Immunostimulatory Effects of Korean Mineral-Rich Seawaters on Cyclophosphamide-Induced Immunosuppression in Mice
Abstract
:1. Introduction
2. Results
2.1. Mineral Elements Contained in Korean Seawaters
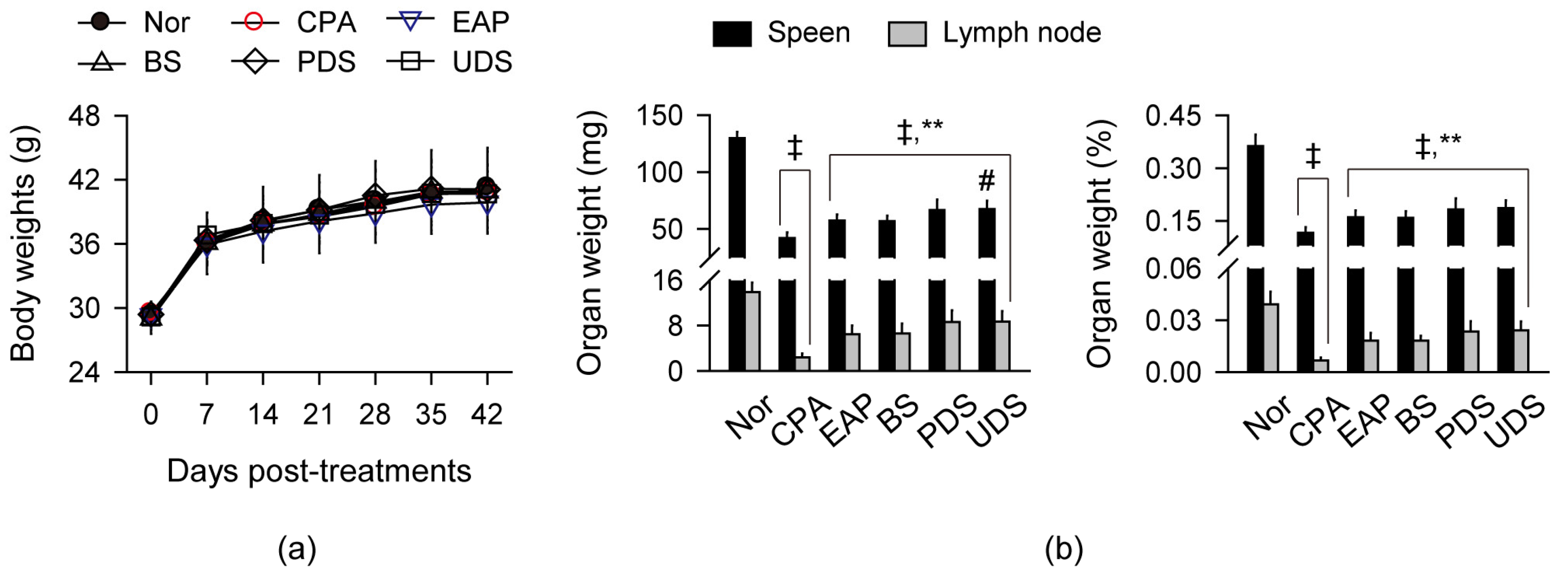
2.2. Changes in Body Weight and Weight of Lymphoid Organs
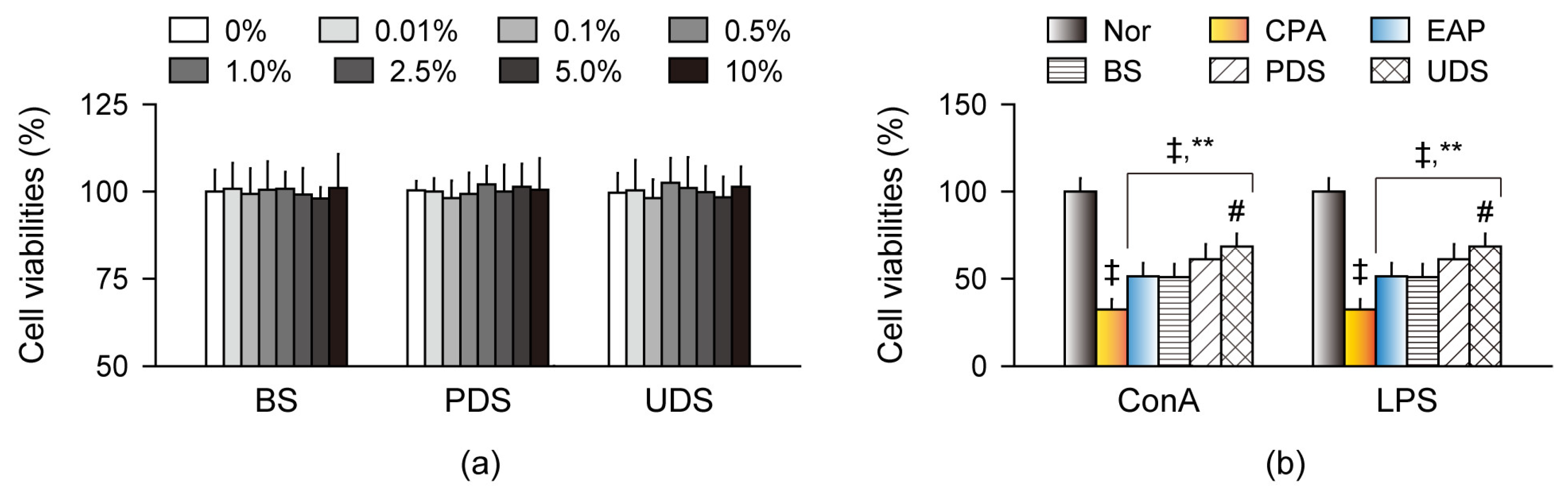
2.3. Effects on Cell Viability and Proliferation in Primary Splenocytes Ex Vivo
2.4. Effects on Natural Killer Cells’ Activity
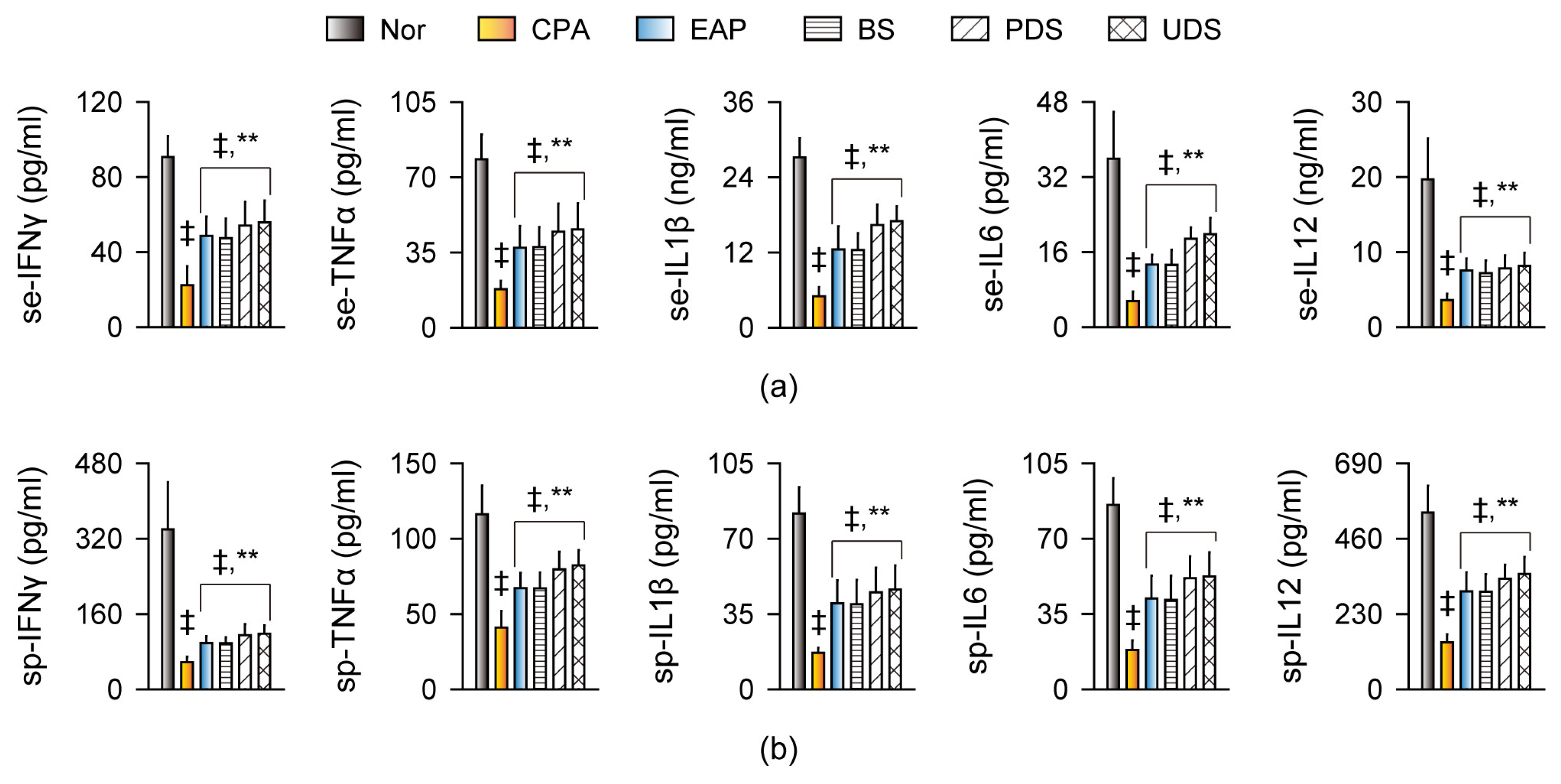
2.5. Effects on Cytokine Production and Gene Expression
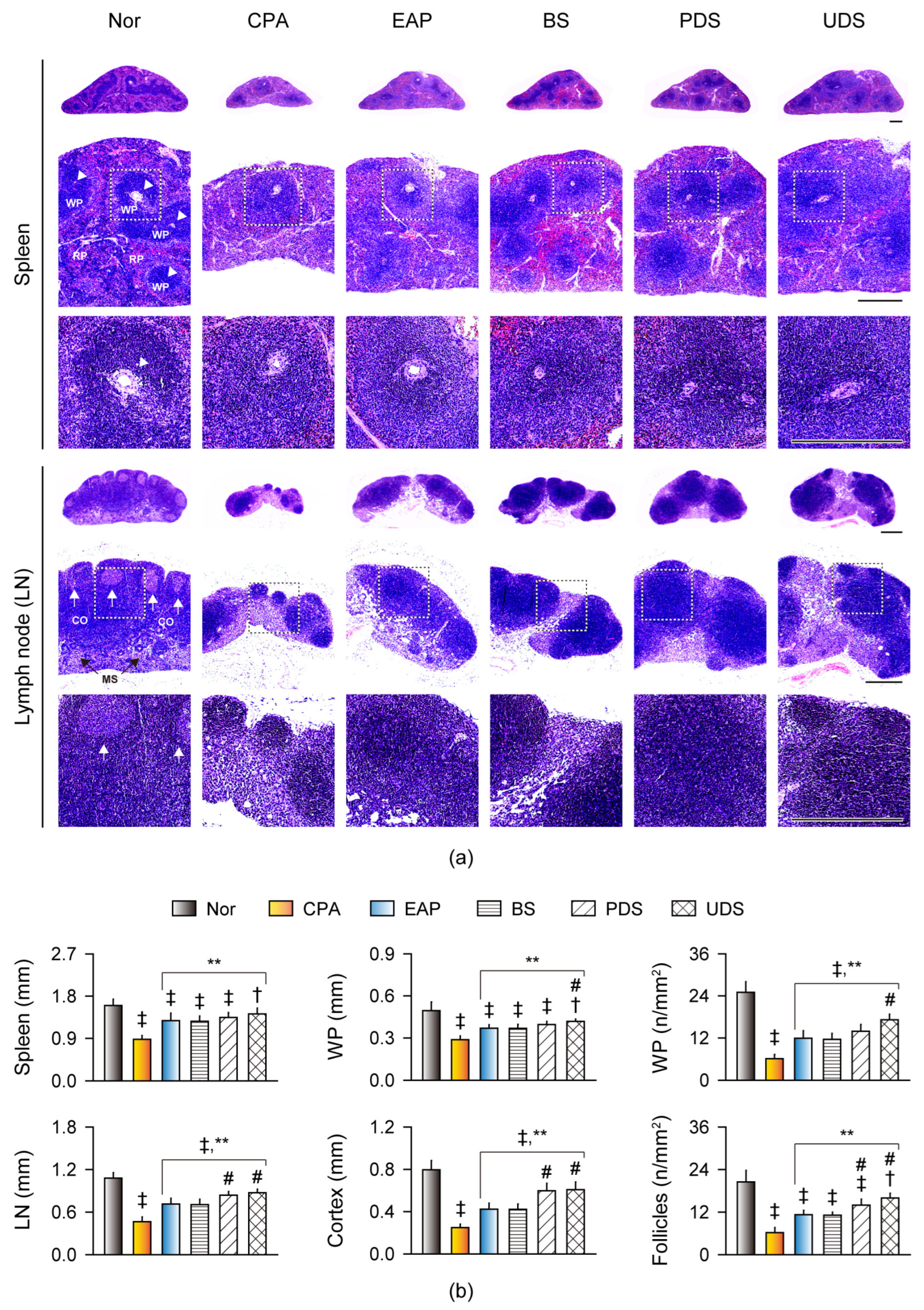
2.6. Histopathological Changes in the Lymphoid Organs
3. Discussion
4. Materials and Methods
4.1. Korean Seawaters and Reagents
4.2. Animal Treatments
4.3. Preparation of Primary Splenocytes
4.4. Cell Viability and Proliferation Assays in Primary Splenocytes
4.5. Assessments of NK Cell Activity
4.6. Assessments of Serum and Splenic Cytokine Levels
4.7. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.8. Histopathological Analysis
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Christian, L.M.; Kiecolt-Glaser, J.K. Stress, age, and immune function: Toward a lifespan approach. J. Behav. Med. 2006, 29, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2016. [Google Scholar]
- Ponce, R. Adverse consequences of immunostimulation. J. Immunotoxicol. 2008, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the immune-boosting functions of vitamins and minerals as nutritional food bioactive compounds: A comprehensive review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, A.; Shafayet Ahmed, S.; Karim, M.R.; Al-Mamun, M.R.; Akhter, S.; Sohel, M.; Hasan, M.; Bellah, S.F.; Amin, M.N. Clinically proven natural products, vitamins and mineral in boosting up immunity: A comprehensive review. Heliyon 2023, 9, e15292. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63, E93–E101. [Google Scholar] [CrossRef]
- Kacar, B.; Garcia, A.K.; Anbar, A.D. Evolutionary History of Bioessential Elements Can Guide the Search for Life in the Universe. Chembiochem 2021, 22, 114–119. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Weyh, C.; Kruger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, S. Sources and deficiency diseases of mineral nutrients in human health and nutrition: A review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Mohd Nani, S.Z.; Majid, F.A.; Jaafar, A.B.; Mahdzir, A.; Musa, M.N. Potential Health Benefits of Deep Sea Water: A Review. Evid.-Based Complement. Altern. Med. eCAM 2016, 2016, 6520475. [Google Scholar] [CrossRef] [PubMed]
- Kumssa, D.B.; Joy, E.J.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Hataguchi, Y.; Tai, H.; Nakajima, H.; Kimata, H. Drinking deep-sea water restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur. J. Clin. Nutr. 2005, 59, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H.; Tai, H.; Nakajima, H. Reduction of allergic skin responses and serum allergen-specific IgE and IgE-inducing cytokines by drinking deep-sea water in patients with allergic rhinitis. Oto-Rhino-Laryngol. Nova 2001, 11, 302–303. [Google Scholar] [CrossRef]
- Kimata, H.; Tai, H.; Nakagawa, K.; Yokoyama, Y.; Nakajima, H.; Ikegami, Y. Improvement of skin symptoms and mineral imbalance by drinking deep sea water in patients with atopic eczema/dermatitis syndrome (AEDS). Acta Med. 2002, 45, 83–84. [Google Scholar] [CrossRef]
- Kawada, M.; Takeuchi, H.; Con, S.A.; Yamamoto, E.; Yasukawa, T.; Nakagawa, K.; Ikegami, Y.; Sugiura, T. Antibacterial activities of refined deep seawater on Helicobacter pylori. Eff. Chromium Immune Syst. 2013, 2, S1. [Google Scholar] [CrossRef]
- Kim, C.G.; Kang, M.; Lee, Y.H.; Min, W.G.; Kim, Y.H.; Kang, S.J.; Song, C.H.; Park, S.J.; Park, J.H.; Han, C.H.; et al. Bathing Effects of Various Seawaters on Allergic (Atopic) Dermatitis-Like Skin Lesions Induced by 2,4-Dinitrochlorobenzene in Hairless Mice. Evid.-Based Complement. Altern. Med. eCAM 2015, 2015, 179185. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Shin, H.D.; Yang, K.J.; Lee, H.S.; Kwon, Y.S.; Ku, S.K. Immunomodulatory effects of Aureobasidium pullulans SM-2001 exopolymers on the cyclophosphamide-treated mice. J. Microbiol. Biotechnol. 2010, 20, 438–445. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, Y.S.; Ku, S.; Lee, H.J. Phellinus baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Li, J.; Wang, H.L.; Cheng, W.M.; Zhang, L.; Ge, J.F. Immunomodulating effects of fractioned polysaccharides isolated from Yu-Ping-Feng-Powder in cyclophosphamide-treated mice. Am. J. Chin. Med. 2006, 34, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Choi, J.S.; Seol, D.J.; Choung, J.J.; Ku, S.K. Immunomodulatory Effects of Kuseonwangdogo-Based Mixed Herbal Formula Extracts on a Cyclophosphamide-Induced Immunosuppression Mouse Model. Evid.-Based Complement. Altern. Med. eCAM 2018, 2018, 6017412. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Suzuki, Y.; Kato, Y.; Takeuchi, N.; Takenaka, H.; Kohno, M. Effect of Extract-Added Water Derived from Deep-Sea Water with Different Hardness on Cognitive Function, Motor Ability and Serum Indexes of Obese Mice. Nutrients 2022, 14, 1794. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Chang, Y.-Y.; Chiu, C.-H.; Yang, K.-T.; Wang, Y.; Fu, S.-G.; Chen, Y.-C. Cardiovascular protection of deep-seawater drinking water in high-fat/cholesterol fed hamsters. Food Chem. 2011, 127, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chang, Y.Y.; Hsu, C.L.; Lin, H.W.; Chang, M.H.; Chen, J.W.; Chen, S.S.; Chen, Y.C. Alleviative effects of deep-seawater drinking water on hepatic lipid accumulation and oxidation induced by a high-fat diet. J. Chin. Med. Assoc. 2013, 76, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; Greene, W.C. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 2004, 5, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Rochman, I.; Paul, W.E.; Ben-Sasson, S.Z. IL-6 increases primed cell expansion and survival. J. Immunol. 2005, 174, 4761–4767. [Google Scholar] [CrossRef]
- Portales, P.; Aries, M.F.; Licu, D.; Pinton, J.; Hernandez-Pion, C.; Gall, Y.; Dupuy, P.; Charveron, M.; Clot, J. Immunomodulation induced by Avene spring water on Th1- and Th2-dependent cytokine production in healthy subjects and atopic dermatitis patients. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.P.; Kim, Y.M.; Son, J.; Kim, C.J.; Kim, E.H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement Altern. Med. 2012, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.J.; Chou, P.Y.; Lin, W.H.; Pan, C.H.; Chien, Y.C.; Chung, Y.L.; Liu, F.C.; Wu, C.H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar] [CrossRef]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef]
- Gao, C.; Wu, H.; Guo, S.; Zhang, Y.; Ma, L. Study on Ultrafiltration-Reverse Osmosis Technology in The Treatment of Deep Ocean Water. IOP Conf. Ser. Earth Environ. Sci. 2021, 632, 032032. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Amantini, C.; Morelli, M.B. Editorial: Calcium signaling in cancer immunity. Front. Immunol. 2023, 14, 1315490. [Google Scholar] [CrossRef]
- Butler, J.E.; Satam, M.; Ekstrand, J. Fluoride: An adjuvant for mucosal and systemic immunity. Immunol. Lett. 1990, 26, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef]
- Al Hasan, S.M.; Hassan, M.; Saha, S.; Islam, M.; Billah, M.; Islam, S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr. 2016, 2, 24. [Google Scholar] [CrossRef]
- Adler-Cohen, C.; Czarnowicki, T.; Dreiher, J.; Ruzicka, T.; Ingber, A.; Harari, M. Climatotherapy at the Dead Sea: An effective treatment modality for atopic dermatitis with significant positive impact on quality of life. Dermatitis 2012, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Lee, J.E.; Jeong, D.G.; Lee, Y.H.; Park, S.I.; Lee, D.G.; Han, C.H.; Kang, S.J.; Song, C.H.; Choi, S.H.; et al. Bathing effects of east saline groundwater concentrates on allergic (atopic) dermatitis-like skin lesions induced by 2,4-dinitrochlorobenzene in hairless mice. Exp. Ther. Med. 2017, 13, 3448–3466. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Shozugawa, K.; Sugimori, K.; Watanabe, Y. A survey of monitoring tap water hardness in Japan and its distribution patterns. Sci. Rep. 2021, 11, 13546. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, S.H.; Yoo, Y.G.; Chu, Y.S.; Shon, Y.H.; Nam, K.S.; Yun, J.W. Inhibitory effect of deep-sea water on differentiation of 3T3-L1 adipocytes. Mar. Biotechnol. 2009, 11, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chun, S.Y.; Lee, D.H.; Lee, K.S.; Nam, K.S. Mineral-enriched deep-sea water inhibits the metastatic potential of human breast cancer cell lines. Int. J. Oncol. 2013, 43, 1691–1700. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Das, S.; Paul, B.; Singh, P.; Giri, S. Mineral Assay in Atomic Absorption Spectroscopy. Beats Nat. Sci. 2014, 1, 1–17. [Google Scholar]
- Yang, L.; Pagliano, E.; Mester, Z. Direct determination of dissolved phosphate and silicate in seawater by ion exclusion chromatography sector field inductively coupled plasma mass spectrometry. Anal. Chem. 2014, 86, 3222–3226. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Major (ppm) | BS | PDS | UDS | Trace (ppb) | BS | PDS | UDS |
|---|---|---|---|---|---|---|---|
| Na (sodium) | 3597.5 | 3557.2 | 3477.6 | Ni (nickel) | 86.4 | 88.4 | 86.3 |
| Cl− (chloride) | 6544.4 | 6499.6 | 6379.9 | Li (lithium) | 32.3 | 31.9 | 32.8 |
| SO42− (sulfate) | 682.9 | 687.6 | 672.8 | Rb (rubidium) | 28.4 | 29.0 | 29.0 |
| K (potassium) | 120.5 | 118.3 | 117.8 | Cu (copper) | 19.3 | 28.3 | 18.7 |
| Ca (calcium) | 120.5 | 137.4 | 132.8 | Co (cobalt) | 15.3 | 16.0 | 15.6 |
| Br− (bromide) | 73.4 | 73.6 | 73.1 | Zn (zinc) | 13.5 | 16.1 | 15.4 |
| NO3− (nitrate) | 66.9 | 64.3 | 61.8 | Se (selenium) | 13.2 | 12.7 | 13.1 |
| F− (fluoride) | 36.4 | 32.4 | 32.4 | V (vanadium) | 11.4 | 11.5 | 11.5 |
| Sr (strontium) | 1.8 | 1.9 | 1.8 | As (arsenic) | 5.2 | 5.0 | 4.9 |
| Mg (magnesium) | 1.4 | 1.5 | 1.3 | Ba (barium) | 3.5 | 1.5 | 1.8 |
| B (boron) | 1.2 | 1.2 | 1.2 | Mo (molybdenum) | 3.1 | 3.1 | 3.1 |
| Fe (iron) | 0.4 | 0.4 | 0.4 | Al (aluminum) | 2.3 | 0.7 | 1.6 |
| Ge (germanium) | 0.3 | 0.3 | 0.3 | Cr (chromium) | 0.5 | 0.6 | 0.6 |
| Ti (titanium) | 0.2 | 0.3 | 0.3 | Mn (manganese) | 0.4 | 0.5 | 0.4 |
| Targets (GenBank IDs) | Sequence (5′–3′) |
|---|---|
| NF-κB (NM008689) | Forward: CAATGGCTACACAGGACCA |
| Reverse: CACTGTCACCTGGAACCAGA | |
| INFγ (NM008337) | Forward: GTTACTGCCACGGCACAGTCATTG |
| Reverse: ACCATCCTTTTGCCAGTTCCTCCAG | |
| TNFα (NM013693) | Forward: CCTGTAGCCCACGTCGTAGC |
| Reverse: TTGACCTCAGCGCTGAGTTG | |
| IL1β (NM008361) | Forward: AGCTGTGGCAGCTACCTGTG |
| Reverse: GCTCTGCTTGTGAGGTGCTG | |
| IL6 (NM031168) | Forward: TCTTGGGACTGATGCTGGTGAC |
| Reverse: GCAGCTCCCTCTTGTTGT | |
| IL12 (NM008354) | Forward: CAGGTGTCTTAGCCAGTCC |
| Reverse: CATAACGCACTAGGTTTGCCGA | |
| GAPDH (NM008084) | Forward: CATCTTCCAGGAGCGAGACC |
| Reverse: TCCACCACCCTGTTGCTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-G.; Choi, J.H.; Ku, S.-K.; Song, C.-H. Immunostimulatory Effects of Korean Mineral-Rich Seawaters on Cyclophosphamide-Induced Immunosuppression in Mice. Mar. Drugs 2024, 22, 234. https://doi.org/10.3390/md22060234
Kim C-G, Choi JH, Ku S-K, Song C-H. Immunostimulatory Effects of Korean Mineral-Rich Seawaters on Cyclophosphamide-Induced Immunosuppression in Mice. Marine Drugs. 2024; 22(6):234. https://doi.org/10.3390/md22060234
Chicago/Turabian StyleKim, Choong-Gon, Jae Ho Choi, Sae-Kwang Ku, and Chang-Hyun Song. 2024. "Immunostimulatory Effects of Korean Mineral-Rich Seawaters on Cyclophosphamide-Induced Immunosuppression in Mice" Marine Drugs 22, no. 6: 234. https://doi.org/10.3390/md22060234
APA StyleKim, C.-G., Choi, J. H., Ku, S.-K., & Song, C.-H. (2024). Immunostimulatory Effects of Korean Mineral-Rich Seawaters on Cyclophosphamide-Induced Immunosuppression in Mice. Marine Drugs, 22(6), 234. https://doi.org/10.3390/md22060234






