolds with Pt(IV) Prodrug Initiator for Postsurgical Tumor Treatment. Research 2022, 2022, 9784510. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser Assisted Bioprinting of Engineered Tissue with High Cell Density and Microscale Organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D Printing Restorative Materials Using a Stereolithographic Technique: A Systematic Review. Dent. Mater. 2021, 37, 336–350. [Google Scholar] [CrossRef]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Ostendorf, A.; Chichkov, B.N. Two-Photon Polymerization: A New Approach to Micromachining. Photonics Spectra 2006, 40, 72. [Google Scholar]
- Nguyen, A.K.; Narayan, R.J. Two-Photon Polymerization for Biological Applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Bártolo, P.J. Stereolithography–Materials, Processes and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Jian, B.; Li, H. Shaping Soft Materials via Digital Light Processing-Based 3D Printing: A Review. Forces Mech. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital Light Processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi Hosseinabadi, H.; Nieto, D.; Yousefinejad, A.; Fattel, H.; Ionov, L.; Miri, A.K. Ink Material Selection and Optical Design Considerations in DLP 3D Printing. Appl. Mater. Today 2023, 30, 101721. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Zhu, W.; Victorine, G.; Lawrence, N.; Chen, S. 3D-Printing of Functional Biomedical Microdevices via Light- and Extrusion-Based Approaches. Small Methods 2018, 2, 1700277. [Google Scholar] [CrossRef]
- Mousavi, A.; Provaggi, E.; Kalaskar, D.M.; Savoji, H. 3D Printing Families: Laser, Powder, and Nozzle-Based Techniques. In 3D Printing in Medicine; Woodhead Publishing: Sawston, UK, 2023; pp. 29–57. [Google Scholar]
- Tosto, C.; Pergolizzi, E.; Blanco, I.; Patti, A.; Holt, P.; Karmel, S.; Cicala, G. Epoxy Based Blends for Additive Manufacturing by Liquid Crystal Display (LCD) Printing: The Effect of Blending and Dual Curing on Daylight Curable Resins. Polymers 2020, 12, 1594. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Qin, Y.; Liang, W.; Yu, H.; Liu, L. Digital Micro-Mirror Device -Based Light Curing Technology and Its Biological Applications. Opt. Laser Technol. 2021, 143, 107344. [Google Scholar] [CrossRef]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- Khiari, Z. Sustainable Upcycling of Fisheries and Aquaculture Wastes Using Fish-Derived Cold-Adapted Proteases. Front. Nutr. 2022, 9, 875697. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- MetaCyc Database Compound Search for Chitosan. Available online: https://biocyc.org/compound?orgid=META&id=Chitosan (accessed on 28 February 2024).
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kitagawa, H. Biosynthesis and Function of Chondroitin Sulfate. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Özbek, S. Evolution of Glycosaminoglycans: Comparative Biochemical Study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef]
- Liao, Y.H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. J. Deliv. Target. Ther. Agents 2005, 12, 327–342. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Godbey, W.T. Chapter 17–Stem Cells, Tissue Engineering, and Regenerative Medicine. In Biotechnology and Its Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 389–409. [Google Scholar]
- Schiraldi, C.; Cimini, D.; De Rosa, M. Production of Chondroitin Sulfate and Chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita-Toyoda, A.; Yamada, S.; Haslam, S.M.; Khoo, K.H.; Sugiura, M.; Morris, H.R.; Dell, A.; Sugahara, K. Structural Determination of Five Novel Tetrasaccharides Containing 3-O-Sulfated D-Glucuronic Acid and Two Rare Oligosaccharides Containing a β-D-Glucose Branch Isolated from Squid Cartilage Chondroitin Sulfate E. Biochemistry 2004, 43, 11063–11074. [Google Scholar] [CrossRef] [PubMed]
- MetaCyc Database Compound Search for Hyaluronan. Available online: https://biocyc.org/compound?orgid=META&id=Hyaluronan (accessed on 28 February 2024).
- MetaCyc Database Compound Search for Chondroitin Sulfate. Available online: https://biocyc.org/compound?orgid=META&id=Chondroitin-Sulfate-A (accessed on 28 February 2024).
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; Del González, M.P.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- MetaCyc Database Compound Search for Alginate. Available online: https://biocyc.org/compound?orgid=META&id=ALGINATE (accessed on 28 February 2024).
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Carrageenans. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–291. [Google Scholar]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- MetaCyc Database Compound Search for Kappa (κ)-Carrageenan. Available online: https://biocyc.org/compound?orgid=META&id=kappa-Carrageenan (accessed on 28 February 2024).
- MetaCyc Database Compound Search for Iota (ι)-Carrageenan. Available online: https://biocyc.org/compound?orgid=META&id=iota-Carrageenan (accessed on 28 February 2024).
- MetaCyc Database Compound Search for Lambda (λ)-Carrageenan. Available online: https://biocyc.org/compound?orgid=META&id=Lambda-Carrageenan (accessed on 28 February 2024).
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and Its Applications in Drug Delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients. In Handbook of Pharmaceutical Excipients, 8th ed.; Libros Digitales-Pharmaceutical Press: London, UK, 2017. [Google Scholar]
- Du, L.; Keplova, L.; Khiari, Z.; Betti, M. Preparation and Characterization of Gelatin from Collagen Biomass Obtained through a PH-Shifting Process of Mechanically Separated Turkey Meat. Poult. Sci. 2014, 93, 989–1000. [Google Scholar] [CrossRef]
- Du, L.; Khiari, Z.; Pietrasik, Z.; Betti, M. Physicochemical and Functional Properties of Gelatins Extracted from Turkey and Chicken Heads. Poult. Sci. 2013, 92, 2463–2474. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Jiang, L.; Zeng, Y.; Han, Y.; Sha, C.; Xie, X.; Li, H.; Zhou, J.; Lin, W. 3D Bioprinting of Collagen-Based Materials for Oral Medicine. Collagen Leather 2023, 5, 23. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of Blue Whiting Skin Gelatines Extracted after Pretreatment with Different Organic Acids. J. Aquat. Food Product. Technol. 2015, 24, 546–555. [Google Scholar] [CrossRef]
- Khiari, Z.; Gonzalez-Gonzalez, C.R. Advances in Food Protein Biotechnology. Prog. Food Biotechnol. 2018, 4, 1–49. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2021, 21, e2000280. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type i Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2019, 42, 240–250. [Google Scholar] [CrossRef]
- Dille, M.J.; Haug, I.J.; Draget, K.I. Chapter 34–Gelatin and Collagen. In Handbook of Hydrocolloids, 3rd ed.; Woodhead Publishing (Elsevier): Duxford, UK, 2021. [Google Scholar]
- Pedroza-González, S.C.; Rodriguez-Salvador, M.; Pérez-Benítez, B.E.; Moisés Alvarez, M.; Santiago, G.T. De Bioinks for 3D Bioprinting: A Scientometric Analysis of Two Decades of Progress. Int. J. Bioprint 2021, 7, 68–91. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Klöck, G.; Pfeffermann, A.; Ryser, C.; Gröhn, P.; Kuttler, B.; Hahn, H.J.; Zimmermann, U. Biocompatibility of Mannuronic Acid-Rich Alginates. Biomaterials 1997, 18, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-Laden 3D Bioprinting Hydrogel Matrix Depending on Different Compositions for Soft Tissue Engineering: Characterization and Evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform Inkjet Printing of Cellular Structures with Bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135, 091011–0910119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tolba, E.; Der, H.C.S.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.R.; Mü Ller, W.E.G. Effect of Bioglass on Growth and Biomineralization of Saos-2 Cells in Hydrogel after 3d Cell Bioprinting. PLoS ONE 2014, 9, e112497. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Chrisey, D.B. Laser-Assisted Printing of Alginate Long Tubes and Annular Constructs. Biofabrication 2013, 5, 015002. [Google Scholar] [CrossRef]
- Shi, P.; Edgar, T.Y.S.; Yeong, W.Y.; Laude, A. Hybrid Three-Dimensional (3D) Bioprinting of Retina Equivalent for Ocular Research. Int. J. Bioprint 2017, 3, 138–146. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An Additive Manufacturing-Based PCL-Alginate-Chondrocyte Bioprinted Scaffold for Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint 2020, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Biegun, M.K.; del Campo, A. 3D Bioprinting of Structural Proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating Rheological Properties and Printability of Collagen Bioinks: The Effects of Riboflavin Photocrosslinking and PH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef]
- Matejkova, J.; Kanokova, D.; Supova, M.; Matejka, R. A New Method for the Production of High-Concentration Collagen Bioinks with Semiautonomic Preparation. Gels 2024, 10, 66. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D Bioprinting of HAP/Collagen Scaffold in Gelation Bath for Bone Tissue Engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds. Tissue Eng. Part. A 2015, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering Zonal Cartilage through Bioprinting Collagen Type II Hydrogel Constructs with Biomimetic Chondrocyte Density Gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.A.; Agrawal, A.; Lee, B.H.; Chua, H.Y.; Low, K.Y.; Lau, B.C.; Machluf, M.; Venkatraman, S. Printing Cell-Laden Gelatin Constructs by Free-Form Fabrication and Enzymatic Protein Crosslinking. Biomed. Microdevices 2015, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Dou, W.; Heerschap, A.; Oosterwijk, E.; Walboomers, X.F.; Święszkowski, W. In Vitro and in Vivo Assessment of a 3D Printable Gelatin Methacrylate Hydrogel for Bone Regeneration Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Distler, T.; Polley, C.; Schneidereit, D.; Seitz, H.; Friedrich, O.; Tihminlioglu, F.; Boccaccini, A.R. 3D Printed Gelatin/Decellularized Bone Composite Scaffolds for Bone Tissue Engineering: Fabrication, Characterization and Cytocompatibility Study. Mater. Today Bio 2022, 15, 100309. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield Stress Determines Bioprintability of Hydrogels Based on Gelatin-Methacryloyl and Gellan Gum for Cartilage Bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D Printed Gelatin/Hydroxyapatite Scaffolds for Stem Cell Chondrogenic Differentiation and Articular Cartilage Repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D Bioprinting of Methacrylated Hyaluronic Acid (MeHA) Hydrogel with Intrinsic Osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis Both In Vitro and In Vivo. Adv. Sci. 2018, 5, 1700550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, L.; Li, L.; Huang, C.; Shi, K.; Meng, X.; Wang, P.; Wu, M.; Li, L.; Cao, H.; et al. 3D-Bioprinted BMSC-Laden Biomimetic Multiphasic Scaffolds for Efficient Repair of Osteochondral Defects in an Osteoarthritic Rat Model. Biomaterials 2021, 279, 121216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, H.; Li, D.; Zhang, Q.; Xu, L.; Ruan, C. Advanced Strategies in the Application of Gelatin-Based Bioink for Extrusion Bioprinting. Biodes Manuf. 2023, 6, 586–608. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative Thermoplastic Chitosan Obtained by Thermo-Mechanical Mixing with Polyol Plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct Bioprinting of Vessel-like Tubular Microfluidic Channels. J. Nanotechnol. Eng. Med. 2013, 4, 020902–0210017. [Google Scholar] [CrossRef]
- Yadav, L.R.; Chandran, S.V.; Lavanya, K.; Selvamurugan, N. Chitosan-Based 3D-Printed Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioǧlu, M. A Bioprintable Form of Chitosan Hydrogel for Bone Tissue Engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Development of Polyelectrolyte Chitosan-Gelatin Hydrogels for Skin Bioprinting. Procedia CIRP 2016, 49, 105–112. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent Advancements in Applications of Chitosan-Based Biomaterials for Skin Tissue Engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Derakhshanfar, S.; Zhong, W.; Li, B.; Lu, F.; Xing, M.; Li, X. Characterization and Application of Carboxymethyl Chitosan-Based Bioink in Cartilage Tissue Engineering. J. Nanomater. 2020, 2020, 2057097. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Zabala, A.; Gálvez-Martín, P.; Marchal, J.A.; Vázquez-Lasa, B.; Gallego, I.; Saenz-del-Burgo, L.; Pedraz, J.L. Chondroitin and Dermatan Sulfate Bioinks for 3D Bioprinting and Cartilage Regeneration. Macromol. Biosci. 2022, 22, 2100435. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.W. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable Shear-Thinning Nanoengineered Hydrogels for Stem Cell Delivery. Nanoscale 2016, 8, 12362–12372. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.M.C.; Silva, J.C.; Serro, A.P.; Cabral, J.M.S.; Sanjuan-Alberte, P.; Ferreira, F.C. 3D Bioprinting of Novel κ-Carrageenan Bioinks: An Algae-Derived Polysaccharide. Bioengineering 2022, 9, 109. [Google Scholar] [CrossRef]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef]
- İlhan, G.T.; Irmak, G.; Gümüşderelioğlu, M. Microwave Assisted Methacrylation of Kappa Carrageenan: A Bioink for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2020, 164, 3523–3534. [Google Scholar] [CrossRef]
- Muscolino, E.; Di Stefano, A.B.; Trapani, M.; Sabatino, M.A.; Giacomazza, D.; Alessi, S.; Cammarata, E.; Moschella, F.; Cordova, A.; Toia, F.; et al. κ-Carrageenan and PVA Blends as Bioinks to 3D Print Scaffolds for Cartilage Reconstruction. Int. J. Biol. Macromol. 2022, 222, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, T.; Pourkamali-Anaraki, F.; Peterson, A.M. Application of Machine Learning in Polymer Additive Manufacturing: A Review. J. Polym. Sci. 2023. [Google Scholar] [CrossRef]
- Ramesh, S.; Deep, A.; Tamayol, A.; Kamaraj, A.; Mahajan, C.; Madihally, S. Advancing 3D Bioprinting through Machine Learning and Artificial Intelligence. Bioprinting 2024, 38, e00331. [Google Scholar] [CrossRef]
- Dou, B.; Zhu, Z.; Merkurjev, E.; Ke, L.; Chen, L.; Jiang, J.; Zhu, Y.; Liu, J.; Zhang, B.; Wei, G.W. Machine Learning Methods for Small Data Challenges in Molecular Science. Chem. Rev. 2023, 123, 8736–8780. [Google Scholar] [CrossRef] [PubMed]
- Ruberu, K.; Senadeera, M.; Rana, S.; Gupta, S.; Chung, J.; Yue, Z.; Venkatesh, S.; Wallace, G. Coupling Machine Learning with 3D Bioprinting to Fast Track Optimisation of Extrusion Printing. Appl. Mater. Today 2021, 22, 100914. [Google Scholar] [CrossRef]
- Tian, S.; Stevens, R.; McInnes, B.T.; Lewinski, N.A. Machine Assisted Experimentation of Extrusion-based Bioprinting Systems. Micromachines 2021, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Song, J.; Song, B.; Lu, W.F. Multi-Objective Optimization Design through Machine Learning for Drop-on-Demand Bioprinting. Engineering 2019, 5, 586–593. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Song, B.; Song, J.; Li, S.; Trau, D.; Lu, W.F. Learning-Based Cell Injection Control for Precise Drop-on-Demand Cell Printing. Ann. Biomed. Eng. 2018, 46, 1267–1279. [Google Scholar] [CrossRef]
- Reina-Romo, E.; Mandal, S.; Amorim, P.; Bloemen, V.; Ferraris, E.; Geris, L. Towards the Experimentally-Informed In Silico Nozzle Design Optimization for Extrusion-Based Bioprinting of Shear-Thinning Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 701778. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Q.; Casillas, J.; Mcanally, M.; Mubtasim, N.; Gollahon, L.S.; Wu, D.; Xu, C. Prediction of Cell Viability in Dynamic Optical Projection Stereolithography-Based Bioprinting Using Machine Learning. J. Intell. Manuf. 2022, 33, 995–1005. [Google Scholar] [CrossRef]


| Bioprinting Technology | Resolution | Cell Viability | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| Jetting | Inkjet | 10–100 µm [21,22,23] | 70–95% [22,23,24] | Non-contact technique, flexible, low cost, reproducible, and simple [25,26,27] | Thermal and shear damage, non-uniform droplet size, unsuitable for viscous and concentrated bio-inks [25,26,27] |
| Laser-Assisted | 10–50 µm [28] | 80–95% [20,29] | High resolution and cell viability, accurate, suitable for printing of high cell densities and compatible with highly viscous biomaterials [25,26,27] | Often there is difficulty to position the bio-ink to the desired location, low stability, may require further chemical modification [25,26,27] | |
| Extrusion | Pneumatic-, Screw- and/or Piston-Driven | 100 µm [30] | 40–80% [30,31] | Simple and affordable, good mechanical resistance, possibility to use multiple materials simultaneously [25,26,27,32,33] | Low resolution and cell viability, possible thermal degradation, specific type of material required (thermoplastic) [25,26,27,32,33] |
| Vat Polymerization | Stereolithography (SLA) | 20–50 µm [34,35,36,37] | 85–95% [38,39] | High resolution, accurate, efficient use of bio-ink, gentler on cells, does not use high temperature and shear stress [40,41,42] | Bioprinted models tend to be fragile, limited availability of bio-ink materials [42] |
| Two-Photon Polymerization (TPP) | 100 nm to tens of µm [43,44,45] | 90–95% [46,47] | Very high resolution and precision, effective in producing 3D micro/nano structures, bioprinting is not limited to the layer-by-layer approach, bioprinting without any geometrical limitations [42,48,49,50,51] | Low throughput, photosensitive materials are very scant, bioprinting process is cumbersome and time-consuming compared to SLA and DLP [42,48,52] | |
| Digital Light Processing (DLP) | 25–50 µm [40,53] | 85–95% [54,55] | High resolution, precision, and bioprinting speed, gentler on cells, does not use high temperature and shear stress [40,41,42,54,56,57] | More expensive than SLA, limitation on the size of the finished products, some issues with the photoactive liquid resins used in the printing process (toxicity and odor) [42,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiari, Z. Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting. Mar. Drugs 2024, 22, 134. https://doi.org/10.3390/md22030134
Khiari Z. Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting. Marine Drugs. 2024; 22(3):134. https://doi.org/10.3390/md22030134
Chicago/Turabian StyleKhiari, Zied. 2024. "Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting" Marine Drugs 22, no. 3: 134. https://doi.org/10.3390/md22030134
APA StyleKhiari, Z. (2024). Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting. Marine Drugs, 22(3), 134. https://doi.org/10.3390/md22030134






