Aurantoside L, a New Tetramic Acid Glycoside with Anti-Leishmanial Activity Isolated from the Marine Sponge Siliquariaspongia japonica
Abstract
:1. Introduction
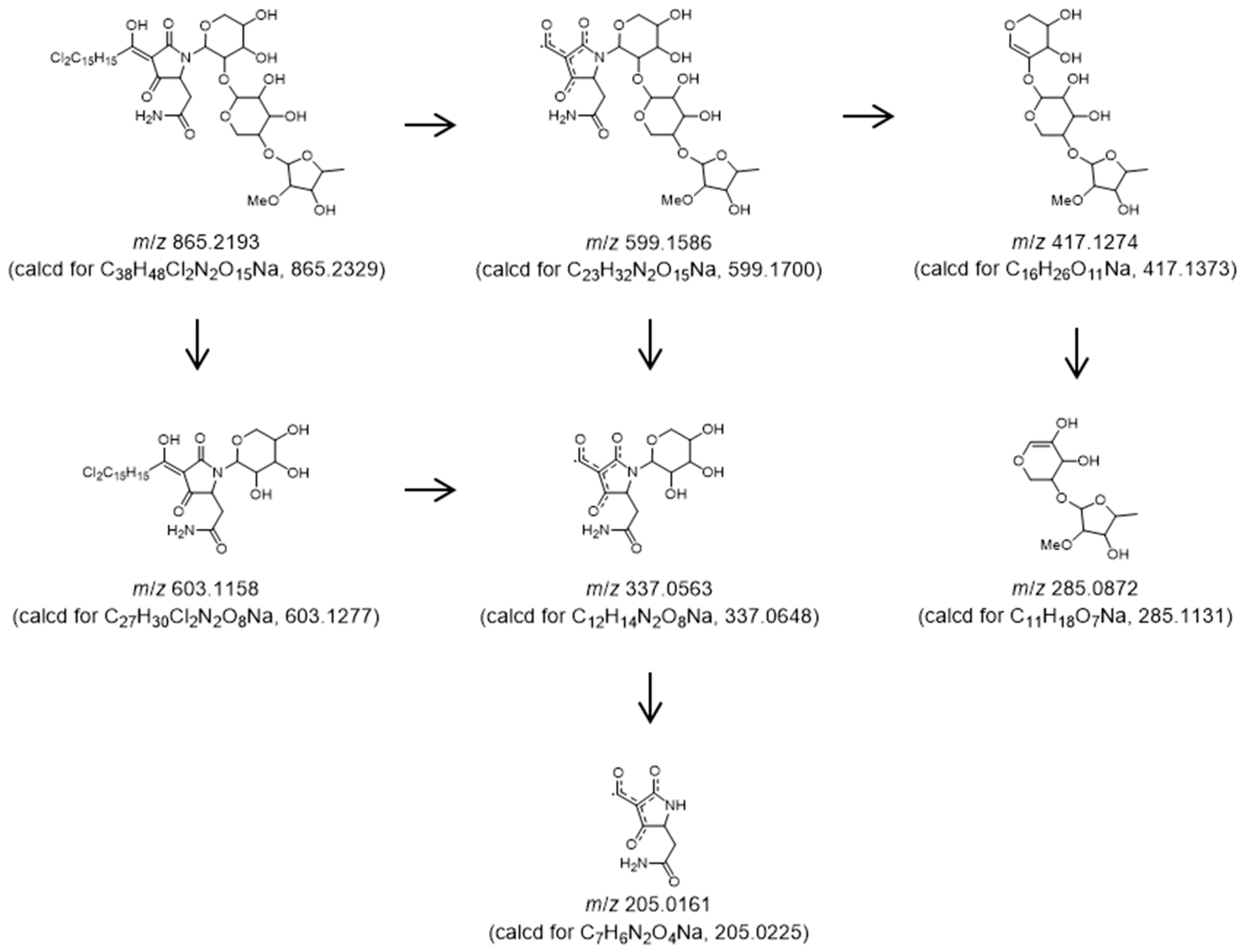
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Isolation
3.4. Anti-Leishmanial Assay
3.5. Anti-Trypanosomal Assay
3.6. Cell Culture
3.7. Cytotoxic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bruza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951. [Google Scholar] [CrossRef] [PubMed]
- Matlashewski, G.; Arana, B.; Kroegar, A.; Battacharya, S.; Sundar, S.; Das, P.; Sinha, P.K.; Rijal, S.; Mondal, D.; Zilberstein, D.; et al. Visceral leishmaniasis: Elimination with existing interventions. Lancet Infect. Dis. 2011, 11, 322. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Primez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; El-Agroudy, E.; Helmy, A.; Ibrahim, T.M.; Shavandi, A.; Bekhit, A.E.A. Leishmania treatment and prevention: Natural and synthesized drugs. Eur. J. Med. Chem. 2018, 160, 229. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Sundar, S. Drug Resistance in Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; Pieper, P.; Borborema, S.E.T.; Thevenard, F.; Lago, J.H.G.; Croft, S.L.; Anderson, E.A. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat. Prod. Rep. 2021, 38, 2214. [Google Scholar] [CrossRef]
- Ueoka, R.; Nakao, Y.; Kawatsu, S.; Yaegashi, J.; Matsumoto, Y.; Matsunaga, S.; Furihata, K.; van Soest, R.W.M.; Fusetani, N. Gracilioethers A-C, Antimalarial Metabolites from the Marine Sponge Agelas gracilis. J. Org. Chem. 2009, 74, 4203. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.-T.; Goto, Y.; Inoue, N.; Kawazu, S.; Matsumoto, Y.; Imahara, Y.; Tarumi, M.; Nakai, H.; Fusetani, N.; Nakao, Y. Cristaxenicin A, an Antiprotozoal Xenicane Diterpenoid from the Deep Sea Gorgonian Acathoprimnoa cristata. J. Org. Chem. 2012, 77, 10962. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Faulkner, D.J. Lithistid Sponges: Star Performers or Hosts to the Stars. Angew. Chem. Int. Ed. 1998, 37, 2162. [Google Scholar] [CrossRef]
- Sata, N.U.; Matsunaga, S.; Fusetani, N.; van Soest, R.W.M. Aurantosides D, E, and F: New Antifungal Tetramic Acid Glycosides from the Marine Sponge Siliquariaspongia japonica. J. Nat. Prod. 1999, 62, 969. [Google Scholar] [CrossRef] [PubMed]
- Sata, N.U.; Wada, S.; Matsunaga, S.; Watabe, S.; van Soest, R.W.M.; Fusetani, N. Rubrosides A−H, New Bioactive Tetramic Acid Glycosides from the Marine Sponge Siliquariaspongia japonica. J. Org. Chem. 1999, 64, 2331. [Google Scholar] [CrossRef]
- Keffer, J.L.; Plaza, A.; Bewley, C.A. Motualevic Acids A−F, Antimicrobial Acids from the Sponge Siliquariaspongia sp. Org. Lett. 2009, 11, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Gustchina, E.; Baker, H.L.; Kelly, M.; Bewley, C.A. Mirabamides A–D, Depsipeptides from the Sponge Siliquariaspongia mirabilis That Inhibit HIV-1 Fusion. J. Nat. Prod. 2007, 70, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A−C and Theopapuamides B−D, Depsipeptides from an Indonesian Sponge That Inhibit HIV-1 Entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, G.E.; Smith, R.A.; Roberts, J.D. Nuclear magnetic resonance spectroscopy. Carbon-13 chemical shifts of chlorinated organic compounds. J. Org. Chem. 1974, 39, 1276. [Google Scholar] [CrossRef]
- Kinns, M.; Sanders, J.K.M. Improved frequency selectivity in nuclear overhauser effect difference spectroscopy. J. Magn. Reson. 1984, 56, 518. [Google Scholar] [CrossRef]
- Royles, B.J.L. Naturally Occurring Tetramic Acids: Structure, Isolation, and Synthesis. Chem. Rev. 1995, 95, 1981. [Google Scholar] [CrossRef]
- Angawi, R.F.; Bavestrello, G.; Calcinai, B.; Dien, H.A.; Donnarumma, G.; Tufano, M.A.; Paoletti, I.; Grimaldi, E.; Chianese, G.; Fattorusso, E.; et al. Aurantoside J: A New Tetramic Acid Glycoside from Theonella swinhoei. Insights into the Antifungal Potential of Aurantosides. Mar. Drugs 2011, 9, 2809. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Fusetani, N.; Kato, Y.; Hirota, H. Aurantosides A and B: Cytotoxic tetramic acid glycosides from the marine sponge Theonella sp. J. Am. Chem. Soc. 1991, 113, 9690. [Google Scholar] [CrossRef]
- Wolf, D.; Schmitz, F.J.; Qiu, F.; Kelly-Borges, M. Aurantoside C, a New Tetramic Acid Glycoside from the Sponge Homophymia conferta. J. Nat. Prod. 1999, 62, 170. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Davis, R.A.; Harper, M.K.; Veltri, C.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Aurantosides G, H, and I: Three New Tetramic Acid Glycosides from a Papua New Guinea Theonella swinhoei. J. Nat. Prod. 2005, 68, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Subramani, R.; Feussner, K.; Aalbersberg, W. Aurantoside K, a New Antifungal Tetramic Acid Glycoside from a Fijian Marine Sponge of the Genus Melophlus. Mar. Drugs 2012, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Shiroiwa, T.; Murayama, S.; Matsunaga, S.; Goto, Y.; Matsumoto, Y.; Fusetani, N. Identification of Renieramycin A as an Antileishmanial Substance in a Marine Sponge Neopetrosia sp. Mar. Drugs 2004, 2, 55. [Google Scholar] [CrossRef]
- Sakurai, T.; Sugimoto, C.; Inoue, N. Identification and molecular characterization of a novel stage-specific surface protein of Trypanosoma congolense epimastigotes. Mol. Biochem. Parasitol. 2008, 161, 1. [Google Scholar] [CrossRef]
- Mo, X.; Li, Q.; Ju, J. Naturally occurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566. [Google Scholar] [CrossRef]
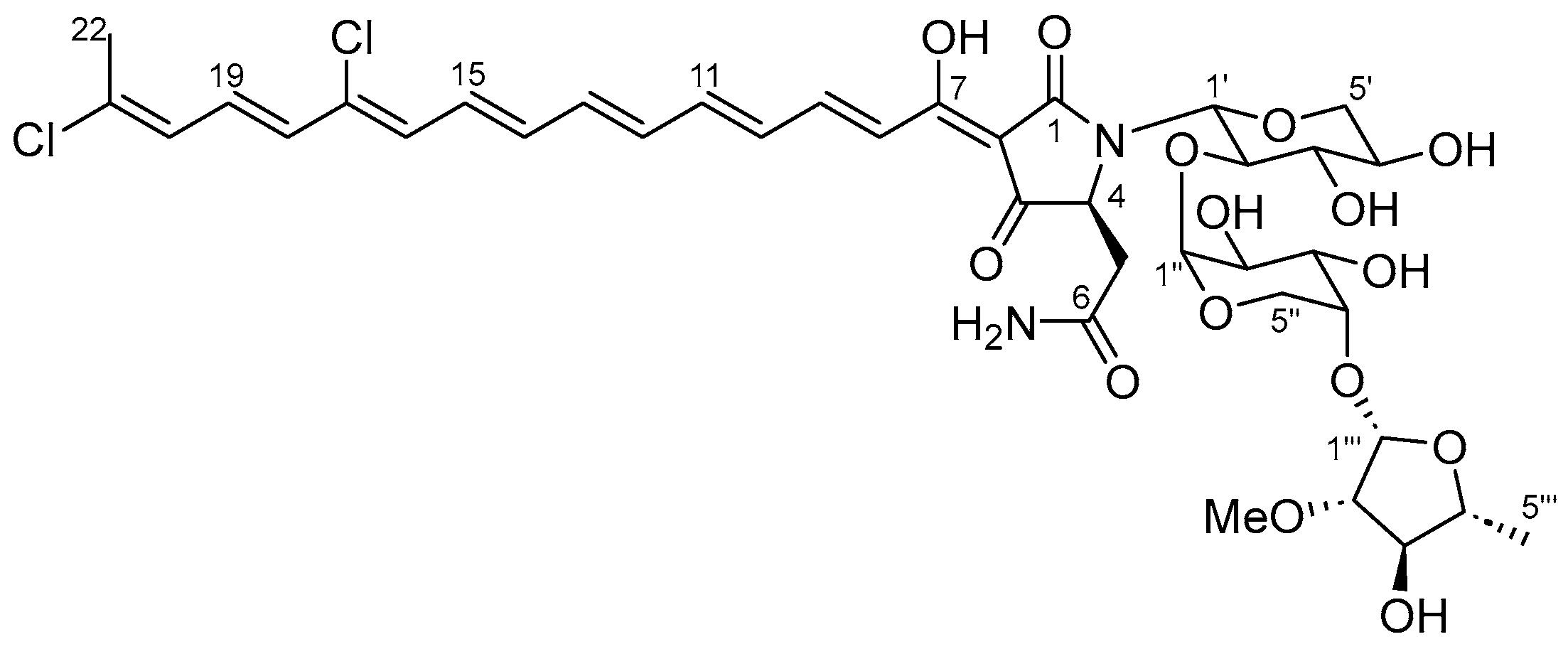
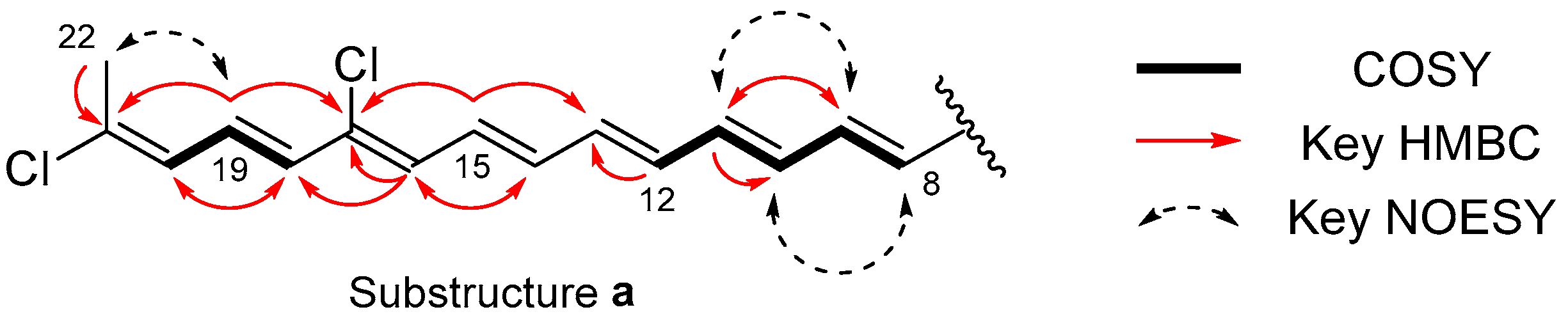
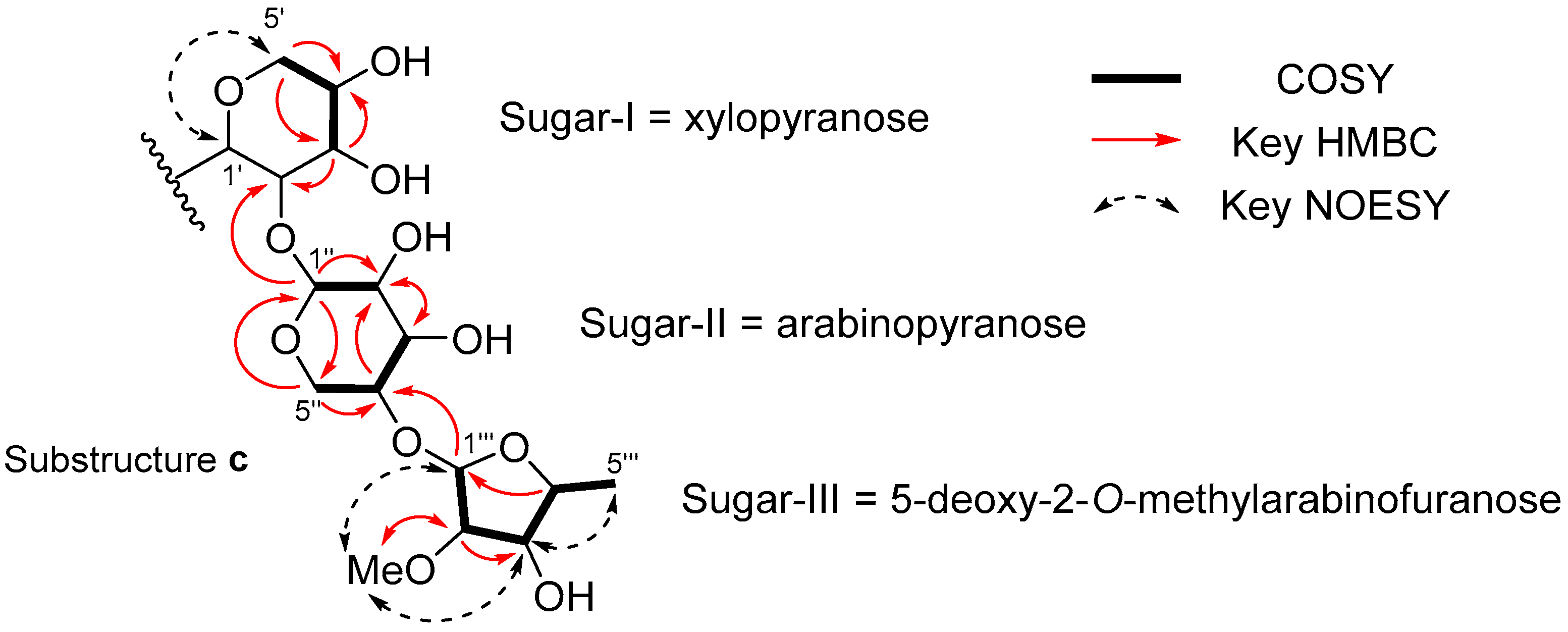
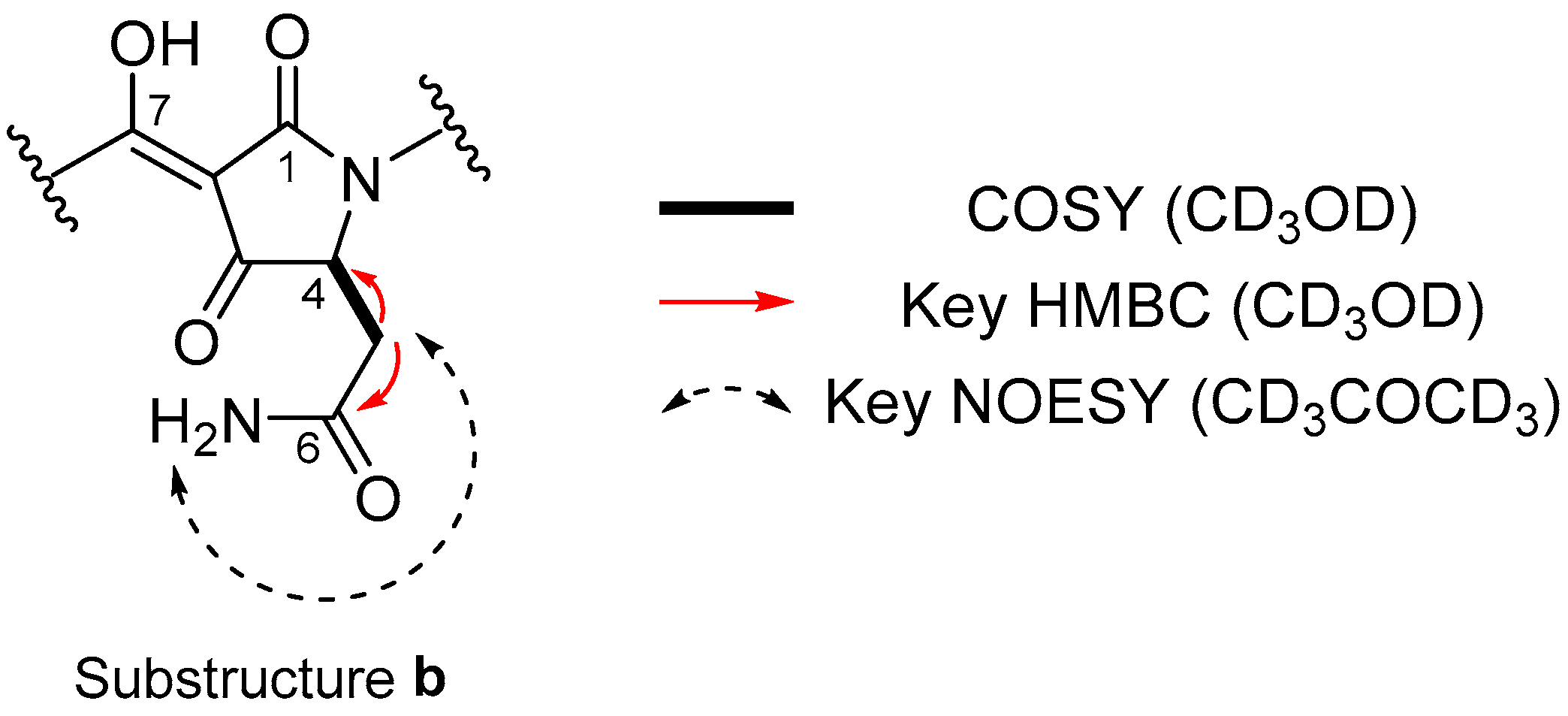
| Position | δC | δH Mult. (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | 4.33 br | 5a, 5b | ||
| 5a | 38.4 | 2.67 dd (15.4, 7.6) | 4, 5b | 6 |
| 5b | 2.81 dd (15.4, 3.6) | 4, 5a | 6 | |
| 6 | 174.5 | |||
| 7 | 175.2 | |||
| 8 | 122.3 | 7.22 d (15.1) | 9 | 7, 10 |
| 9 | 146.5 | 7.60 dd (15.1, 11.5) | 8, 10 | 7, 11 |
| 10 | 133.6 | 6.61 dd (14.5, 11.5) | 9, 11 | 12 |
| 11 | 145.3 | 6.89 dd (14.5, 11.6) | 10, 12 | 9 |
| 12 | 135.9 | 6.56 m | 11 | 13 |
| 13 | 140.5 | 6.72 dd (16.1, 11.6) | 11, 15 | |
| 14 | 137.7 | 6.59 m | 16 | |
| 15 | 132.9 | 6.86 dd (14.2, 11.6) | 13, 17 | |
| 16 | 131.3 | 6.55 d (11.6) | 14, 17, 18 | |
| 17 | 134.7 | |||
| 18 | 131.8 | 6.44 d (14.4) | 19 | 16, 17, 20 |
| 19 | 128.0 | 6.75 dd (14.4, 11.5) | 18, 20 | 17, 21 |
| 20 | 129.0 | 6.39 d (11.5) | 19, 22 | 18, 21, 22 |
| 21 | 136.4 | |||
| 22 | 21.8 | 2.26 brs | 20 | 20, 21 |
| 1′ | 86.3 | 4.52 br | ||
| 2′ | 81.4 | 4.52 br | ||
| 3′ | 79.4 | 3.48 dd (8.8, 8.8) | 4′ | 2′, 4′ |
| 4′ | 70.6 | 3.62 m | 3′, 5′a, 5′b | |
| 5′a | 69.3 | 3.21 dd (11.0, 10.8) | 4′, 5′b | 3′, 4′ |
| 5′b | 3.88 dd (10.8, 4.8) | 4′, 5′a | 4′ | |
| 1″ | 104.0 | 5.04 br | 2″ | 2′, 2″, 5″ |
| 2″ | 71.7 | 3.80 dd (9.5, 2.9) | 1″ | 3″ |
| 3″ | 70.9 | 3.79 dd (9.5, 2.9) | 4″ | 2″ |
| 4″ | 76.1 | 3.91 m | 3″, 5″a, 5″b | 2″ |
| 5″a | 61.6 | 3.59 dd (12.9, 2.3) | 4″, 5″b | 1″, 3″, 4″ |
| 5″b | 3.74 dd (12.9, 0.9) | 4″, 5″a | ||
| 1‴ | 98.9 | 5.09 d (4.4) | 2‴ | 4″, 2‴, 3‴ |
| 2‴ | 87.4 | 3.67 dd (8.1, 4.4) | 1‴, 3‴ | 1‴, 3‴, OMe |
| 3‴ | 79.9 | 3.90 dd (8.1, 7.1) | 2‴, 4‴ | 1‴, 2‴, 4‴, 5‴ |
| 4‴ | 79.7 | 3.76 qd (7.1, 6.4) | 3‴, 5‴ | 1‴, 3‴ |
| 5‴ | 21.0 | 1.32 d (6.4) | 4‴ | 4‴ |
| OCH3 | 58.5 | 3.35 s | 2‴ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyadomari, Y.; Goto, Y.; Suganuma, K.; Kawazu, S.-i.; Becking, L.E.; Fusetani, N.; Nakao, Y. Aurantoside L, a New Tetramic Acid Glycoside with Anti-Leishmanial Activity Isolated from the Marine Sponge Siliquariaspongia japonica. Mar. Drugs 2024, 22, 171. https://doi.org/10.3390/md22040171
Oyadomari Y, Goto Y, Suganuma K, Kawazu S-i, Becking LE, Fusetani N, Nakao Y. Aurantoside L, a New Tetramic Acid Glycoside with Anti-Leishmanial Activity Isolated from the Marine Sponge Siliquariaspongia japonica. Marine Drugs. 2024; 22(4):171. https://doi.org/10.3390/md22040171
Chicago/Turabian StyleOyadomari, Yasumoto, Yasuyuki Goto, Keisuke Suganuma, Shin-ichiro Kawazu, Leontine E. Becking, Nobuhiro Fusetani, and Yoichi Nakao. 2024. "Aurantoside L, a New Tetramic Acid Glycoside with Anti-Leishmanial Activity Isolated from the Marine Sponge Siliquariaspongia japonica" Marine Drugs 22, no. 4: 171. https://doi.org/10.3390/md22040171









