Nutraceutical and Medicinal Importance of Marine Molluscs
Abstract
:1. Introduction
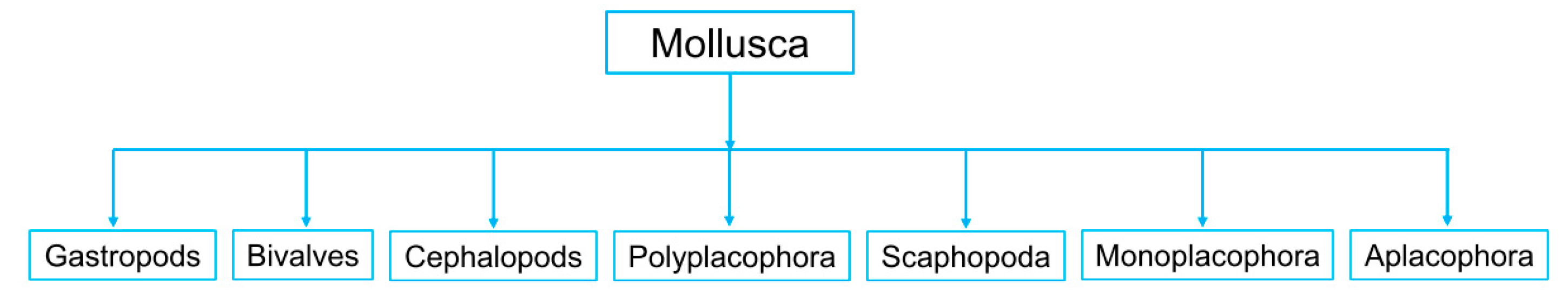
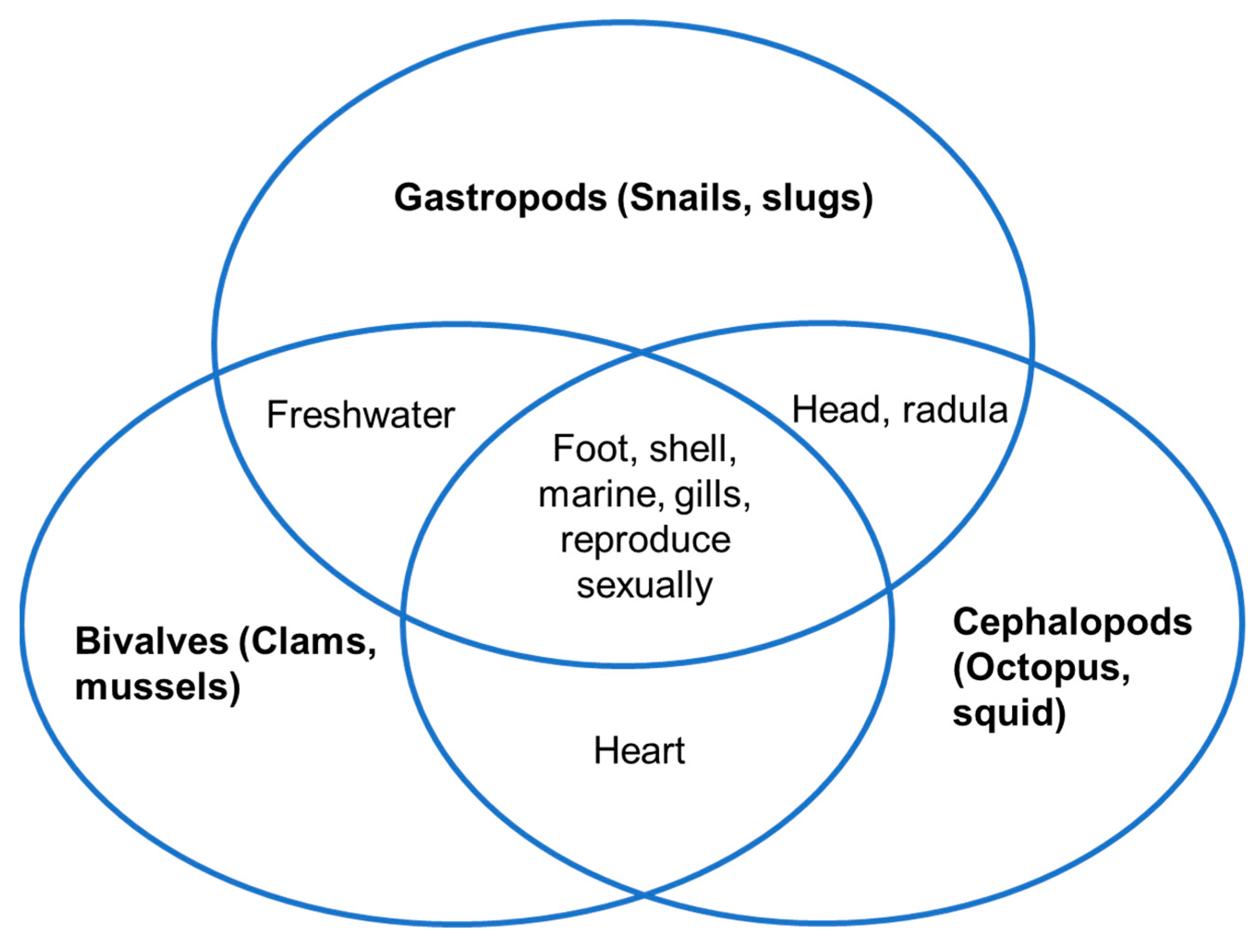
2. Definition and Types of Marine Molluscs
3. Marine Molluscs and Chemical Ecology
4. Extraction Techniques of Bioactive Compounds from Marine Molluscs
4.1. Conventional Techniques
4.2. Non-Conventional Techniques
4.2.1. Enzyme-Assisted Extraction (EAE)
4.2.2. Microwave-Assisted Extraction (MAE)
4.2.3. Subcritical Water Extraction (SWE)
4.2.4. Supercritical Fluid Extraction (SC-CO2)
4.2.5. Ultrasound-Assisted Extraction (UAE)
| Extraction Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Enzyme-assisted extraction (EAE) | (i) It is a non-toxic, environmentally beneficial method. (ii) It enables the production of large yields of bioactive compounds. (iii) It transforms raw materials that are insoluble in water into those that are soluble. (iv) The approach is rather inexpensive due to the utilization of food-grade enzymes. | (i) Enzyme treatment is often a lengthy process that might take hours or even days. | [107,108] |
| Microwave-assisted extraction (MAE) | (i) Minimal solvent usage and treatment duration. (ii) Elevated extraction yields. | (i) It is only possible to utilize solvents with strong dielectric characteristics. (ii) The most thermolabile compounds may degrade over time when using open vessels. (iii) Substantial energy use. | [109,110] |
| Subcritical water extraction (SWE) | (i) Use of non-toxic solvents. | (i) Expensive prices for the necessary high-pressure apparatus. (ii) Thermolabile compounds may degrade because of high-temperature extractions. | [111,112] |
| Supercritical fluid extraction (SC-CO2) | (i) Increased selectivity due to the ability to control a compound’s solubility in a supercritical fluid. (ii) The extraction is solvent-free as the CO2 is eliminated and leaves no trace. (iii) Ideal for extracting thermolabile compounds. | (i) Substantial expenses for the necessary high-pressure apparatus. (ii) Toxic modifiers, such as methanol, are necessary for the extraction of polar chemical compounds. (iii) May require more time than the other available methods. | [113,114] |
| Ultrasound-assisted extraction (UAE) | (i) Minimal solvent usage and treatment duration. (ii) A high degree of cell disruption efficiency. (iii) High extraction yields. (iv) Inexpensive. | (i) It works best with solvents with low vapor pressure, low viscosity, and low surface tension. (ii) Oversonication has the potential to degrade extract quality. | [115,116] |
5. Nutraceutical Importance of Marine Molluscs
5.1. Proteins and Peptides
5.2. Polysaccharides
5.3. Lipids
5.4. Polyphenolic Compounds Pigments
5.5. Marine Enzymes
5.6. Minerals and Vitamins
6. Medicinal Importance of Marine Molluscs
6.1. Anti-Microbial Properties
6.2. Anti-Cancer Properties
6.3. Anti-Inflammatory and Analgesic Properties
6.4. Antioxidant Properties
7. Limitations, Gaps, and New Perspectives
- (1)
- Understand how these compounds exert their effects on biological systems, whether it is by targeting specific molecular pathways, receptors, enzymes, or other mechanisms;
- (2)
- Identify potential therapeutic targets for drug development. This knowledge can lead to the creation of new drugs or therapeutic interventions that have specific effects on biological processes;
- (3)
- Optimize treatment regimens, dosages, and combinations. This knowledge can help improve the efficacy of treatments and reduce potential side effects.
8. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, G. Marine biodiversity characteristics. C. R. Biol. 2011, 334, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future. Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 1–38. [Google Scholar] [CrossRef]
- Cayol, J.L.; Ollivier, B.; Alazard, D.; Amils, R.; Godfroy, A.; Piette, F.; Prieur, D. The extreme conditions of life on the planet and exobiology. In Environmental Microbiology: Fundamentals and Applications; Springer: Dordrecht, The Netherlands, 2015; Volume 10, pp. 353–394. [Google Scholar] [CrossRef]
- Tan, L.T. Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Mar. Drugs 2023, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.J.; Puglisi, M.P.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2006, 23, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.L.; Sink, J.K. Phylum Mollusca. In Field Guide to the Offshore Marine Invertebrates of South Africa; Malachite Marketing and Media: Pretoria, South Africa, 2018; Volume 1, pp. 253–391. [Google Scholar] [CrossRef]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.M.; Liu, Y. Marine Mollusks: Food with Benefits. Compr. Rev. Food Sci. Food Saf. 2019, 18, 548–564. [Google Scholar] [CrossRef]
- Boulajfene, W. Nutritional and Health Benefits of Marine Mollusks. In Marine Biochemistry; Taylor & Francis: Abingdon, UK, 2022; pp. 435–453. [Google Scholar] [CrossRef]
- Avila, C.; Angulo-Preckler, C. Bioactive Compounds from Marine Heterobranchs. Mar. Drugs 2020, 18, 657. [Google Scholar] [CrossRef]
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone snails: A big store of conotoxins for novel drug discovery. Toxins 2017, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.; Redl, E.; Wollesen, T.; Todt, C.; Wanninger, A. Aplacophoran Mollusks Evolved from Ancestors with Polyplacophoran-like Features. Curr. Biol. 2013, 23, 2130–2134. [Google Scholar] [CrossRef]
- Kijewska, A.; Koroza, A.; Grudlewska-Buda, K.; Kijewski, T.; Wiktorczyk-Kapischke, N.; Zorena, K.; Skowron, K. Molluscs—A ticking microbial bomb. Front. Microbiol. 2023, 13, 1061223. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Benkendorff, K.; Green, T.; Speck, P. Marine Snails and Slugs: A Great Place to Look for Antiviral Drugs. J. Virol. 2015, 89, 8114–8118. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Saba, S. Bivalve Culture Optimisation of Three Autochthonous Species in a Central-Western Mediterranean Lagoon. Ph.D. Thesis, Universita Degli Stud Di Sassari, Sassari, Italy, 2011; pp. 1–182. [Google Scholar]
- Chakraborty, K.; Joy, M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: An overview. Food Res. Int. 2020, 137, 109637. [Google Scholar] [CrossRef]
- Eghianruwa, Q.A.; Osoniyi, O.R.; Maina, N.; Wachira, S. Bioactive Peptides from Marine Molluscs—A Review. Int. J. Biochem. Res. Rev. 2019, 27, 1–12. [Google Scholar] [CrossRef]
- D’Souza, S.L.; Shenoy, K.B. Marine molluscs of India-a review on their diversity and distribution. J. Coast. Conserv. 2023, 27, 67. [Google Scholar] [CrossRef]
- Vinther, J. The origins of molluscs. Palaeontology 2015, 58, 19–34. [Google Scholar] [CrossRef]
- Haszprunar, G.; Schander, C.; Halanych, K.M. Relationships of higher molluscan taxa. In Phylogeny and Evolution of the Mollusca; Oxford Academic Press: Oxford, UK, 2008; pp. 19–32. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Venkatesan, V. Marine molluscan diversity in India—Exploitation, Conservation. In Course Manual Summer School on Advanced Methods for Fish Stock Assessment and Fisheries Management; ICAR-Central Marine Fisheries Research Institute: New Delhi, India, 2017; Volume 2, pp. 56–81. [Google Scholar]
- Wanninger, A.; Wollesen, T. The evolution of molluscs. Biol. Rev. 2019, 94, 102–115. [Google Scholar] [CrossRef]
- Ekin, İ. Molluscs: Their usage as nutrition, medicine, aphrodisiac, cosmetic, jewelry, cowry, pearl, accessory and so on from the history to today. Middle East. J. Sci. 2018, 4, 45–51. [Google Scholar] [CrossRef]
- Haszprunar, G.; Wanninger, A. Molluscs. Curr. Biol. 2012, 22, R510–R514. [Google Scholar] [CrossRef] [PubMed]
- Pyron, M.; Brown, K.M. Introduction to Mollusca and the Class Gastropoda. In Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 383–421. [Google Scholar] [CrossRef]
- Voronezhskaya, E.E.; Croll, R.P.; Schmidt-Rhaesa, A.; Harzsch, S.; Purschke, G. Structure and Evolution of Invertebrate Nervous Systems; Oxford University Press: Oxford, UK, 2016; Volume 20, pp. 196–221. [Google Scholar] [CrossRef]
- Venkatesan, V.; Mohamed, K.S. Gastropod classification and taxonomy. In Summer School on Recent Advances in Marine Biodiversity Conservation and Management; Central Marine Fisheries Research Institute: New Delhi, India, 2015; pp. 38–41. [Google Scholar]
- Cunha, T.J.; Fernández-Simón, J.; Petrula, M.; Giribet, G.; Moles, J. Photographic Checklist, DNA Barcoding, and New Species of Sea Slugs and Snails from the Faafu Atoll, Maldives (Gastropoda: Heterobranchia and Vetigastropoda). Diversity 2023, 15, 219. [Google Scholar] [CrossRef]
- Giribet, G. Bivalvia. In Phylogeny and Evolution of the Mollusca; Oxford Academic Press: Oxford, UK, 2008; pp. 105–141. [Google Scholar] [CrossRef]
- Nasution, S.; Effendi, I.; Nedi, S.; Mardalisa, M. Species Diversity of Marine Bivalves from the Strait of Rupat Island Riau Province, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 934, 012071. [Google Scholar] [CrossRef]
- Ramadhaniaty, M.; Syawali; Karina, S.; Muhammadar. Biodiversity of bivalves in the mangrove ecosystem in Kampung Jawa, Banda Aceh. IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 787–793. [Google Scholar] [CrossRef]
- Amodio, P.; Boeckle, M.; Schnell, A.K.; Ostojíc, L.; Fiorito, G.; Clayton, N.S. Grow Smart and Die Young: Why Did Cephalopods Evolve Intelligence? Trends Ecol. Evol. 2019, 34, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Haimovici, M.; Santos, R.A.D.; Fischer, L.G. Class Cephalopoda. In Compedium of Brazilian Sea Shells; Evangraf: Porto Alegre, Brazil, 2009; pp. 611–658. [Google Scholar] [CrossRef]
- Schmidt, C.V.; Mouritsen, O.G. Cephalopods as Challenging and Promising Blue Foods: Structure, Taste, and Culinary Highlights and Applications. Foods 2022, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Vitti, J.J. Cephalopod Cognition in an Evolutionary Context: Implications for Ethology. Biosemiotics 2013, 6, 393–401. [Google Scholar] [CrossRef]
- Budelmann, B.U. Cephalopoda. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 785–793. [Google Scholar] [CrossRef]
- Venkatesan, V.; Vidya, R.; Mohamed, K.S. Molluscan Taxonomy. J. Molluscan Stud. 2017, 13, 189–218. [Google Scholar]
- Correia, M.D.; Coelho, C.A.; Sovierzoski, H.H. Polyplacophora (Mollusca) from reef ecosystems and associations with macroalgae on the coast of Alagoas, Northeastern Brazil. Zoologia 2015, 32, 289–295. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Schwabe, E.; Saito, H.; Samadi, S.; Giribet, G. Evolution in the deep sea: A combined analysis of the earliest diverging living chitons (Mollusca:Polyplacophora:Lepidopleurida). Invertebr. Syst. 2010, 24, 560–572. [Google Scholar] [CrossRef]
- Reynolds, P.D. The Scaphopoda. Adv. Mar. Biol. 2002, 42, 137–236. [Google Scholar] [CrossRef] [PubMed]
- Sumner-Rooney, L.H.; Schrödl, M.; Lodde-Bensch, E.; Lindberg, D.R.; Heß, M.; Brennan, G.P.; Sigwart, J.D. A neurophylogenetic approach provides new insight to the evolution of Scaphopoda. Evol. Dev. 2015, 17, 337–346. [Google Scholar] [CrossRef]
- Haszprunar, G.; Ruthensteiner, B. Monoplacophora (Tryblidia)-some unanswered questions. Am. Malacol. Bull. 2013, 31, 189–194. [Google Scholar] [CrossRef]
- Kano, Y.; Kimura, S.; Kimura, T.; Warén, A. Living Monoplacophora: Morphological conservatism or recent diversification? Zool. Scr. 2012, 41, 471–488. [Google Scholar] [CrossRef]
- Glaubrecht, M.; Maitas, L.; Salvini-Plawen, L.V. Aplacophoran Mollusca in the natural history museum Berlin. An annotated catalogue of Thiele’s type specimens, with a brief review of “Aplacophora” classification. Zoosyst. Evol. 2005, 81, 145–166. [Google Scholar] [CrossRef]
- Saxena, A. Taxonomy of Phylum Mollusca. In Libre Texts Biology; Discovery Publishing House: New Delhi, India, 2005; Volume 1, pp. 1–21. Available online: https://biblio.sg/book/text-book-mollusca-saxena/d/1383665160 (accessed on 11 August 2023).
- Jones, T.B. General Biology. In Libre Texts Biology; Discovery Publishing House: New Delhi, India, 2023; p. 13722. Available online: https://bio.libretexts.org/@go/page/13722 (accessed on 11 August 2023).
- Pohnert, G. Chemical Defense Strategies of Marine Organisms. In The Chemistry of Pheromones and Other Semiochemicals I.; Springer: Berline/Heidelberg, Germany, 2004; Volume 239, pp. 179–219. [Google Scholar] [CrossRef]
- Kamio, M.; Yambe, H.; Fusetani, N. Chemical cues for intraspecific chemical communication and interspecific interactions in aquatic environments: Applications for fisheries and aquaculture. Fish. Sci. 2022, 88, 203–239. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Tiscar, P.G.; Mosca, F. Defense mechanisms in farmed marine molluscs. Vet. Res. Commun. 2004, 28, 57–62. [Google Scholar] [CrossRef]
- Derby, C.D. Escape by Inking and Secreting: Marine Molluscs Avoid Predators Through a Rich Array of Chemicals and Mechanisms. Biol. Bull. 2007, 213, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Fiorotti, H.B.; Figueiredo, S.G.; Campos, F.V.; Pimenta, D.C. Cone snail species off the Brazilian coast and their venoms: A review and update. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, 1–19. [Google Scholar] [CrossRef]
- Pathak, S. Marine Bioprospecting: Bioactive compounds from Cnidarians and Molluscs: A Review. In Proceedings of the National Conference on Innovations in Biological Sciences, Gujarat, India, 10 January 2020; pp. 227–238. Available online: https://ssrn.com/abstract=3567752 (accessed on 13 August 2023).
- Avila, C. Molluscan natural products as biological models: Chemical ecology, histology, and laboratory culture. In Molluscs: Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 43, pp. 1–23. [Google Scholar] [CrossRef]
- Turner, A.H.; Craik, D.J.; Kaas, Q.; Schroeder, C.I. Bioactive compounds isolated from neglected predatory marine gastropods. Mar. Drugs 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Ragi, A.S.; Leena, P.P.; Nair, S.M. Study of lipids and amino acid composition of marine Gastropod, tibia curta collected from the southwest coast of India. World J. Pharm. Pharm. Sci. 2016, 5, 1058–1076. [Google Scholar]
- Weissburg, M.J.; Ferner, M.C.; Pisut, D.P.; Smee, D.L. Ecological consequences of chemically mediated prey perception. J. Chem. Ecol. 2002, 23, 1953–1970. [Google Scholar] [CrossRef]
- Ferner, M.C.; Weissburg, M.J. Slow-moving predatory gastropods track prey odors in fast and turbulent flow. J. Exp. Biol. 2005, 208, 809–819. [Google Scholar] [CrossRef]
- Weissburg, M.; Beauvais, J. The smell of success: The amount of prey consumed by predators determines the strength and range of cascading non-consumptive effects. PeerJ 2015, 2015, e1426. [Google Scholar] [CrossRef] [PubMed]
- Kicklighter, C.E.; Hay, M.E. Integrating prey defensive traits: Contrasts of marine worms from temperate and tropical habitats. Ecol. Monogr. 2006, 76, 195–215. [Google Scholar] [CrossRef]
- Paul, V.J.; Arthur, K.E.; Ritson-Williams, R.; Ross, C.; Sharp, K. Chemical Defenses: From Compounds to Communities. Biol. Bull. 2007, 213, 226–251. [Google Scholar] [CrossRef]
- Antunes, A.; Efferth, T. Chemical Ecology of Marine Organisms. In Biodiversity, Natural Products and Cancer Treatment; World Scientific: Singapore, 2014; pp. 107–146. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L. Perspectives on the marine animal forests of the world. In Perspectives on the Marine Animal Forests of the World; Springer: Cham, Switzerland, 2021; pp. 1–530. [Google Scholar] [CrossRef]
- Hay, M.E. Marine chemical ecology: What’s known and what’s next? J. Exp. Mar. Biol. Ecol. 1996, 200, 103–134. [Google Scholar] [CrossRef]
- Sadjadi, N. Chemical Ecology of Biocompounds in Molluscs. Biol. Resour. Water 2018, 11, 213–245. [Google Scholar] [CrossRef]
- Bornancin, L.; Bonnard, I.; Mills, S.C.; Banaigs, B. Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Nat. Prod. Rep. 2017, 34, 644–676. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; White, A.M.; Suciati; Katavic, P.L.; Krishnaraj, R.R.; Winters, A.E.; Mollo, E.; Cheney, K.L.; Garson, M.J. Chemoecological studies on marine natural products: Terpene chemistry from marine mollusks. Pure Appl. Chem. 2014, 86, 995–1002. [Google Scholar] [CrossRef]
- Sreeja, K.L.; Sreejamole, K.L.; Radhakrishnan, C.K. Preliminary qualitative chemical evaluation of the extracts from mussel Perna viridis. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 38–42. Available online: https://www.researchgate.net/publication/235990003 (accessed on 17 April 2024).
- Zhukova, N.V. Fatty acids of marine mollusks: Impact of diet, bacterial symbiosis and biosynthetic potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Alkanani, T.; Parrish, C.C.; Thompson, R.J.; McKenzie, C.H. Role of fatty acids in cultured mussels, Mytilus edulis, grown in Notre Dame Bay, Newfoundland. J. Exp. Mar. Biol. Ecol. 2007, 348, 33–45. [Google Scholar] [CrossRef]
- Ekin, I.; Başhan, M. Fatty acid composition of selected tissues of Unio elongatulus (Bourguignat, 1860) (Mollusca: Bivalvia) collected from Tigris River, Turkey. Turk. J. Fish. Aquat. Sci. 2010, 10, 445–451. [Google Scholar] [CrossRef]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef] [PubMed]
- Anedda, R.; Siliani, S.; Melis, R.; Loi, B.; Baroli, M. Lipid metabolism of sea urchin Paracentrotus lividus in two contrasting natural habitats. Sci. Rep. 2021, 11, 17174. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; Liu, L.; Cai, S.; Ye, Q.; Xue, B.; Wang, X.; Zhang, S.; Chen, F.; Cai, C.; et al. Role of functional fatty acids in modulation of reproductive potential in livestock. J. Anim. Sci. Biotechnol. 2023, 14, 24. [Google Scholar] [CrossRef]
- Hu, E.; Wang, R.; Pan, C.; Yang, W. Fatty acids: Composition and functions for reproduction. In Aquaculture Research Progress; Nova Science Publisher: Hauppauge, NY, USA, 2009; Volume 3, pp. 128–146. Available online: https://www.researchgate.net/publication/221703645_Fatty_acids_composition_and_functions_for_reproduction (accessed on 17 April 2024).
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Brett, M.T.; Müller-Navarra, D.C. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Kiran, N.; Siddiqui, G.; Khan, A.N.; Ibrar, K.; Tushar, P. Extraction and Screening of Bioactive Compounds with Antimicrobial Properties from Selected Species of Mollusk and Crustacean. J. Clin. Cell Immunol. 2014, 5, 1000189. [Google Scholar] [CrossRef]
- Odeleye, T.; White, W.L.; Lu, J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review. Food Funct. 2019, 10, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Ghenebzia, I.; Hemmami, H.; Amor, B.I.; Zeghoud, S.; Seghir, B.B.; Hammoudi, R. Different methods of extraction of bioactive compounds and their effect on biological activity: A review. Int. J. Second. Metab. 2023, 10, 469–494. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Valles-Regino, R.; Mouatt, P.; Rudd, D.; Yee, L.H.; Benkendorff, K. Extraction and quantification of bioactive Tyrian purple precursors: A comparative and validation study from the hypobranchial gland of a muricid Dicathais orbita. Molecules 2016, 21, 1672. [Google Scholar] [CrossRef]
- Daso, A.P.; Okonkwo, O.J. Conventional Extraction Techniques: Soxhlet and Liquid-Liquid Extractions and Evaporation. Anal. Sep. Sci. 2015, 5, 1437–1468. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Satdive, R.K. Comparison of techniques for the extraction of the anti-cancer drug camptothecin from Nothapodytes foetida. J. Chromatogr. A 2005, 1063, 9–13. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and isolation of phenolic compounds. Methods Mol. Biol. 2012, 864, 427–464. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC—Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Kechaou, E.S.; Dumay, J.; Donnay-Moreno, C.; Jaouen, P.; Gouygou, J.P.; Bergé, J.P.; Amar, R.B. Enzymatic hydrolysis of cuttlefish (Sepia officinalis) and sardine (Sardina pilchardus) viscera using commercial proteases: Effects on lipid distribution and amino acid composition. J. Biosci. Bioeng. 2009, 107, 158–164. [Google Scholar] [CrossRef]
- Majik, M.S.; Gawas, U.B. Recent advances in extraction of natural compounds. In New Horizons in Natural Compound Research; Academic Press: Cambridge, MA, USA, 2023; pp. 17–33. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Zhu, B.W.; Tong, L.; Wu, H.T.; Qin, L.; Tan, H.; Chi, Y.L.; Qu, J.Y.; Murata, Y. Extraction of lipid from scallop (Patinopecten yessoensis) viscera by enzyme-assisted solvent and supercritical carbon dioxide methods. Int. J. Food Sci. Technol. 2010, 45, 1787–1793. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Nunes, M.A.G.; Barbosa, I.S.; Santos, G.L.; Peso-Aguiar, M.C.; Korn, M.G.A.; Flores, E.M.M.; Dressler, V.L. Evaluation of microwave and ultrasound extraction procedures for arsenic speciation in bivalve mollusks by liquid chromatography-inductively coupled plasma-mass spectrometry. Spectrochim. Acta Part B Spectrosc. 2013, 86, 108–114. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Alameda-Cuesta, Á.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Rapid microwave-assisted extraction method for the analysis of common antidepressants and metabolites in marine organisms. Microchem. J. 2023, 195, 109471. [Google Scholar] [CrossRef]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of polysaccharides extraction from Pacific oyster (Crassostrea gigas) using subcritical water: Structural characterization and biological activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef]
- Lee, H.J.; Saravana, P.S.; Cho, Y.N.; Haq, M.; Chun, B.S. Extraction of bioactive compounds from oyster (Crassostrea gigas) by pressurized hot water extraction. J. Supercrit. Fluids 2018, 141, 120–127. [Google Scholar] [CrossRef]
- Mohammadi, S.; Alfaro, A.C.; Baroutian, S.; Seyfoddin, A. Extraction of bioactive compounds from black-footed abalone (Haliotis iris) using subcritical water extraction. J. Chem. Technol. Biotechnol. 2022, 97, 3511–3519. [Google Scholar] [CrossRef]
- Jamalluddin, N.A.; Ismail, N.; Mutalib, S.R.A.; Sikin, A.M. Sc-CO2 extraction of fish and fish by-products in the production of fish oil and enzyme. Bioresour. Bioprocess. 2022, 9, 21. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Rudd, D.; Benkendorff, K. Supercritical CO2 extraction of bioactive Tyrian purple precursors from the hypobranchial gland of a marine gastropod. J. Supercrit. Fluids 2014, 94, 1–7. [Google Scholar] [CrossRef]
- Deolindo, C.T.P.; Kleemann, C.R.; Bosch-Orea, C.; Molognoni, L.; Daguer, H.; Hoff, R.B.; Costa, A.C.O. Sample pooling and incurred samples improve analytical throughput and quality control of lipophilic phycotoxins screening in bivalve mollusks. Anal. Bioanal. Chem. 2023, 415, 5023–5034. [Google Scholar] [CrossRef] [PubMed]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Fonseca, T.G.; Bebianno, M.J.; Alonso, E. Ultrasound-assisted extraction as an easy-to-perform analytical methodology for monitoring ibuprofen and its main metabolites in mussels. Anal. Bioanal. Chem. 2022, 414, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, S.H.; Ahn, G.; Kim, K.N.; Cha, S.H.; Kim, S.K.; Jeon, B.T.; Park, P.J.; Lee, K.W.; Jeon, Y.J. Effect of enzyme-assisted extract of Sargassum coreanum on induction of apoptosis in HL-60 tumor cells. J. Appl. Phycol. 2012, 24, 675–684. [Google Scholar] [CrossRef]
- Heng, M.Y.; Tan, S.N.; Yong, J.W.H.; Ong, E.S. Emerging green technologies for the chemical standardization of botanicals and herbal preparations. TrAC—Trends Anal. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Sawston, UK, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; Adebo, O.A.; Piater, L.; Phoku, J.Z.; Njobeh, P.B. Subcritical Water Extraction and Its Prospects for Aflatoxins Extraction in Biological Materials. In Aflatoxin-Control, Analysis, Detection and Health Risks; Intech Open: London, UK, 2017; pp. 229–250. [Google Scholar] [CrossRef]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent Advances in the Processing of Agri-food By-products by Subcritical Water. Food Bioproc. Technol. 2023, 16, 2705–2724. [Google Scholar] [CrossRef]
- Author, C.; Mohamed, A.; Abdulamir, A.; Abas, H. A Review on Supercritical Fluid Extraction as New Analytical Method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Linares, G.; Rojas, M.L. Ultrasound-Assisted Extraction of Natural Pigments from Food Processing By-Products: A Review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional Value and Food Safety of Bivalve Molluscan Shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Benkendorff, K.; Rudd, D.; Nongmaithem, B.D.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the traditional medical uses of Muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef] [PubMed]
- Lah, R.A.; Smith, J.; Savins, D.; Dowell, A.; Bucher, D.; Benkendorff, K. Investigation of nutritional properties of three species of marine turban snails for human consumption. Food Sci. Nutr. 2017, 5, 14–30. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent advances in marine-based nutraceuticals and their health benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef]
- Patra, D.D. Marine-Based Nutraceuticals vis-à-vis Therapeutic Potential: A Summary. Examines Mar. Biol. Oceanogr. 2021, 4, 1–5. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, W.S.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Shahidi, F. Marine Bioactives and Their Application in the Food Industry: A Review. Appl. Sci. 2023, 13, 12088. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Moruf, R.O.; Ogunbambo, M.M.; Taiwo, M.A.; Afolayan, O.A. Marine Bivalves as a Dietary Source of High-Quality Lipid: A Review with Special Reference to Natural n-3 Long Chain Polyunsaturated Fatty Acids. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2021, 78, 11–18. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipids and fatty acids of nudibranch mollusks: Potential sources of bioactive compounds. Mar Drugs 2014, 12, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 604045. [Google Scholar] [CrossRef]
- Tran, Q.T.; Le, T.T.T.; Pham, M.Q.; Do, T.L.; Vu, M.H.; Nguyen, D.C.; Bach, L.G.; Bui, L.M.; Pham, Q.L. Fatty acid, lipid classes and phospholipid molecular species composition of the marine clam Meretrix lyrata (Sowerby 1851) from Cua Lo Beach, Nghe An Province, Vietnam. Molecules 2019, 24, 895. [Google Scholar] [CrossRef]
- Fokina, N.N.; Ruokolainen, T.R.; Nemova, N.N. Lipid Composition Modifications in the Blue Mussels (Mytilus edulis L.) from the White Sea. Org. Mol. Malacol. 2017, 7, 143–159. [Google Scholar] [CrossRef]
- Tsoupras, A.; Iatrou, C.; Frangia, C.; Demopoulos, C. The Implication of Platelet Activating Factor in Cancer Growth and Metastasis: Potent Beneficial Role of PAF-Inhibitors and Antioxidants. Infect. Disord. Drug Targets 2012, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chromodoris sp. and Phyllidia coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Tabakaev, A.V. Phospholipids from Soft Tissues of the Bivalve Mollusk Anadara broughtonii. Chem. Nat. Compd. 2016, 52, 299–300. [Google Scholar] [CrossRef]
- Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar. Drugs 2021, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between fatty acid profile and anti-inflammatory activity in common Australian seafood by-products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Therapeutic Potential of Polyphenols and Other Micronutrients of Marine Origin. Mar. Drugs 2023, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood Discards: A Potent Source of Enzymes and Biomacromolecules with Nutritional and Nutraceutical Significance. Front. Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine health-promoting compounds: Recent trends for their characterization and human applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kusaykin, M.I.; Zakharenko, A.M.; Menshova, R.V.; Khanh, H.H.N.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B Enzym. 2014, 102, 154–160. [Google Scholar] [CrossRef]
- Berezkina, A.Y.; Avdiuk, K.V.; Gudzenko, O.V.; Kharkhota, M.A.; Utevsky, A.Y. Bacterial enzymes associated with gastropod mollusc nacella concinna from the water area of the argentine islands (West antarctica). Probl. Cryobiol. Cryomed. 2020, 30, 295. [Google Scholar] [CrossRef]
- Setyati, W.A.; Pringgenies, D.; Soenardjo, N.; Pramesti, R. Enzyme-producing symbiotic bacteria in gastropods and bivalves molluscs: Candidates for bioindustry materials. Biodiversitas 2023, 24, 20–25. [Google Scholar] [CrossRef]
- Peña, O.I.G.; Zavala, M.Á.L.; Ruelas, H.C. Pharmaceuticals market, consumption trends and disease incidence are not driving the pharmaceutical research on water and wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef]
- Wynendaele, E.; Furman, C.; Wielgomas, B.; Larsson, P.; Hak, E.; Block, T.; Van Calenbergh, S.; Willand, N.; Markuszewski, M.; Odell, L.R.; et al. Sustainability in drug discovery. Med. Drug Discov. 2021, 12, 100107. [Google Scholar] [CrossRef]
- Pati, P.; Sahu, B.K.; Panigrahy, R.C. Marine molluscs as a potential drug cabinet: An overview. Indian J. Geo-Mar. Sci. 2015, 44, 961–970. [Google Scholar]
- Fernebro, J. Fighting bacterial infections—Future treatment options. Drug Resist. Updates 2011, 14, 125–139. [Google Scholar] [CrossRef]
- Mitta, G.; Vandenbulcke, F.; Roch, P. Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 2000, 486, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Lamers, M.H. Novel antibiotics targeting bacterial replicative dna polymerases. Antibiotics 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Knapp, O.; McArthur, J.R.; Adams, D.J. Conotoxins targeting neuronal voltage-gated sodium channel subtypes: Potential analgesics? Toxins 2012, 4, 1236–1260. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Aleksijević, L.H.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Liu, L.; Benkendorff, K. Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Mar. Drugs 2020, 18, 570. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Talapatra, S.N.; Swarnakar, S. Bioactive Compounds from Marine Invertebrates for Potential Medicines—An Overview. Int. Lett. Nat. Sci. 2015, 34, 42–61. [Google Scholar]
- Wen, L.Y.K. Lactobacillus rhamnosus GG down regulates autophagy in intestinal mononuclear cells to enhance effector T cell responses induced by rotavirus vaccine. J. Clin. Cell Immunol. 2013, 2, 177. [Google Scholar] [CrossRef]
- Pereira, R.B.; Andrade, P.B.; Valentão, P. Chemical diversity and biological properties of secondary metabolites from sea hares of Aplysia genus. Mar. Drugs 2016, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert Opin. Drug Discov. 2019, 14, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, A.; Pandiyan, R.; Kandasamy, P.; Antoniraj, M.G.; Navabshan, I.; Sakthivel, B.; Dharmaraj, S.; Chinnaiyan, S.K.; Ashokkumar, V.; Ngamcharussrivichai, C. Marine biome-derived secondary metabolites, a class of promising antineoplastic agents: A systematic review on their classification, mechanism of action and future perspectives. Sci. Total Environ. 2022, 836, 155445. [Google Scholar] [CrossRef] [PubMed]
- Soomro, S. Oxidative Stress and Inflammation. Open J. Immunol. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Ciotu, C.I.; Fischer, M.J.M. Novel Analgesics with Peripheral Targets. Neurotherapeutics 2020, 17, 784–825. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; El-Aty, A.M.A. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef]
- Holmes, D. Conotoxins: How a deadly snail could help ease pain. Lancet Neurol. 2014, 13, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Treschow, A.P.; Hodges, L.D.; Wright, P.F.A.; Wynne, P.M.; Kalafatis, N.; Macrides, T.A. Novel anti-inflammatory ω-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed]
- Pachaiyappan, A.; Muthuvel, A.; Sadhasivam, G.; Sankar, V.J.V.; Sridhar, N.; Kamar, M. In vitro antioxidant activity of different gastropods, bivalves and echinoderm by solvent extraction method. Int. J. Pharm. Sci. Res. 2014, 5, 2539–2545. [Google Scholar] [CrossRef]
- Salas, S.; Chakraborty, K. An unreported polyether macrocyclic lactone with antioxidative and anti-lipoxygenase activities from the Babylonidae gastropod mollusc Babylonia spirata. Med. Chem. Res. 2018, 27, 2446–2453. [Google Scholar] [CrossRef]
- Joy, M.; Chakraborty, K.; Raola, V.K. New sterols with anti-inflammatory potentials against cyclooxygenase-2 and 5-lipoxygenase from Paphia malabarica. Nat. Prod. Res. 2017, 31, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.; Chakraborty, K. Specialized oxygenated heterocyclics from Villorita cyprinoides with cyclooxygenase-2 and 5-lipoxygenase inhibitory properties. Food Res. Int. 2018, 106, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Thebti, A.; Meddeb, A.; Salem, I.B.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.I. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics 2023, 12, 225. [Google Scholar] [CrossRef]
- Amerikova, M.; El-Tibi, I.P.; Maslarska, V.; Bozhanov, S.; Tachkov, K. Antimicrobial activity, mechanism of action, and methods for stabilisation of defensins as new therapeutic agents. Biotechnol. Biotechnol. Equip. 2019, 33, 671–682. [Google Scholar] [CrossRef]
- Miljanich, G.P. Ziconotide: Neuronal Calcium Channel Blocker for Treating Severe Chronic Pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Pettit, G.R.; Kamel, E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J. Biol. Chem. 1990, 265, 17141–17149. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Otto, C.S.; Scheuer, P.J.; Dunbar, D.C. Kahalalides: Bioactive Peptides from a Marine Mollusk Elysia rufescens and Its Algal Diet Bryopsis sp. J. Org. Chem 1996, 61, 6594–6600. [Google Scholar] [CrossRef] [PubMed]
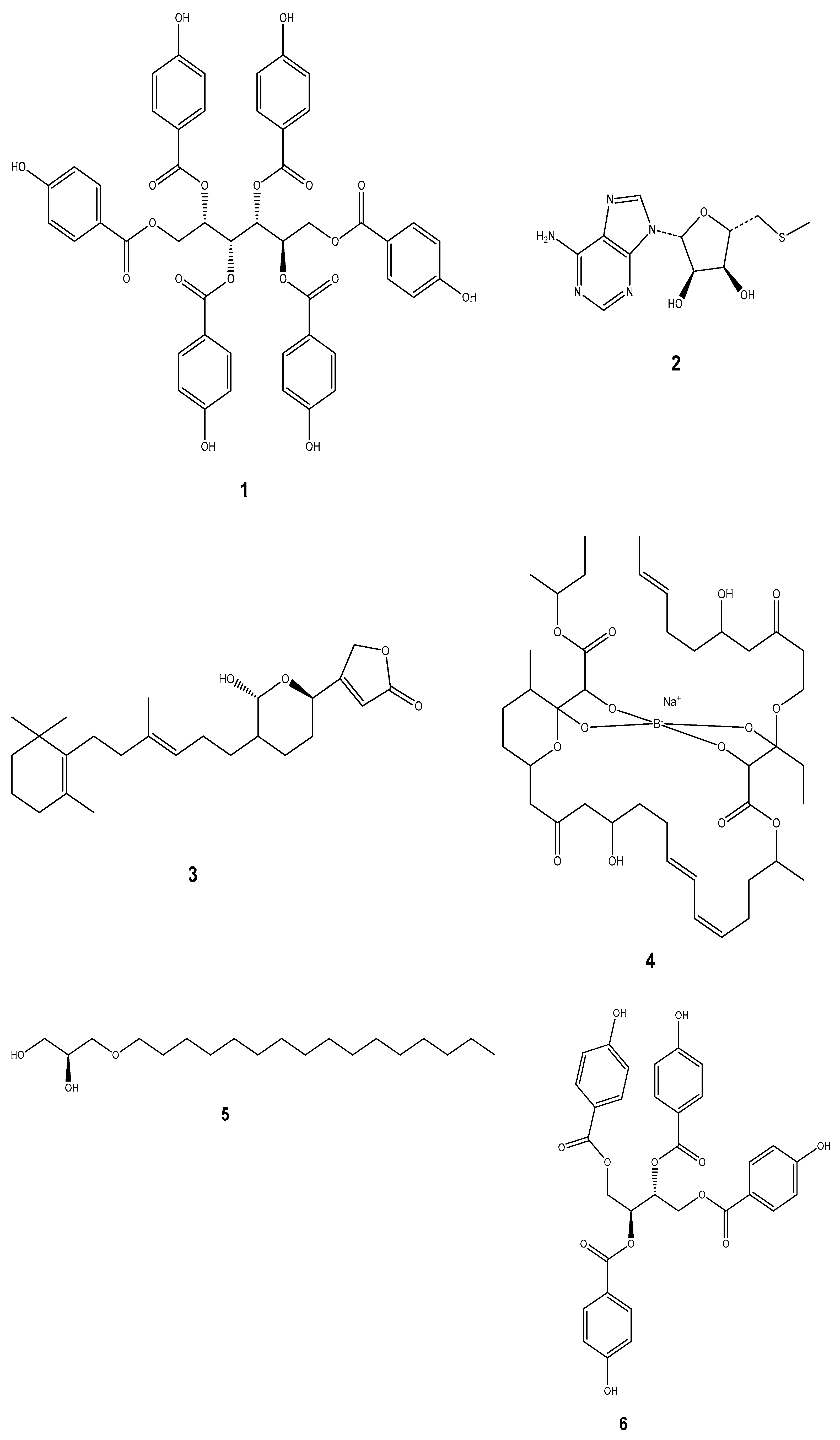

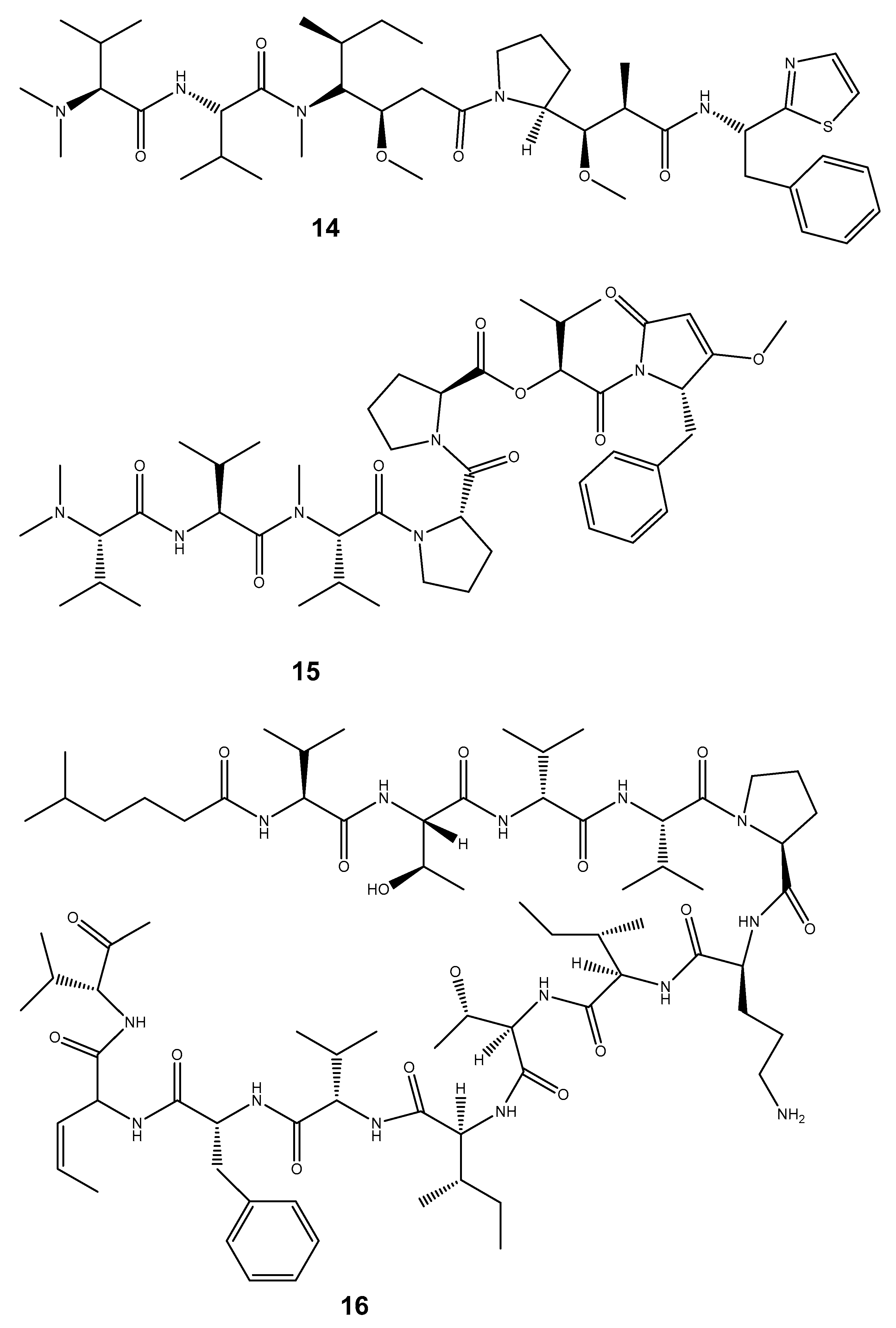
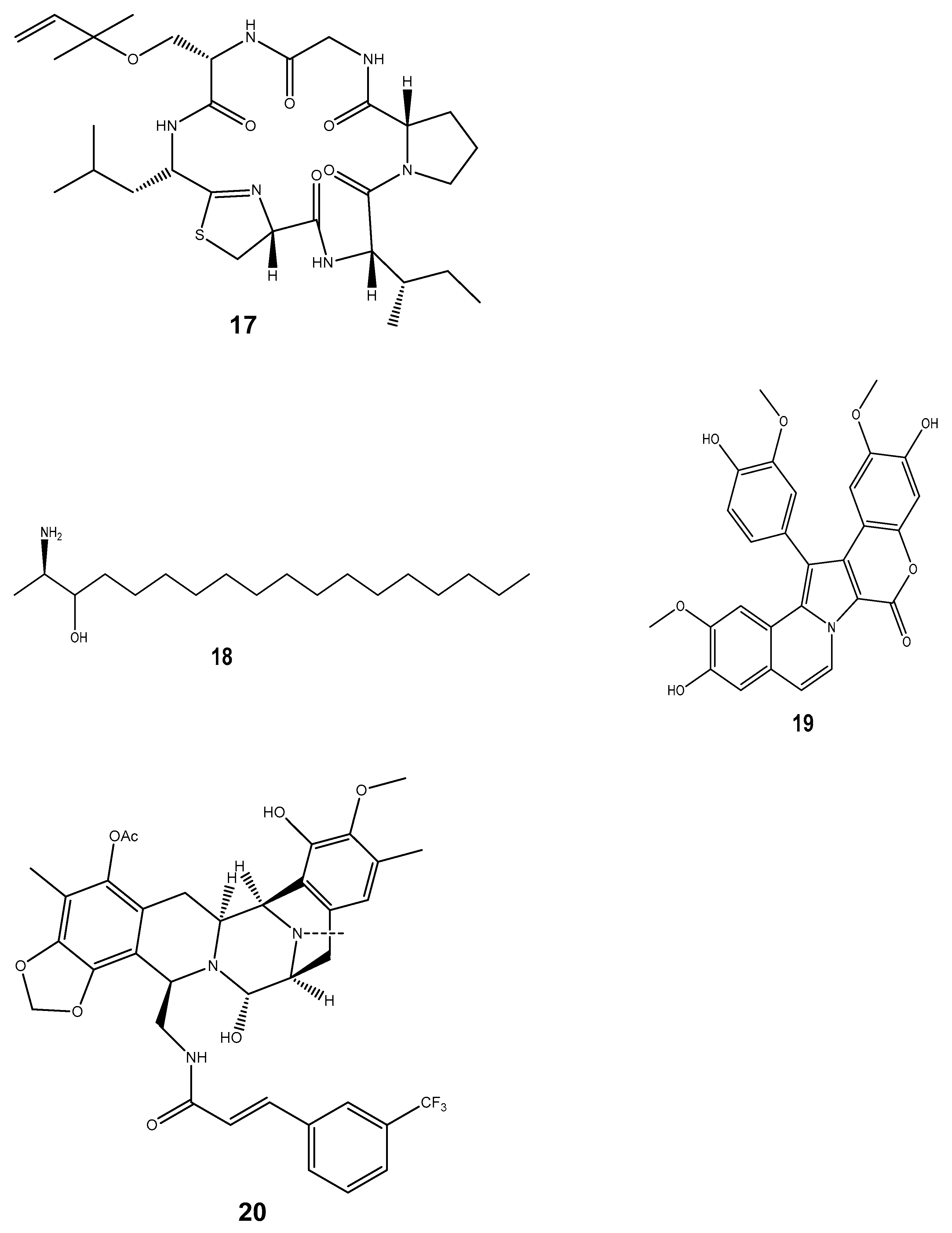
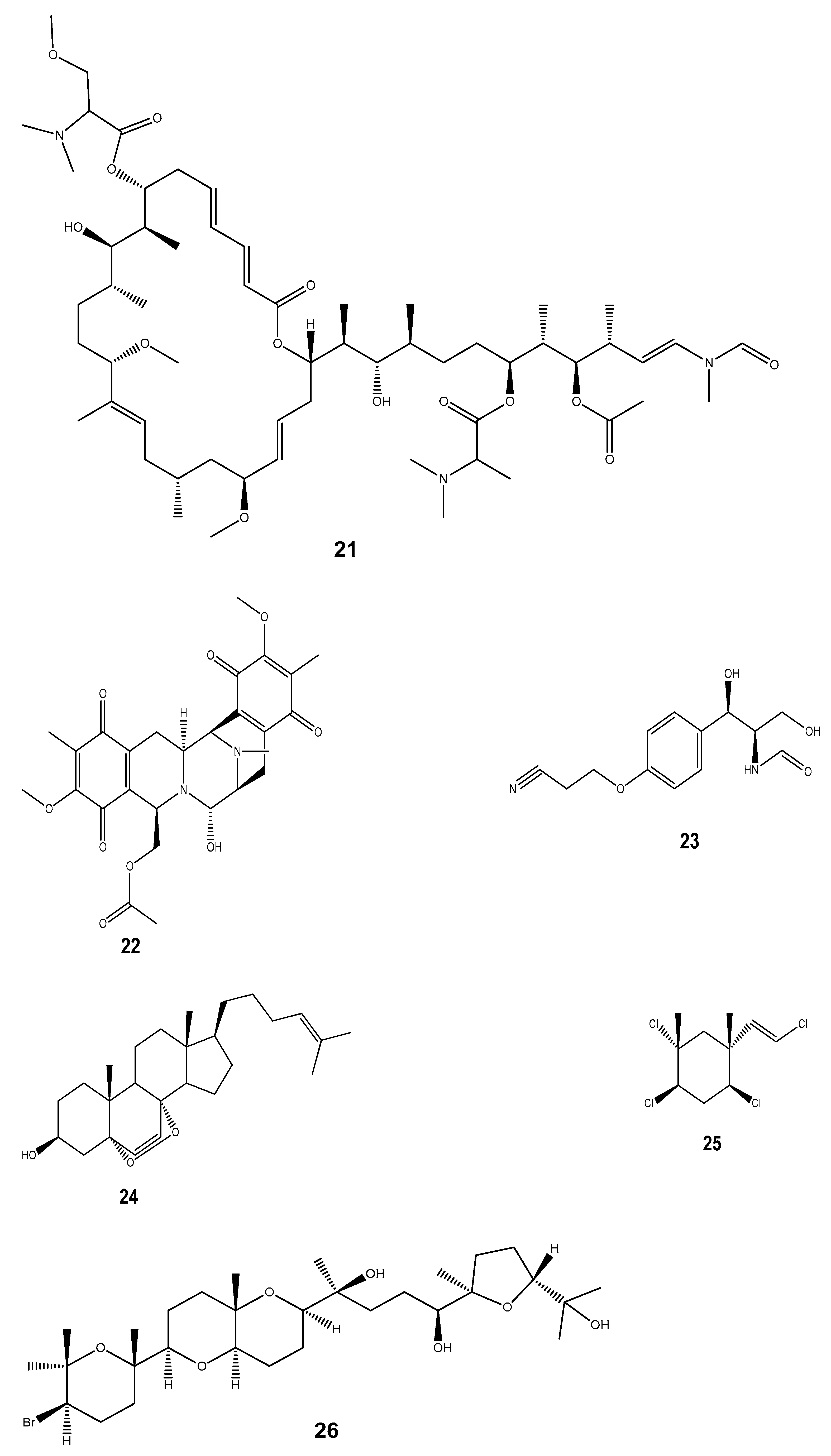
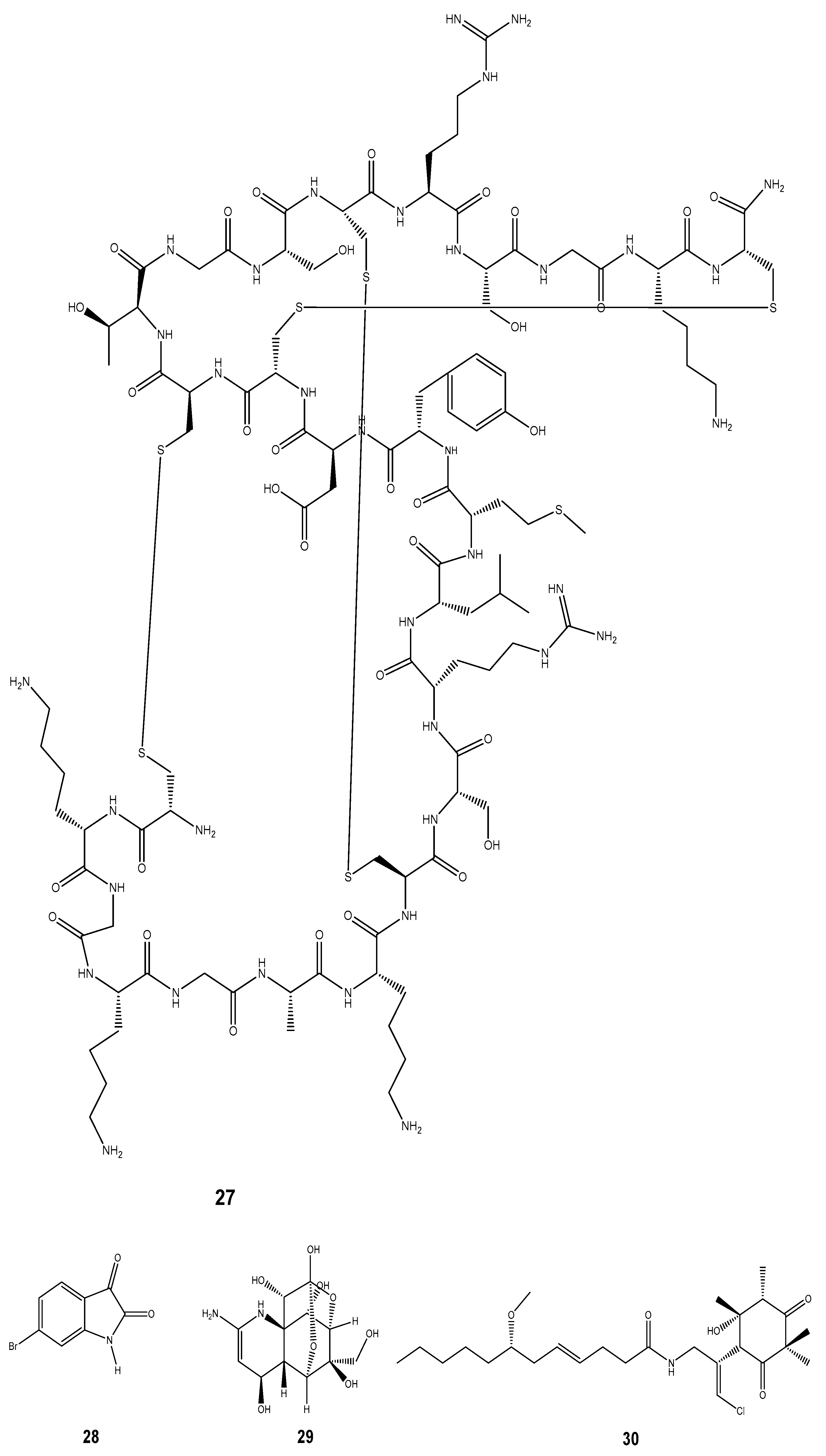

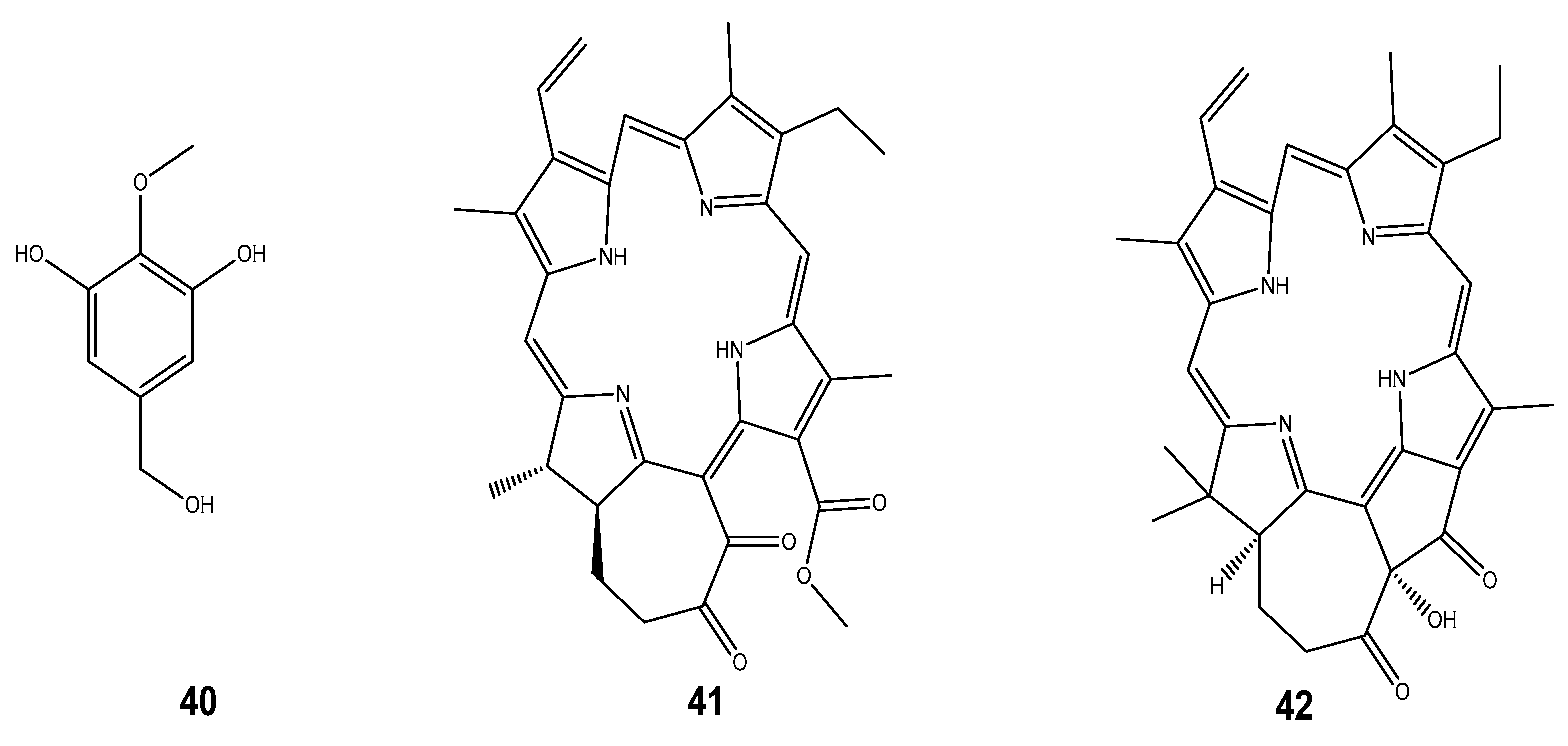
| Compound | Mollusc Species | Therapeatic Effect | Associated Company/institution | Mode of Action | References |
|---|---|---|---|---|---|
| Ziconotide (ω-conotoxin) (27) | Conus geographus and Conus magus | Anti-inflammatory and analgesic | Elan corporation | Disrupts the calcium channel at the neuromuscular junction that is involved in the transmission of nerve impulses. The pain sensitivity is associated with calcium channels. | [173] |
| Dolastatin 10 (14) | Dollabella auricularia | Anti-cancer | Celltrion pharmaceutical company | Interferes with and hinders mitotic cell division. Due to its potent capacity to block the mitotic cell cycle, it may be able to specifically target cancer cells. | [174,175] |
| Kahalalide-F (16) | Elysia rufescens | Anti-cancer | Pharmamar | Induces oncosis in cancerous cells via the lysosomal induction and permeabilization of the cell membrane. Furthermore, the compound also suppresses the expression of genes involved in DNA replication and cell proliferation, which may prevent tumour development and spread. | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngandjui, Y.A.T.; Kereeditse, T.T.; Kamika, I.; Madikizela, L.M.; Msagati, T.A.M. Nutraceutical and Medicinal Importance of Marine Molluscs. Mar. Drugs 2024, 22, 201. https://doi.org/10.3390/md22050201
Ngandjui YAT, Kereeditse TT, Kamika I, Madikizela LM, Msagati TAM. Nutraceutical and Medicinal Importance of Marine Molluscs. Marine Drugs. 2024; 22(5):201. https://doi.org/10.3390/md22050201
Chicago/Turabian StyleNgandjui, Yvan Anderson Tchangoue, Tsotlhe Trinity Kereeditse, Ilunga Kamika, Lawrence Mzukisi Madikizela, and Titus Alfred Makudali Msagati. 2024. "Nutraceutical and Medicinal Importance of Marine Molluscs" Marine Drugs 22, no. 5: 201. https://doi.org/10.3390/md22050201
APA StyleNgandjui, Y. A. T., Kereeditse, T. T., Kamika, I., Madikizela, L. M., & Msagati, T. A. M. (2024). Nutraceutical and Medicinal Importance of Marine Molluscs. Marine Drugs, 22(5), 201. https://doi.org/10.3390/md22050201







