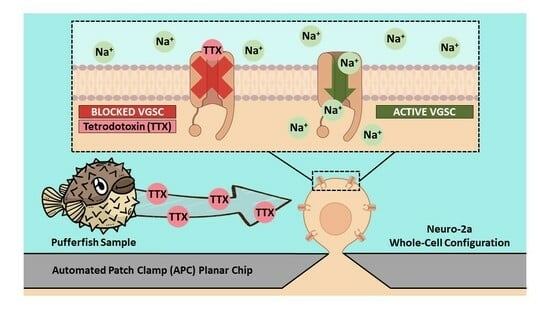
Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples
Abstract
1. Introduction
2. Results
2.1. Suitability of Neuro-2a Cells for TTX Detection Using APC
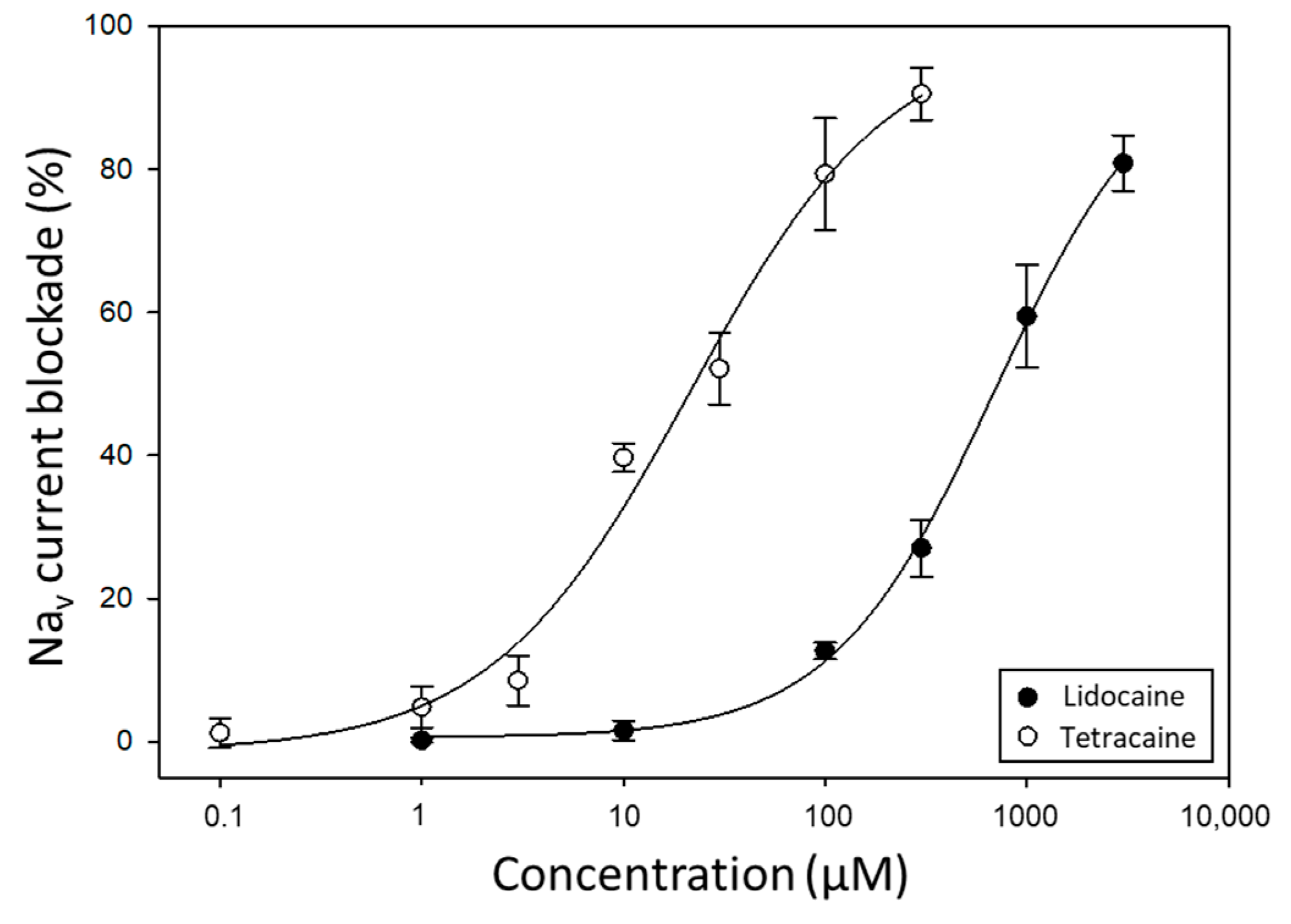
2.2. Evaluation of Pufferfish Matrix Effects on Nav Function and Blockade by TTX
2.3. Analysis of TTX in Lagocephalus Sceleratus Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Pufferfish Samples and Tetrodotoxin (TTX) Extraction
4.3. Neuro-2a Cell Line Maintenance
4.4. Automated Patch Clamp Recording
4.5. Colorimetric Cell-Based Assay (CBA)
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New invasive nemertean species (Cephalothrix simula) in England with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Bio. Ecol. 1998, 116, 249–256. [Google Scholar] [CrossRef]
- Hwang, P.A.; Tsai, Y.H.; Lin, S.J.; Hwang, D.F. The gastropods possessing TTX and/or PSP. Food Rev. Int. 2007, 23, 321–340. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–775. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 Annual report of the American association of poison control center’s national poison data system (NPDS): 38th annual report. Clin. Toxicol. 2021, 59, 1282–1501. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sanchez, V.; Rodrígez Loureo, P.; Alfonso Rancaño, A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef]
- Ulman, A.; Yildiz, T.; Demirel, N.; Canak, O.; Yemişken, E.; Pauly, D. The biology and ecology of the invasive silver-cheeked toadfish (Lagocephalus sceleratus), with emphasis on the Eastern Mediterranean. NeoBiota 2021, 68, 145–175. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Filiz, H.; Er, M. Akdeniz’in yeni misafiri [New guests in the Mediterranean Sea]. Deniz Magazin Dergisi 2004, 3, 52–54. [Google Scholar]
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglu, M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin. Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004. Laying Down Specific Rules for the Organisation of Official Controls on Products of Animal Origin Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0854 (accessed on 29 November 2023).
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. J. EFSA 2017, 15, 4752. [Google Scholar]
- Quilliam, M.A.; Thomson, B.A.; Scott, G.J.; Siu, K.M. Ion-spray mass spectrometry of marine neurotoxins. Rapid Commun. Mass Spectrom. 1989, 3, 145–150. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Wekell, M.M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: Semiautomated assay for saxitoxins, brevetoxins, and ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative methods for the detection of emerging marine toxins: Biosensors, biochemical assays and cell-based assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef]
- Cañete, E.; Diogène, J. Comparative study of the use of neuroblastoma cells (Neuro-2a) and neuroblastoma-glioma hybrid cells (NG108-15) for the toxic effect quantification of marine toxins. Toxicon 2008, 52, 541–550. [Google Scholar] [CrossRef]
- Alkassar, M.; Leonardo, S.; Diogène, J.; Campàs, M. Immobilisation of Neuro-2a cells on electrodes and electrochemical detection of MTT formazan crystals to assess their viability. Bioelectrochemistry 2022, 148, 108274. [Google Scholar] [CrossRef] [PubMed]
- Reverté, J.; Alkassar, M.; Diogène, J.; Campàs, M. Detection of ciguatoxins and tetrodotoxins with biosensors and other smart bioanalytical systems. Foods 2023, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Kogure, K.; Tampline, M.; Simidu, U.; Colwell, R.R. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Alkassar, M.; Sanchez-Henao, A.; Reverté, J.; Barreiro, L.; Rambla-Alegre, M.; Leonardo, S.; Mandalakis, M.; Peristeraki, P.; Diogène, J.; Campàs, M. Evaluation of toxicity equivalency factors of tetrodotoxin analogues with a Neuro-2a cell-based assay and application to puffer fish from Greece. Mar. Drugs 2023, 21, 432. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.Y.; Chen, B.; Chen, W.B.; Yang, F.; Miao, T.Y.; Peng, J. Preparation and application of tetrodotoxin DNA aptamer. Food Sci. 2014, 35, 205–208. [Google Scholar]
- Reverté, L.; Rambla-Alegre, M.; Leonardo, S.; Bellés, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogène, J.; Campàs, M. Development and validation of a maleimide-based enzyme-linked immunosorbent assay for the detection of tetrodotoxin in oysters and mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Dong, Y.; Han, X.; Wang, S.; Liang, X. Aptamers and aptasensors for highly specific recognition and sensitive detection of marine biotoxins: Recent advances and perspectives. Toxins 2018, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Campàs, M.; Reverté, J.; Rambla-Alegre, M.; Campbell, K.; Gerssen, A.; Diogène, J. A fast magnetic bead-based colorimetric immunoassay for the detection of tetrodotoxins in shellfish. Food Chem. Toxicol. 2020, 140, 111315. [Google Scholar] [CrossRef] [PubMed]
- Shkembi, X.; Skouridou, V.; Svobodova, M.; Leonardo, S.; Bashammakh, A.S.; Alyoubi, A.O.; Campàs, M.; O’Sullivan, C.K. Hybrid antibody-aptamer assay for detection of tetrodotoxin in pufferfish. Anal. Chem. 2022, 93, 14810–14819. [Google Scholar] [CrossRef]
- Mattei, C.; Legros, C. The voltage-gated sodium channel: A major target of marine neurotoxins. Toxicon 2014, 91, 84–95. [Google Scholar] [CrossRef]
- Ferreiro, S.F.; Vilariño, N.; Carrera, C.; Louzao, M.C.; Cantalapiedra, A.G.; Santamarina, G.; Cifuentes, J.M.; Vieira, A.C.; Botana, L.M. Subacute cardiovascular toxicity of the marine phycotoxin Azaspiracid-1 in rats. Toxicol 2016, 151, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Inserra, M.; Israel, M.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple sodium channel isoforms mediate the pathological effects of Pacific ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef]
- Finol-Urdaneta, R.K.; Zhorov, B.S.; Baden, D.G.; Adams, D.J. Brevetoxin versus brevenal modulation of human Nav1 channels. Mar. Drugs 2023, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Evaluation of toxicity equivalent factors of paralytic shellfish poisoning toxins in seven human sodium channels types by an automated high throughput electrophysiology system. Arch. Toxicol. 2016, 90, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Randall, A.; McNaughton, N.; Green, P. Properties of voltage-gated Na+ channels in the human rhabdomyosarcoma cell-line SJ-RH30: Conventional and automated patch clamp analysis. Pharmacol. Res. 2006, 54, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Albert, R. The Horwitz ratio (HorRat): A useful index of method performance with respect to precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Guidelines for Standard Method Performance Requirements. In AOAC Official Methods of Analysis; AOAC International: Rockville, Maryland, 2016; Appendix F; pp. 1–18. [Google Scholar]
- Reverté, L.; Campbell, K.; Rambla-Alegre, M.; Elliott, C.T.; Diogène, J.; Campàs, M. Immunosensor array platforms based on self-assembled dithiols for the electrochemical detection of tetrodotoxins in puffer fish. Anal. Chim. Acta 2017, 989, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.F.; Brooks, J.M.; Strassmaier, T.; Haedo, R.J.; Puryear, C.B.; Roth, B.L.; Ouk, K.; Pin, S.S. Application of high-throughput automated patch-clamp electrophysiology to study voltage-gated ion channel function in primary cortical cultures. SLAS Discov. 2020, 25, 447–457. [Google Scholar] [CrossRef]
- Ritzau-Jost, A.; Nerlich, J.; Kaas, T.; Krueger, M.; Tsintsadze, T.; Eilers, J.; Barbour, B.; Smith, S.M.; Hallermann, S. Direct whole-cell patch-clamp recordings from small boutons in rodent primary neocortical neuron cultures. Cell Rep. 2023, 4, 102168. [Google Scholar] [CrossRef]
- Masters, J. Human cancer cell lines: Fact and fantasy. Nat. Rev. Mol. Cell Biol. 2000, 1, 233–236. [Google Scholar] [CrossRef]
- Noreng, S.; Li, T.; Payandeh, J. Structural Pharmacology of voltage-gated sodium channels. J. Mol. Biol. 2021, 433, 166967. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Chiba, Y.; Wakamori, M.; Yamada, T.; Tsunogae, S.; Cho, Y.; Sakakibara, R.; Imazu, T.; Tokoro, S.; Satake, Y.; et al. Differential binding of tetrodotoxin and its derivatives to voltage-sensitive sodium channel subtypes (Nav 1.1 to Nav 1.7). Br. J. Pharmacol. 2017, 174, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Clare, J.J.; Tate, S.N.; Nobbs, M.; Romanos, M.A. Voltage-gated sodium channels as therapeutic targets. Drug Discov. Today 2000, 5, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Denomme, N.; Lukowski, A.L.; Hull, J.M.; Jameson, M.B.; Bouza, A.A.; Narayan, A.R.H.; Isom, L.L. The voltage-gated sodium channel inhibitor, 4,9-anhydrotetrodotoxin, blocks human Nav1.1 in addition to Nav1.6. Neurosci. Lett. 2020, 724, 134853. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.M.; Huang, C.F.; Chao, C.C.; Lu, D.Y.; Kuo, C.S.; Cheng, T.H.; Chang, L.Y.; Chou, C.H. Voltage-gated K+ channels play a role in cAMP-stimulated neuritogenesis in mouse neuroblastoma N2A cells. J. Cell Physiol. 2011, 226, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Saify, Z.S.; Jamali, K.S.; Naz, S.; Hassan, S.; Siddiqui, S. Vitex negundo induces an anticonvulsant effect by inhibiting voltage gated sodium channels in murine Neuro 2A cell line. Pak. J. Pharm. Sci. 2018, 31, 297–303. [Google Scholar] [PubMed]
- Badisa, R.B.; Wi, S.; Jones, Z.; Mazzio, E.; Zhou, Y.; Rosenberg, J.T.; Latinwo, L.M.; Grant, S.C.; Goodman, C.B. Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: Potential mechanism for its resistance in cell death. Cell Death Discov. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-M.; Cho, H.-Y.; Chiang, C.-W.; Chuang, T.-H.; Wu, S.-N.; Tu, Y.-F. Characterization in inhibitory effectiveness of carbamazepine in voltage-gated Na+ and erg-mediated K+ currents in a mouse neural crest-derived (Neuro-2a) cell line. Int. J. Mol. Sci. 2022, 23, 7892. [Google Scholar] [CrossRef]
- Wu, P.-M.; Lai, P.-C.; Cho, H.-Y.; Chuang, T.-H.; Wu, S.-N.; Tu, Y.-F. Effective perturbations by phenobarbital on INa, IK(erg), IK(M) and IK(DR) during pulse train stimulation in neuroblastoma Neuro-2a cells. Biomedicines 2022, 10, 1968. [Google Scholar] [CrossRef]
- LePage, K.T.; Dickey, R.W.; Gerwick, W.H.; Jester, E.L.; Murray, T.F. On the use of Neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit. Rev. Neurobiol. 2005, 17, 27–50. [Google Scholar] [CrossRef]
- Meadows, S.L.; Malhotra, J.; Loukas, A.; Thyagarajan, V.; Kazen-Gillespie, K.A.; Koopman, M.C.; Kriegler, S.; Isom, L.L.; Ragsdale, D.S. Functional and biochemical analysis of a sodium channel β1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J. Neurosci. 2002, 22, 10699–10709. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, G.; Beck, H.; Schramm, J.; Elger, C.E.; Urban, B.W. Electrophysiological characterization of Na+ currents in acutely isolated human hippocampal dentate granule cells. J. Physiol. 1998, 509, 139–150. [Google Scholar] [CrossRef]
- Raposo-Garcia, S.; Cao, A.; Costas, C.; Louzao, M.C.; Vilariño, N.; Vale, C.; Botana, L.M. Mouse N2a neuroblastoma assay: Uncertainties and comparison with alternative cell-based assays for ciguatoxin detection. Mar. Drugs 2023, 21, 590. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, P.; Vijayaragavan, K.; Chahine, M. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br. J. Pharmacol. 2009, 142, 576–584. [Google Scholar] [CrossRef]
- Obergrussberger, A.; Brüggemann, A.; Goetze, T.A.; Rapedius, M.; Haarmann, C.; Rinke, I.; Becker, N.; Oka, T.; Ohtsuki, A.; Stengel, T.; et al. Automated patch clamp meets high-throughput screening: 384 cells recorded in parallel on a planar patch clamp module. SLAS Technol. 2016, 21, 779–793. [Google Scholar] [CrossRef]
- John, V.H.; Main, M.J.; Powell, A.J.; Gladwell, Z.M.; Hick, C.; Sidhu, H.S.; Clare, J.J.; Tate, S.; Trezise, D.J. Heterologous expression and functional analysis of rat Nav1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7-23. Neuropharmacology 2004, 46, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Heinemann, S.H.; Stuhmer, W.; Pusch, M.; Conti, F.; Imoto, K.; Numa, S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991, 293, 93–96. [Google Scholar] [CrossRef]
- Chahine, M.; Bennett, P.B.; George, A.L., Jr.; Horn, R. Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary beta1, beta2 and beta3 subunits. Pflügers Arch.-Eur. J. Appl. Physiol. 1994, 427, 136–142. [Google Scholar] [CrossRef]
- Smith, M.R.; Smith, R.D.; Plummer, N.W.; Meisler, M.H.; Goldin, A.L. Functional analysis of the mouse Scn8a sodium channel. J. Neurosci. 1998, 18, 6093–6102. [Google Scholar] [CrossRef]
- Klugbauer, N.; Lacinova, L.; Flockerzi, V.; Hofmann, F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995, 14, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Yacout, G.A.; El-Samra, M.; Ali, A.; Kotb, S.M. Toxicity of the Red Sea pufferfish Pleuranacanthus sceleratus ‘El-Karad’. Ecotoxicol. Environ. Saf. 2003, 56, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Christidis, G.; Mandalakis, M.; Anastasiou, T.I.; Tserpes, G.; Peristeraki, P.; Somarakis, S. Keeping Lagocephalus sceleratus off the table: Sources of variation in the quantity of TTX, TTX analogues, and risk of tetrodotoxication. Toxins 2021, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Kosker, A.R.; Karakus, M.; Katikou, P.; Dal, I.; Durmus, M.; Ucar, Y.; Ayas, D.; Özogul, F. Monthly variation of tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught from Antalya Bay, Mediterranean Sea. Mar. Drugs 2023, 21, 527. [Google Scholar] [CrossRef]
- Anastasiou, T.I.; Kagiampaki, E.; Kondylatos, G.; Tselepides, A.; Peristeraki, P.; Mandalakis, M. Assessing the toxicity of Lagocephalus sceleratus pufferfish from the Southeastern Aegean Sea and the relationship of tetrodotoxin with gonadal hormones. Mar. Drugs 2023, 21, 520. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Evaluation of hepatic uptake clearance of tetrodotoxin in the puffer fish Takifugu rubripes. Toxicon 2008, 52, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Murakami, Y.; Emoto, Y.; Ngy, L.; Taniyama, S.; Yagi, M.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to non-toxic cultured specimens of the pufferfish Takifugu rubripes. Toxicon 2009, 53, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Takatani, T.; Arakawa, O. Transfer profile of orally and intramuscularly administered tetrodotoxin to artificial hybrid specimens of the pufferfish Takifugu rubripes and Takifugu porphyreus. J. Food Hyg. Soc. Jpn. 2012, 53, 33–38. [Google Scholar] [CrossRef][Green Version]
- Akbora, H.D.; Kunter, I.; Erçetïn, T.; Elagöz, A.M.; Çïçek, B.A. Determination of tetrodotoxin (TTX) levels in various tissues of the silver cheeked puffer fish (Lagocephalus sceleratus (Gmelin, 1789)) in Northern Cyprus Sea (Eastern Mediterranean). Toxicon 2020, 175, 1–6. [Google Scholar] [CrossRef]
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar] [CrossRef]
- Satake, Y.; Adachi, M.; Tokoro, S.; Yotsu-Yamashita, M.; Isobe, M.; Nishikawa, T. Synthesis of 5- and 8-deoxytetrodotoxin. Chem. Asian J. 2014, 9, 1922–1932. [Google Scholar] [CrossRef]
- Leonardo, S.; Rambla-Alegre, M.; Samdal, I.A.; Miles, C.O.; Kilcoyne, J.; Diogène, J.; O’Sullivan, C.K.; Campàs, M. Immunorecognition magnetic supports for the development of an electrochemical immunoassay for azaspiracid detection in mussels. Biosens. Bioelectron. 2017, 92, 200–206. [Google Scholar] [CrossRef]
- Reverté, L.; de la Iglesia, P.; del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of tetrodotoxins in puffer fish by a self-assembled monolayer-based immunoassay and comparison with surface plasmon resonance, LC-MS/MS, and mouse bioassay. Anal. Chem. 2015, 87, 10839–10847. [Google Scholar] [CrossRef]




| Tissue | Hill Slope | IC50 Value (nM) | R2 |
|---|---|---|---|
| Muscle | 0.997 ± 0.068 | 4.927 ± 2.350 | 0.9950 |
| Skin | 0.910 ± 0.120 | 5.017 ± 1.587 | 0.9961 |
| Liver | 1.167 ± 0.204 | 5.118 ± 0.995 | 0.9886 |
| Gonads | 0.801 ± 0.149 | 4.264 ± 0.668 | 0.9859 |
| No tissue | 1.089 ± 0.097 | 6.380 ± 0.860 | 0.9912 |
| Technique | Muscle | Skin | Liver | Gonads | Reference |
|---|---|---|---|---|---|
| APC | 1.59 | 3.32 | 13.80 | 22.46 | This work |
| CBA | 2.78 | 3.68 | 18.53 | 26.63 | This work |
| LC-MS/MS * | 1.43 | 2.02 | 3.96 | 34.62 | [37] |
| LC-HRMS * | 1.23 | 2.42 | 7.67 | 39.44 | [37] |
| mELISA | 2.53 | 3.50 | 28.30 | 33.55 | [37] |
| Electrochemical immunosensor | 1.45 | 2.11 | 16.67 | 33.90 | [40] |
| Optical SPR immunosensor | 3.51 | 4.42 | 24.82 | 30.50 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campàs, M.; Reverté, J.; Tudó, À.; Alkassar, M.; Diogène, J.; Sureda, F.X. Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples. Mar. Drugs 2024, 22, 176. https://doi.org/10.3390/md22040176
Campàs M, Reverté J, Tudó À, Alkassar M, Diogène J, Sureda FX. Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples. Marine Drugs. 2024; 22(4):176. https://doi.org/10.3390/md22040176
Chicago/Turabian StyleCampàs, Mònica, Jaume Reverté, Àngels Tudó, Mounira Alkassar, Jorge Diogène, and Francesc X. Sureda. 2024. "Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples" Marine Drugs 22, no. 4: 176. https://doi.org/10.3390/md22040176
APA StyleCampàs, M., Reverté, J., Tudó, À., Alkassar, M., Diogène, J., & Sureda, F. X. (2024). Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples. Marine Drugs, 22(4), 176. https://doi.org/10.3390/md22040176