Marine Prostanoids with Cytotoxic Activity from Octocoral Clavularia spp.
Abstract
:1. Introduction
2. Results
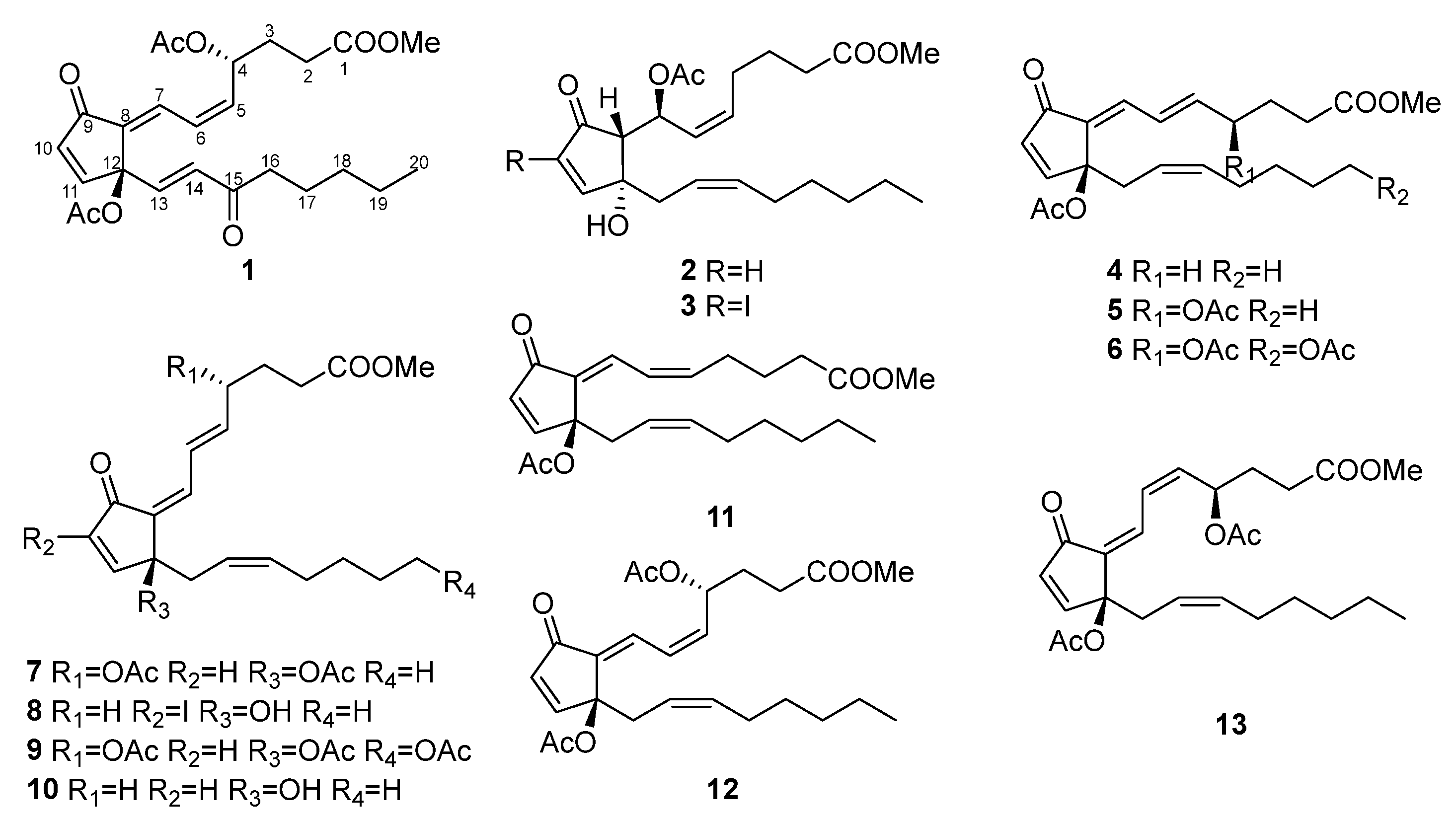
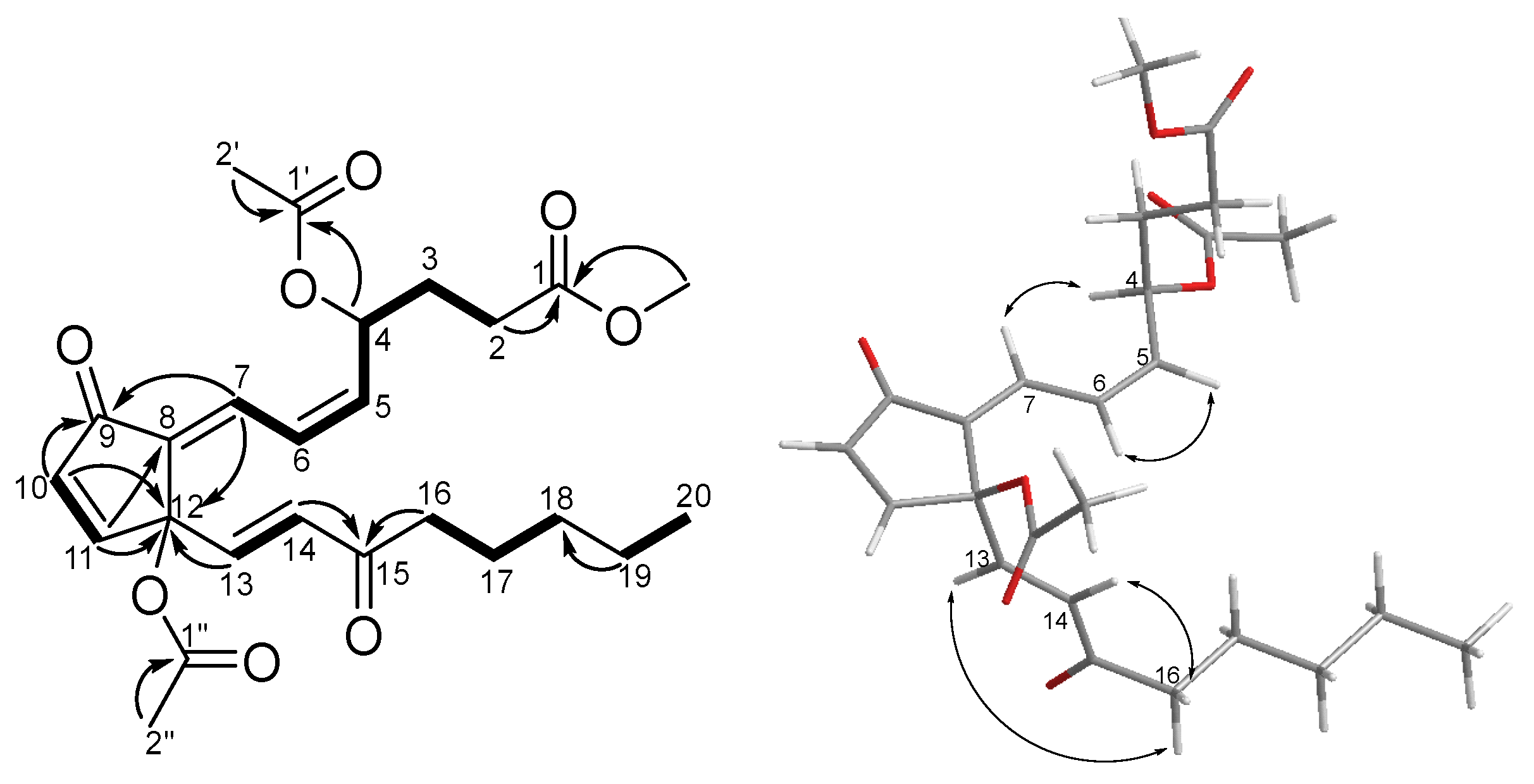
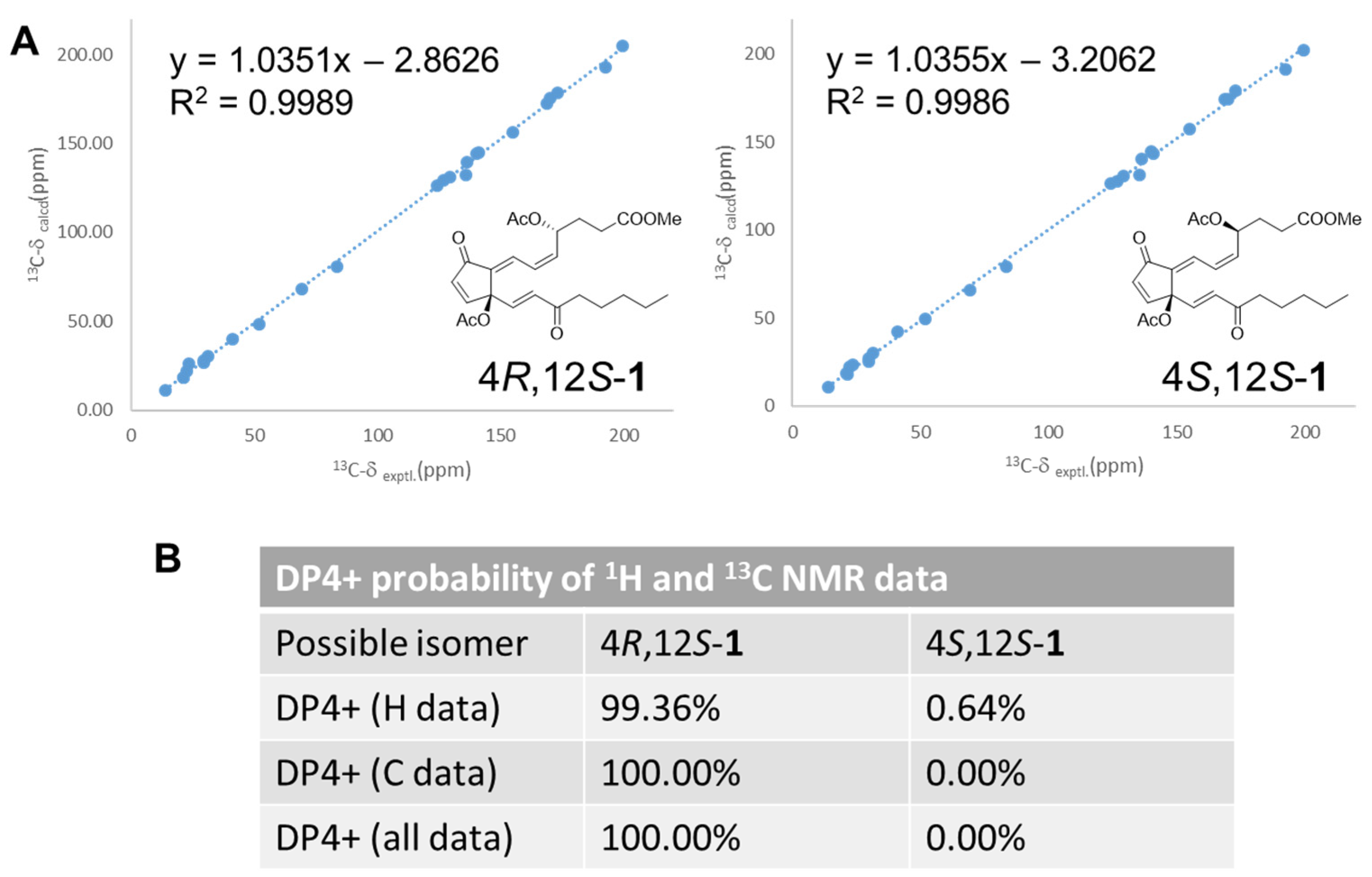
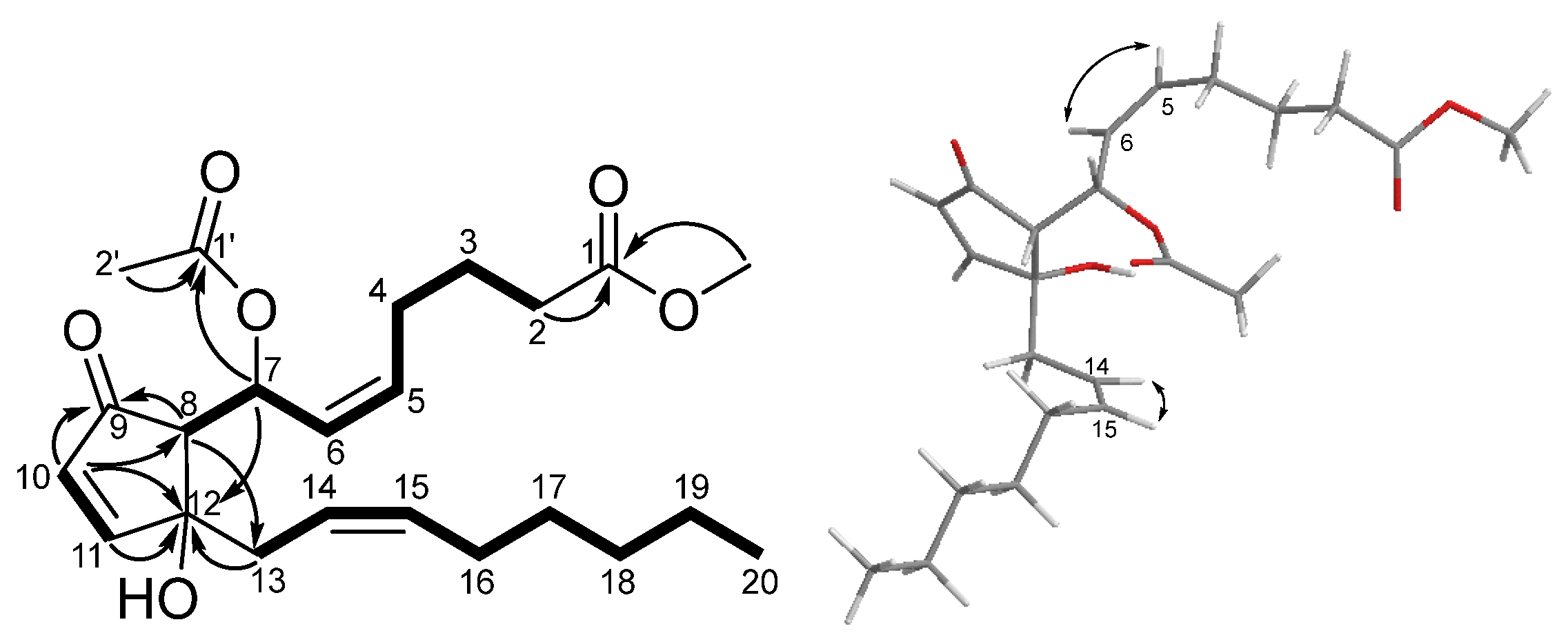
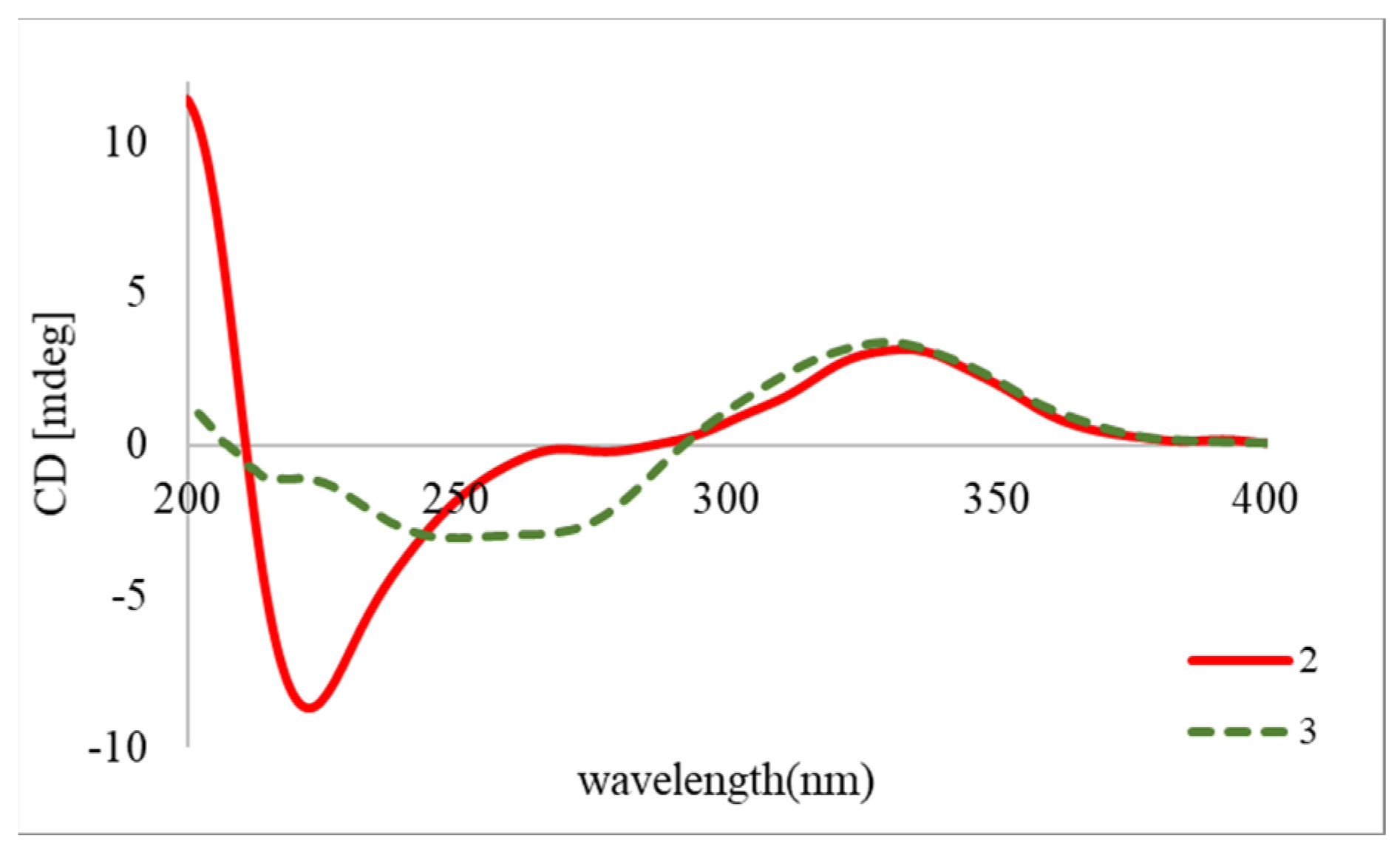
2.1. Structure Elucidation of Isolated Compounds
2.2. Cytotoxic Evaluation of Isolated Compounds
2.3. The Predicted Possible Effects of Thirteen Compounds on Human Tumor Cell Lines for Cytotoxicity
2.4. The Predicted Possible Effects of Thirteen Compounds on NO Production in Macrophages
2.5. The Predicted Pharmacological Potentials of Nine Compounds
3. Discussion
3.1. The Structure and Activity Relationship of Isolated Prostanoids
3.2. The Predicted Possible Cytotoxicity of Thirteen Compounds for Human Tumor Cell Lines
3.3. The Pharmacological Potentials of Nine Compounds
4. Materials and Methods
4.1. General
4.2. Animal Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data
4.5. Parameter of NMR and ECD Calculations
4.6. Cytotoxicity Assays
4.7. In Silico Prediction and Evaluation of Isolated Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitagawa, I.; Kobayashi, M.; Yasuzawa, T.; Son, B.W.; Yoshihara, M. New prostanoids from soft coral. Tetrahedron 1985, 41, 995–1005. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Chia, M.-C.; Wang, S.K.; Chen, H.-J.; El-Gamal, A.A.H.; Dai, C.-F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-Y.; Chuang, Y.-T.; Chang, H.-W.; Lin, Z.-Y.; Chen, C.-Y.; Cheng, Y.-B. Chemical constituents from soft coral Clavularia spp. demonstrate antiproliferative effects on oral cancer cells. Mar. Drugs 2023, 21, 529. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Lo, I.-W.; Wang, S.-K.; Dai, C.-F. New Cytotoxic steroids from the soft coral Clavularia viridis. Steroids 2007, 72, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Wen, Z.-H.; Wang, S.-K.; Duh, C.-Y. New anti-inflammatory steroids from the Formosan soft coral Clavularia viridis. Steroids 2008, 73, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Kaneta, S.; Mori, K.; Yamada, Y.; Honda, A.; Mori, Y. Bromovulone I and iodovulone I, unprecedented brominated and iodinated marine prostanoids with antitumour activity isolated from the Japanese stolonifer Clavularia viridis Quoy and Gaimard. J. Chem. Soc. Chem. Commun. 1986, 15, 981–982. [Google Scholar] [CrossRef]
- Watanabe, K.; Sekine, M.; Takahashi, H.; Iguchi, K. New halogenated marine prostanoids with cytotoxic activity from the Okinawan soft coral Clavularia viridis. J. Nat. Prod. 2001, 64, 1421–1425. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Cheng, Y.-B.; Lin, Y.-C.; Guh, J.-H.; Teng, C.-M.; Ko, C.-L. New prostanoids with cytotoxic activity from Taiwanese octocoral Clavularia viridis. J. Nat. Prod. 2004, 67, 542–546. [Google Scholar] [CrossRef]
- Duh, C.-Y.; El-Gamal, A.A.H.; Chu, C.-J.; Wang, S.-K.; Dai, C.-F. New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea. J. Nat. Prod. 2002, 65, 1535–1539. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Khalil, A.T.; Chiou, S.-H.; Kuo, Y.C.; Cheng, Y.-B.; Liaw, C.-C.; Shen, Y.-C. Bioactive marine prostanoids from octocoral Clavularia viridis. Chem. Biodivers. 2008, 5, 784–792. [Google Scholar] [CrossRef]
- Cancer Registry Annual Report; Health Promotion Administration, Ministry of Health and Welfare: Taipei, Taiwan, 2019.
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Rudik, A.V.; Pogodin, P.V.; Savosina, P.I.; Tarasova, O.A.; Dmitriev, A.V.; Ivanov, S.M.; Biziukova, N.Y.; Druzhilovskiy, D.S.; Filimonov, D.A.; et al. CLC-Pred 2.0: A freely available web application for In Silico prediction of human cell line cytotoxicity and molecular mechanisms of action for druglike compounds. Int. J. Mol. Sci. 2023, 24, 1689. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, D.; Isvoran, A.; Ianovici, N. Predictions of the biological effects of several acyclic monoterpenes as chemical constituents of essential oils extracted from plants. Molecules 2023, 28, 4640. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.J.; Kniss, D.A. Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J. Leukoc. Biol. 1999, 65, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, A.J.; Spraggins, R.L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla chemistry of coelenterates. XV. Tetrahedron Lett. 1969, 59, 5185–5188. [Google Scholar] [CrossRef]
- Baker, B.J.; Okuda, R.K.; Yu, P.T.K.; Scheuer, P.J. Punaglandins: Halogenated antitumor eicosanoids from the octocoral Telesto riisei. J. Am. Chem. Soc. 1985, 107, 2976–2977. [Google Scholar] [CrossRef]
- Grozav, A.; Balacescu, O.; Balacescu, L.; Cheminel, T.; Berindan-Neagoe, I.; Therrien, B. Synthesis, anticancer activity, and genome profiling of thiazolo arene ruthenium complexes. J. Med. Chem. 2015, 58, 8475–8490. [Google Scholar] [CrossRef]
- Kelavkar, U.P.; Nixon, J.B.; Cohen, C.; Dillehay, D.; Eling, T.E.; Badr, K.F. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis 2001, 22, 1765–1773. [Google Scholar] [CrossRef]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tsamo, A.T.; Melong, R.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol. Res. 2015, 48, 57. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.P.; Glynn, S.A.; Billiar, T.R.; Wink, D.A.; Chang, J.C. Targeting nitric oxide: Say NO to metastasis. Clin. Cancer Res. 2023, 29, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Liu, Y.; Guevara, M.L.; Blanco, E.; Choi, D.S.; Qian, W.; Patel, T.; Rodriguez, A.A.; Cusimano, J.; Weiss, H.L.; et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, S.; Xu, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide 2019, 83, 33–39. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Bhat, G.J. Formulation and nanotechnology-based approaches for solubility and bioavailability enhancement of zerumbone. Medicina 2020, 56, 557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Liu, Y.; Ma, R.; Guan, Y.; Chen, X.; Wu, Z.; Chen, T. Anti-Parkinsonian therapy: Strategies for crossing the blood-brain barrier and nano-biological effects of nanomaterials. Nanomicro Lett. 2022, 14, 105. [Google Scholar] [CrossRef]
- Syed, S.B.; Arya, H.; Fu, I.H.; Yeh, T.K.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef]
- Gonzalez, E.; Jain, S.; Shah, P.; Torimoto-Katori, N.; Zakharov, A.; Nguyễn, Ð.T.; Sakamuru, S.; Huang, R.; Xia, M.; Obach, R.S.; et al. Development of robust quantitative structure-activity relationship models for CYP2C9, CYP2D6, and CYP3A4 catalysis and inhibition. Drug Metab. Dispos. 2021, 49, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, E.; Laurent, C.; Lagarde, N.; Fabrega, S.; Nay, L.; Villoutreix, B.O.; Jelsch, C.; Nicot, A.B.; Loriot, M.A.; Miteva, M.A. Machine learning-driven identification of drugs inhibiting cytochrome P450 2C9. PLoS Comput. Biol. 2022, 18, e1009820. [Google Scholar] [CrossRef]
- Rishton, G.M. Molecular diversity in the context of leadlikeness: Compound properties that enable effective biochemical screening. Curr Opin. Chem. Biol. 2008, 12, 340–351. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chen, N.F.; Chen, W.F.; Hung, H.C.; Lee, H.P.; Lin, Y.Y.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; Wen, Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-I.; Tang, J.-Y.; Liu, Y.-S.; Wang, H.-R.; Lee, S.-Y.; Yen, C.-Y.; Chang, H.-W. Roe protein hydrolysates of giant grouper (Epinephelus lanceolatus) inhibit cell proliferation of oral cancer cells involving apoptosis and oxidative stress. BioMed Res. Int. 2016, 2016, 8305073. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Wu, Q.-Y.; Hu, H.-Y.; Chen, Y.-H.; Liu, W.-L. Evidence of ATP assay as an appropriate alternative of MTT assay for cytotoxicity of secondary effluents from WWTPs. Ecotoxicol. Environ. Saf. 2015, 122, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.-P.; Chuang, Y.-T.; Yang, K.-H.; Chang, F.-R.; Sheu, J.-H.; Hou, M.-F.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ren, S.; Dai, Q.; Shen, T.; Li, X.; Li, J.; Xiao, W. InflamNat: Web-based database and predictor of anti-inflammatory natural products. J. Cheminform. 2022, 14, 30. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
| 1 a | 2 a | |||
|---|---|---|---|---|
| δH (J in Hz) | δc, Type | δH (J in Hz) | δc, Type | |
| 1 | 172.9, C | 174.0, C | ||
| 2 | 2.38, m | 29.7, CH2 | 2.34, t (7.5) | 33.4, CH2 |
| 1.29, m | ||||
| 3 | 2.05, m | 29.7, CH2 | 1.72, m | 24.5, CH2 |
| 1.95, m | ||||
| 4 | 5.75, m | 69.3, CH | 2.22, m | 27.0, CH2 |
| 5 | 5.84, dd (10.9, 8.6) | 140.0, CH | 5.57, dt (10.6, 7.5) | 133.3, CH |
| 6 | 6.35, m | 124.4, CH | 5.85, dd (10.6, 9.5) | 129.6, CH |
| 7 | 7.39, d (12.5) | 126.6, CH | 5.94, dd (9.5, 3.6) | 68.3, CH |
| 8 | 135.7, C | 2.55, d (3.6) | 57.2, CH | |
| 9 | 192.7, C | 204.8, C | ||
| 10 | 6.55, d (6.1) | 136.4, CH | 6.17, d (5.8) | 133.7, CH |
| 11 | 7.48, d (6.1) | 155.0, CH | 7.43, d (5.8) | 165.4, CH |
| 12 | 83.5, C | 79.5, C | ||
| 13 | 6.77, d (16.0) | 141.1, CH | 2.56, dd (13.9, 8.3) | 39.3, CH2 |
| 2.34, m | ||||
| 14 | 6.31, d (16.0) | 129.2, CH | 5.36, dt (10.5, 6.9) | 121.8, CH |
| 15 | 199.6, C | 5.65, dt (10.5, 7.4) | 135.7, CH | |
| 16 | 2.53, t (7.4) | 41.2, CH2 | 2.00, m | 27.4, CH2 |
| 17 | 1.59, m | 23.5, CH2 | 1.35, m | 29.1, CH2 |
| 18 | 1.29, m b | 31.3, CH2 | 1.27, m | 31.5, CH2 |
| 19 | 1.29, m b | 22.4, CH2 | 1.30, m | 22.5, CH2 |
| 20 | 0.88, t (7.0) | 13.9, CH3 | 0.89, t (6.8) | 14.0, CH3 |
| 1-OMe | 3.70, s | 51.9, CH3 | 3.68, s | 51.6, CH3 |
| 1′ | 170.0, C | 170.3, C | ||
| 2′ | 2.04, s | 21.3, CH3 | 1.99, s | 21.2, CH3 |
| 1″ | 168.8, C | |||
| 2″ | 2.09, s | 21.2, CH3 | ||
| Compound/Tumor Cell | Ca9-22 |
|---|---|
| 1 | 4.85 ± 0.52 |
| 3 | 3.96 ± 0.06 |
| 4 | 12.4 ± 0.41 |
| 5 | 5.13 ± 0.11 |
| 6 | 4.34 ± 0.06 |
| 7 | 5.15 ± 0.18 |
| 8 | 10.65 ± 0.64 |
| 9 | 9.57 ± 0.32 |
| 11 | 12.72 ± 0.88 |
| 12 | 4.70 ± 0.20 |
| 13 | 10.82 ± 0.36 |
| cisplatin a | 1.08 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-Y.; Hsu, I.-C.; Huang, S.-Y.; Chuang, Y.-T.; Ke, T.-Y.; Chang, H.-W.; Chu, T.-H.; Chen, C.-Y.; Cheng, Y.-B. Marine Prostanoids with Cytotoxic Activity from Octocoral Clavularia spp. Mar. Drugs 2024, 22, 219. https://doi.org/10.3390/md22050219
Cheng M-Y, Hsu I-C, Huang S-Y, Chuang Y-T, Ke T-Y, Chang H-W, Chu T-H, Chen C-Y, Cheng Y-B. Marine Prostanoids with Cytotoxic Activity from Octocoral Clavularia spp. Marine Drugs. 2024; 22(5):219. https://doi.org/10.3390/md22050219
Chicago/Turabian StyleCheng, Ming-Ya, I-Chi Hsu, Shi-Ying Huang, Ya-Ting Chuang, Tzi-Yi Ke, Hsueh-Wei Chang, Tian-Huei Chu, Ching-Yeu Chen, and Yuan-Bin Cheng. 2024. "Marine Prostanoids with Cytotoxic Activity from Octocoral Clavularia spp." Marine Drugs 22, no. 5: 219. https://doi.org/10.3390/md22050219
APA StyleCheng, M.-Y., Hsu, I.-C., Huang, S.-Y., Chuang, Y.-T., Ke, T.-Y., Chang, H.-W., Chu, T.-H., Chen, C.-Y., & Cheng, Y.-B. (2024). Marine Prostanoids with Cytotoxic Activity from Octocoral Clavularia spp. Marine Drugs, 22(5), 219. https://doi.org/10.3390/md22050219







