Anti-Inflammatory Effect of Fucoidan from Costaria costata Inhibited Lipopolysaccharide-Induced Inflammation in Mice
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of FCC
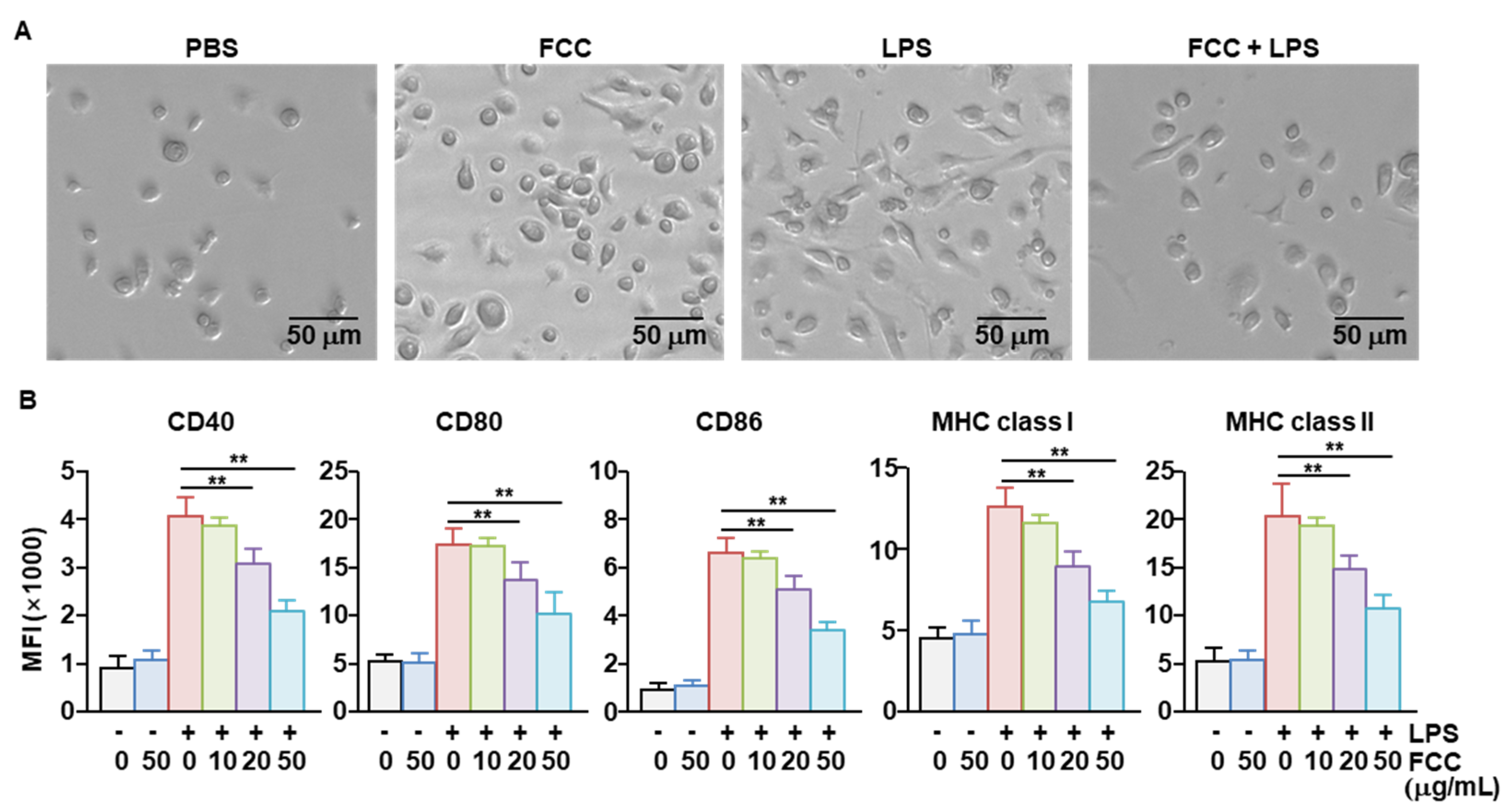
2.2. FCC Inhibited the Activation of Bone Marrow-Derived DCs (BMDCs)
2.3. FCC Inhibited Pro-Inflammatory Cytokine Secretion in LPS-Stimulated BMDCs
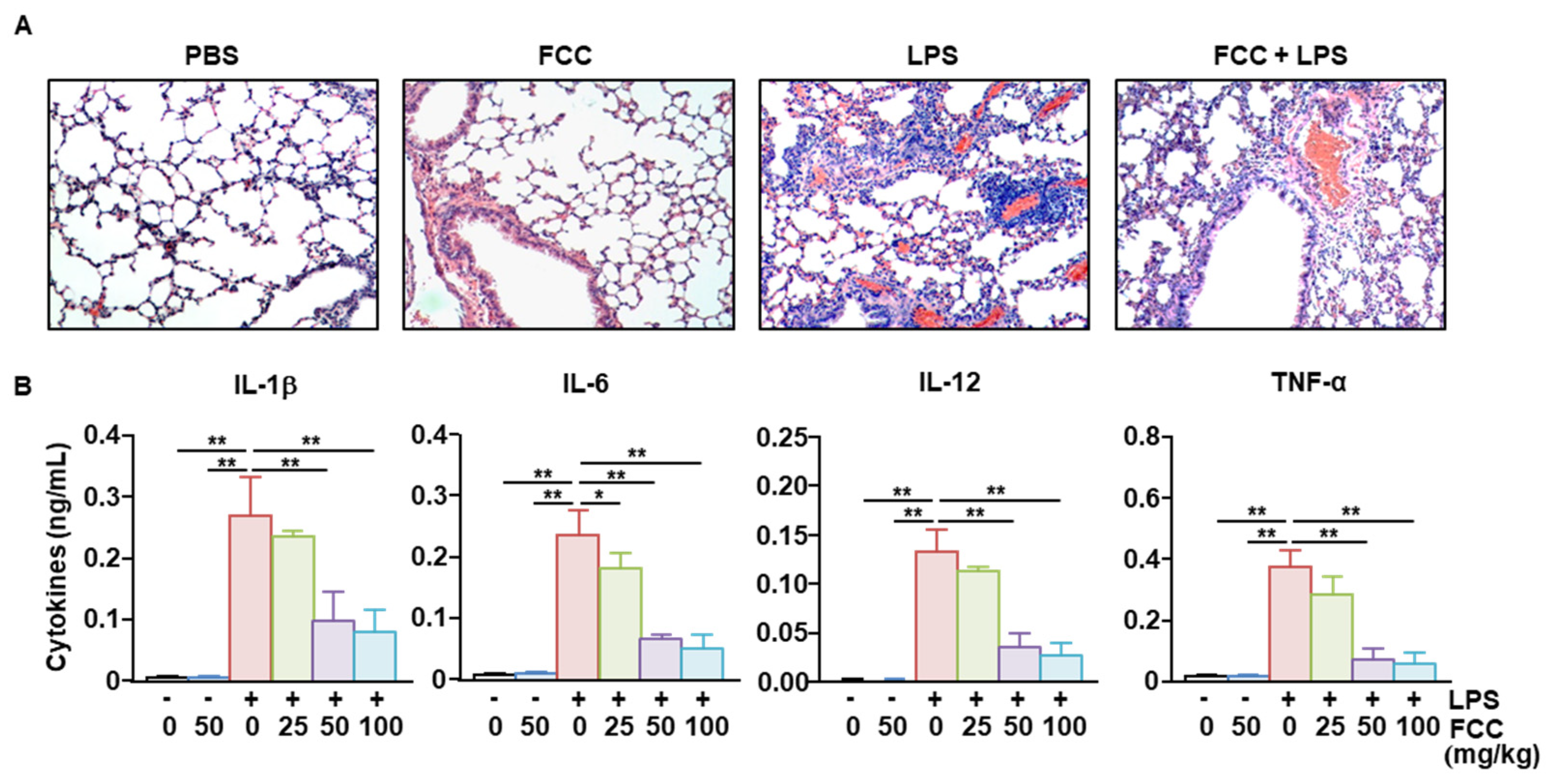
2.4. FCC Suppressed LPS-Induced Acute Lung Inflammation
2.5. Oral Administration of FCC-Suppressed LPS-Induced Sepsis in Mice
3. Discussion
4. Materials and Methods
4.1. Collection of C. costata
4.2. Isolation of FCC
4.3. FCC Purification
4.4. Monosaccharide Composition
4.5. Reagents
4.6. Animals
4.7. BMDC Generation
4.8. Treatment and Analysis of BMDCs
4.9. Flow Cytometric Analysis
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. LPS Injection and FCC Treatment
4.12. Hematoxylin and Eosin (H&E) Staining
4.13. LPS-Induced Sepsis Model
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Olivo, A.O.; Zamora-Gasga, V.M.; Medina-Torres, L.; Perez-Larios, A.; Sayago-Ayerdi, S.G.; Sanchez-Burgos, J.A. Biofunctionalization of natural extracts, trends in biological activity and kinetic release. Adv. Colloid Interface Sci. 2023, 318, 102938. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef]
- Sun, H.; Sun, K.; Sun, J. Recent Advances of Marine Natural Indole Products in Chemical and Biological Aspects. Molecules 2023, 28, 2204. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Lomartire, S.; Pereira, L.; Valado, A.; Marques, J.C.; Goncalves, A.M.M. Seaweeds as Nutraceutical Elements and Drugs for Diabetes Mellitus: Future Perspectives. Mar. Drugs 2024, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Q.; Xu, X.; Li, G.; Tian, C.; Zhang, T. Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin. Herb. Med. 2022, 14, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; He, Y.; Ren, D.; Kow, F.; Qiao, Z.; Liu, S.; Yu, X. Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydr. Polym. 2014, 107, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.L.; Ji, Y.B.; Yang, B. Sulfated modification, characterization and monosaccharide composition analysis of Undaria pinnatifida polysaccharides and anti-tumor activity. Exp. Ther. Med. 2020, 20, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. A Review on Seaweeds and Seaweed-Derived Polysaccharides: Nutrition, Chemistry, Bioactivities, and Applications. Food Rev. Int. 2024, 40, 1312–1347. [Google Scholar] [CrossRef]
- Emmanuel Ofosu Mensah, O.N.K. Pritam Kumar Panda, Parise Adadi, Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Granja, S.; Neves, N.M.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oliveira, C.; Li, Q.; Ferreira, A.S.; Nunes, C.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Wang, C.; Silva, T.H.; et al. Fucoidan from Fucus vesiculosus Inhibits Inflammatory Response, Both In Vitro and In Vivo. Mar. Drugs 2023, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, J.; Jing, X.; Ding, X.; Yu, Y.; Zhao, Q. Fucoidan induces apoptosis and inhibits proliferation of hepatocellular carcinoma via the p38 MAPK/ERK and PI3K/Akt signal pathways. Cancer Manag. Res. 2020, 12, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Formulation, optimization and in vivo evaluation of fucoidan-based cream with anti-inflammatory properties. Mar. Drugs 2021, 19, 643. [Google Scholar] [CrossRef] [PubMed]
- A current view on inflammation. Nat. Immunol. 2017, 18, 825. [CrossRef]
- McInnes, I.B.; Gravallese, E.M. Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 2021, 21, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273, Table of Contents. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Chudnovskiy, A.; Pasqual, G.; Victora, G.D. Studying interactions between dendritic cells and T cells in vivo. Curr. Opin. Immunol. 2019, 58, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Cao, X. Dendritic cells in the regulation of immunity and inflammation. Semin. Immunol. 2018, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Mattiotti, M.; de Cal, M.; Ronco, C.; Zanella, M.; De Rosa, S. Endotoxin in Sepsis: Methods for LPS Detection and the Use of Omics Techniques. Diagnostics 2022, 13, 79. [Google Scholar] [CrossRef]
- Copeland, S.; Warren, H.S.; Lowry, S.F.; Calvano, S.E.; Remick, D.; on behalf of the Inflammation and the Host Response to Injury Investigators. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005, 12, 60–67. [Google Scholar] [CrossRef]
- Manikandan, R.; Parimalanandhini, D.; Mahalakshmi, K.; Beulaja, M.; Arumugam, M.; Janarthanan, S.; Palanisamy, S.; You, S.; Prabhu, N.M. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar] [CrossRef]
- Hadj Ammar, H.; Lajili, S.; Ben Said, R.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. DARU J. Pharm. Sci. 2015, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, N.; Oyama, N.; Chino, T.; Utsunomiya, A.; Hida, Y.; Hasegawa, M. Dietary supplement product composed of natural ingredients as a suspected cause of erythema multiforme: A case report and identification for the confident false positivity of lymphocyte transformation test. J. Dermatol. 2019, 46, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.G.; Lee, D. Oral Administration of East Asian Herbal Medicine for Peripheral Neuropathy: A Systematic Review and Meta-Analysis with Association Rule Analysis to Identify Core Herb Combinations. Pharmaceuticals 2021, 14, 1202. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly changes in the content and monosaccharide composition of fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Arijón, M.; Ponce, N.M.; Solana, V.; Dellatorre, F.G.; Latour, E.A.; Stortz, C.A. Monthly fluctuations in the content and monosaccharide composition of fucoidan from Undaria pinnatifida sporophylls from northern Patagonia. J. Appl. Phycol. 2021, 33, 2433–2441. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lim, D.-J.; Lee, Y.-H.; Park, Y.-I. Variation in fucoidan contents and monosaccharide compositions of Korean Undaria pinnatifida (Harvey) Suringar (Phaeophyta). Algae 2006, 21, 157–160. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.; Shevchenko, N.; Semenova, T.; Sukhoverkhov, S.; Zvyagintseva, T. Compositional heterogeneity of sulfated polysaccharides synthesized by the brown alga Costaria costata. Chem. Nat. Compd. 2011, 47, 96–97. [Google Scholar] [CrossRef]
- Do, H.; Kang, N.S.; Pyo, S.; Billiar, T.R.; Sohn, E.H. Differential regulation by fucoidan of IFN-gamma-induced NO production in glial cells and macrophages. J. Cell Biochem. 2010, 111, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.T.; Kim, J.S.; Kim, W.S.; Lee, J.S.; Jeon, Y.J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Bosnjak, B.; Do, K.T.H.; Forster, R.; Hammerschmidt, S.I. Imaging dendritic cell functions. Immunol. Rev. 2022, 306, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC Class II Presentation in Autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef]
- Netea, M.G.; Kullberg, B.J.; Van der Meer, J.W. Circulating cytokines as mediators of fever. Clin. Infect. Dis. 2000, 31 (Suppl. S5), S178–S184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ning, B.T. Signaling pathways and intervention therapies in sepsis. Signal Transduct. Target. Ther. 2021, 6, 407. [Google Scholar] [CrossRef]
- Rainsford, K. Anti-inflammatory drugs in the 21st century. In Inflammation in the Pathogenesis of Chronic Diseases: The COX-2 Controversy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 3–27. [Google Scholar]
- Henry, D. Side-effects of non-steroidal anti-inflammatory drugs. Bailliere’s Clin. Rheumatol. 1988, 2, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Bird, H.; Giannoudis, P.V. Nonsteroidal anti-inflammatory drugs: Prostaglandins, indications, and side effects. Int. J. Interferon Cytokine Mediat. Res. 2011, 2011, 19–27. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]

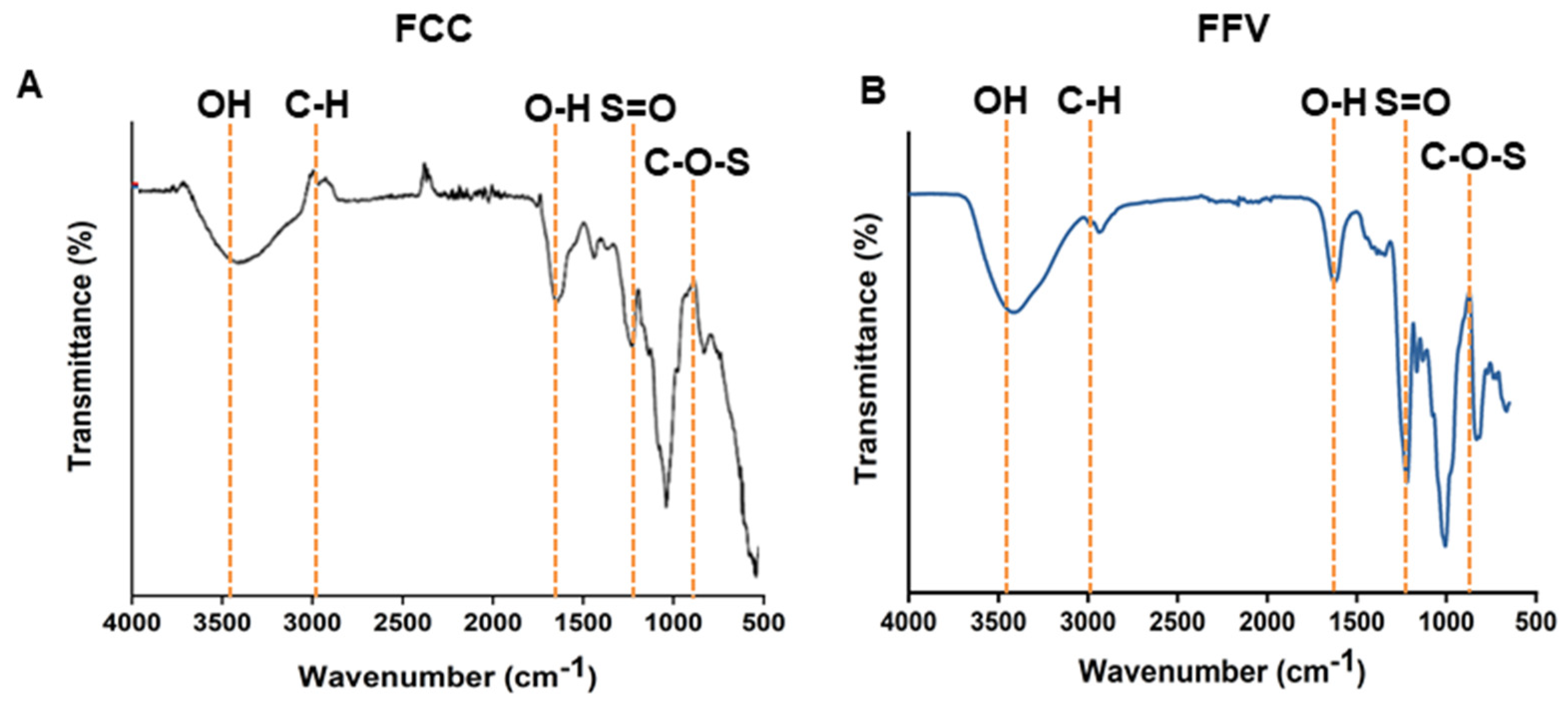


| Polysaccharide Source | Composition of Neutral Sugar a | Uronic Acid b | SO42− c | M.W. (KDa) | ||||
|---|---|---|---|---|---|---|---|---|
| Fucose | Xylose | Glucose | Mannose | Galactose | ||||
| Fucoidan from Fucus vesiculosus (%) | 29.8 ± 1.3 | 2.4 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 | 3.2 ± 0.1 | 5.5 ± 0.21 | 24.5 ± 1.2 | |
| Fucoidan from Costaria costata (%) | 25.4 ± 2.1 | 2.3 ± 0.3 | 3.1 ± 0.2 | 5.2 ± 0.3 | 4.1 ± 0.2 | 3.8 ± 0.4 | 26.7 ± 0.9 | 420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lee, P.C.W.; Jin, J.-O. Anti-Inflammatory Effect of Fucoidan from Costaria costata Inhibited Lipopolysaccharide-Induced Inflammation in Mice. Mar. Drugs 2024, 22, 401. https://doi.org/10.3390/md22090401
Zhang W, Lee PCW, Jin J-O. Anti-Inflammatory Effect of Fucoidan from Costaria costata Inhibited Lipopolysaccharide-Induced Inflammation in Mice. Marine Drugs. 2024; 22(9):401. https://doi.org/10.3390/md22090401
Chicago/Turabian StyleZhang, Wei, Peter C. W. Lee, and Jun-O Jin. 2024. "Anti-Inflammatory Effect of Fucoidan from Costaria costata Inhibited Lipopolysaccharide-Induced Inflammation in Mice" Marine Drugs 22, no. 9: 401. https://doi.org/10.3390/md22090401






