pH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria palmata: A Sequential Approach
Abstract
1. Introduction
2. Results
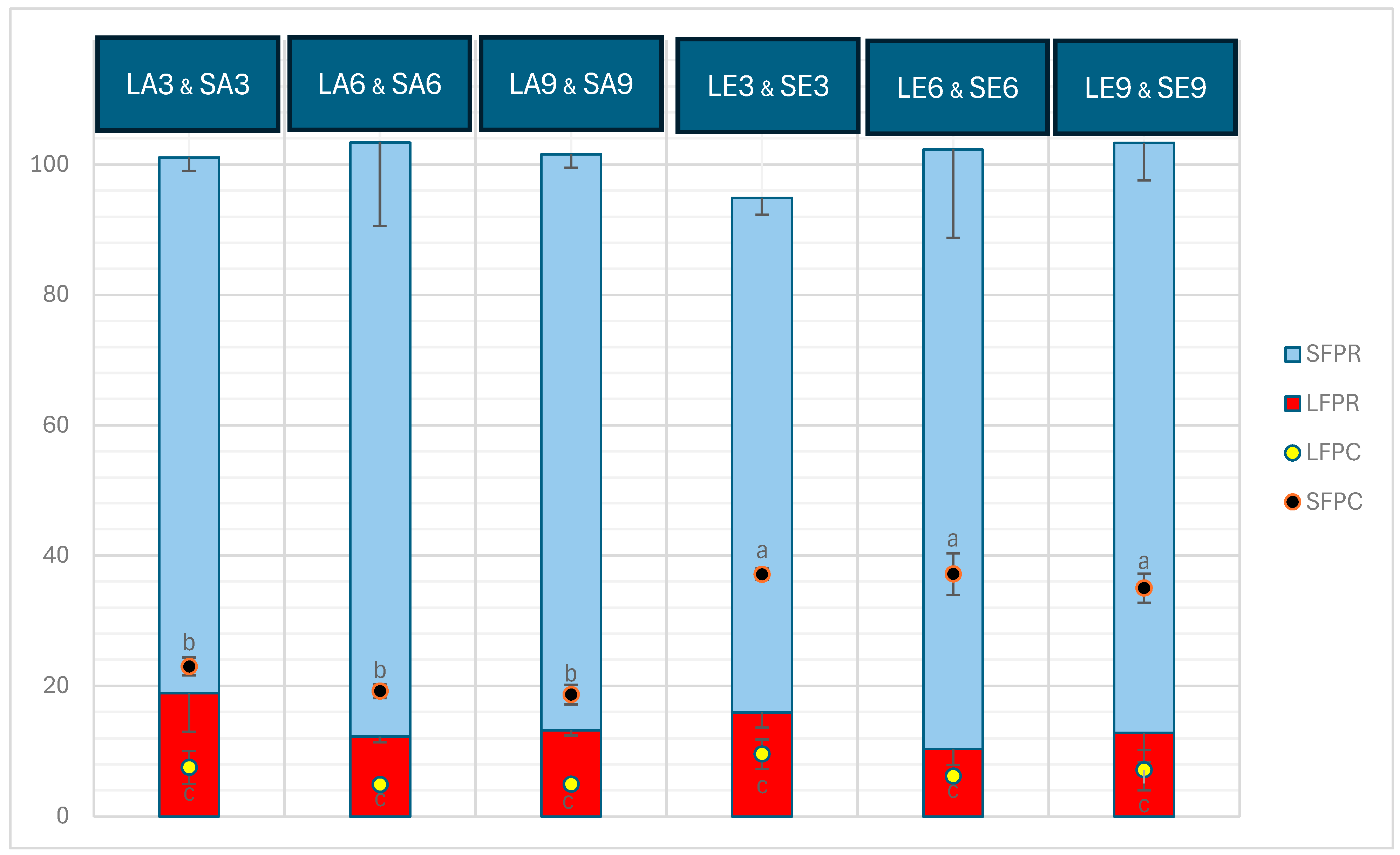
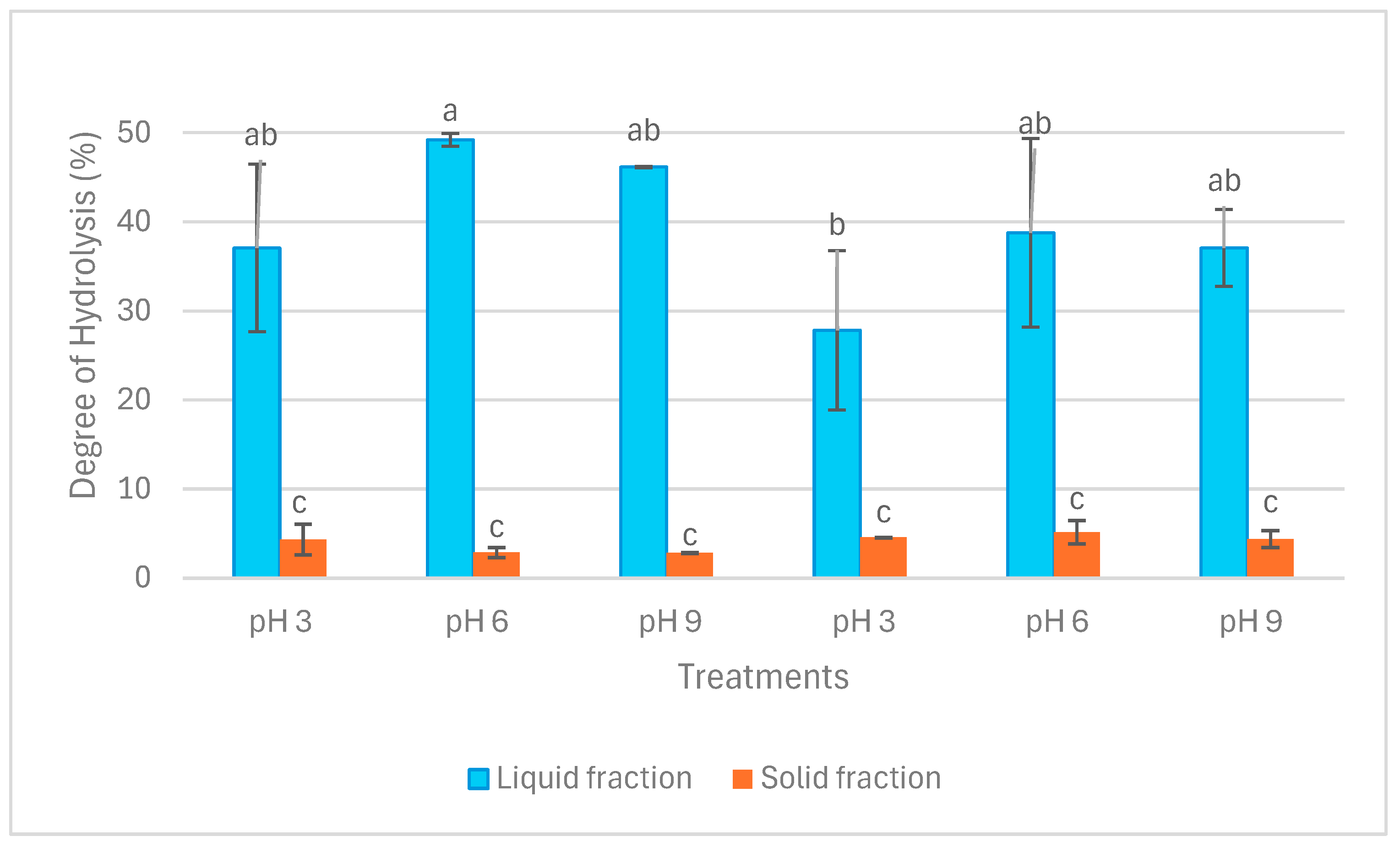
2.1. Protein Content, Protein Recovery, and Degree of Hydrolysis (DH)
2.2. Amino Acid Composition
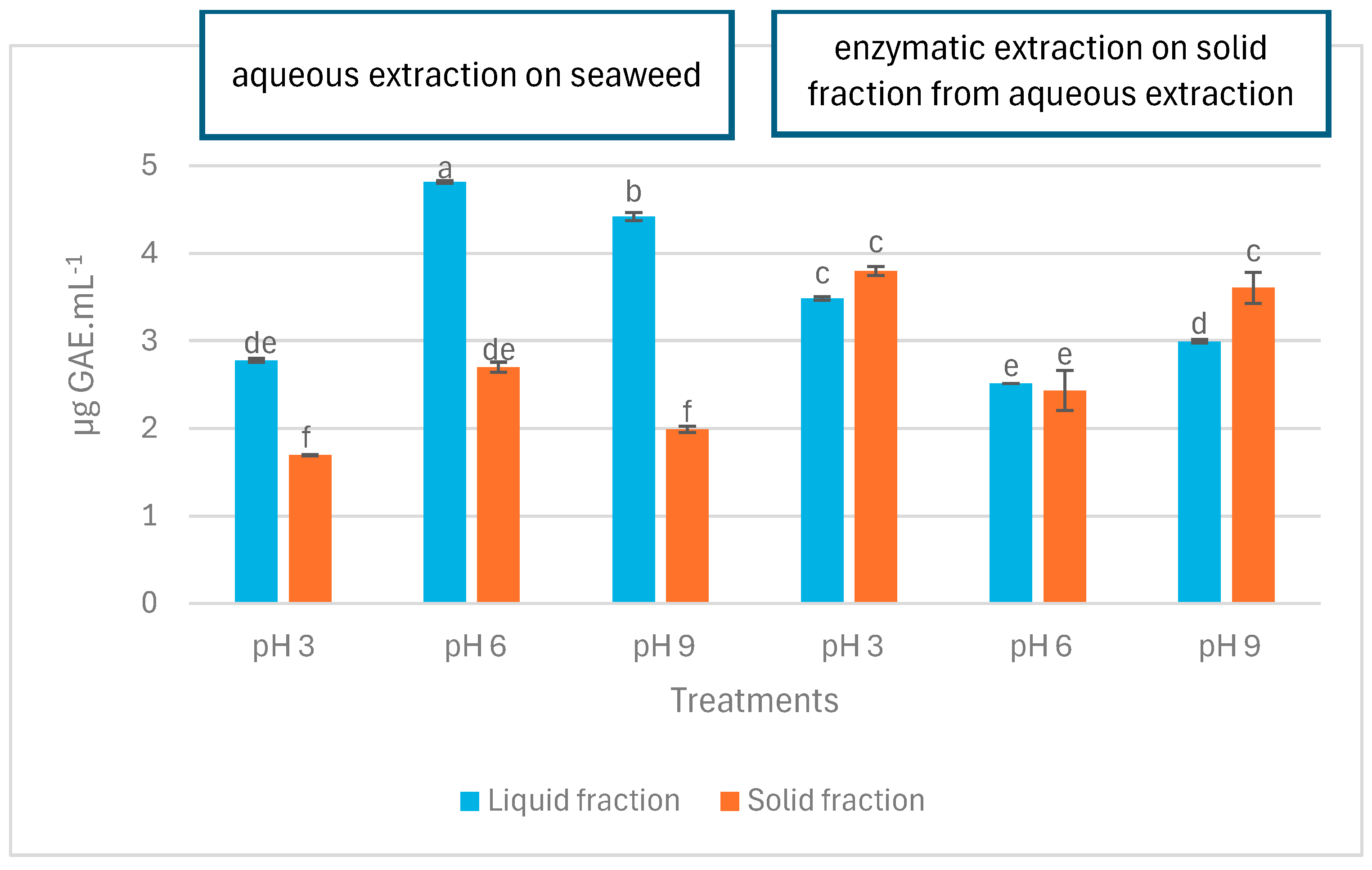
2.3. Total Phenolic Content
2.4. Antioxidant Properties
3. Discussion
3.1. Protein Content, Protein Recovery, and Degree of Hydrolysis
3.2. Amino Acid Composition
3.3. Total Phenolic Content
3.4. Antioxidant Properties
4. Materials and Methods
4.1. Seaweed Biomass Preparation
4.2. Enzymes and Chemicals
4.3. Aqueous Extraction
4.4. Enzymatic Hydrolysis
4.5. Protein Content and Recovery
4.6. Degree of Hydrolysis (DH)
4.7. Amino Acid Profile
4.8. Total Phenolic Content
4.9. DPPH Radical Scavenging Activity
4.10. Fe2+ Chelating Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ANOVA | analysis of variance |
| BHT | butylated hydroxytoluene |
| DH | degree of hydrolysis |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DTT | dithiothreitol |
| EAA | essential amino acids |
| EDTA | ethylenediaminetetraacetic acid |
| HCl | hydrochloric acid |
| HPLC | high-performance liquid chromatography |
| IC50 | the concentration needed to inhibit free radicals or chelate metal ions by 50% |
| LA3 | liquid fraction obtained after aqueous extraction at pH 3 |
| LA6 | liquid fraction obtained after aqueous extraction at pH 6 |
| LA9 | liquid fraction obtained after aqueous extraction at pH 9 |
| LAPU | leucine aminopeptidase units |
| LE3 | liquid fraction obtained after enzymatic extraction at pH 3 |
| LE6 | liquid fraction obtained after enzymatic extraction at pH 6 |
| LE9 | liquid fraction obtained after enzymatic extraction at pH 9 |
| LFPC | liquid fraction’s protein content |
| LFPR | liquid fraction’s protein recovery |
| OPA | o-phthaldialdehyde |
| Na2CO3 | sodium carbonate |
| NaHCO₃ | sodium bicarbonate |
| NaOH | sodium hydroxide |
| SA3 | solid fraction obtained after aqueous extraction at pH 3 |
| SA6 | solid fraction obtained after aqueous extraction at pH 6 |
| SA9 | solid fraction obtained after aqueous extraction at pH 9 |
| SE3 | solid fraction obtained after enzymatic extraction at pH 3 |
| SE6 | solid fraction obtained after enzymatic extraction at pH 6 |
| SE9 | solid fraction obtained after enzymatic extraction at pH 9 |
| SFPC | solid fraction’s protein content |
| SFPR | solid fraction’s protein recovery |
| TAA | total amino acids |
| TPC | total phenolic content |
References
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K. Seaweeds as Prospective Marine Resources for the Development of Bioactive Pharmacophores and Nutraceuticals. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Ambati, R.R., Ravishankar, G.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 369–396. ISBN 978-3-030-92174-3. [Google Scholar]
- Lomartire, S.; Gonçalves, A.M.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar. Drugs 2023, 21, 261. [Google Scholar] [CrossRef]
- Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and Anti-Inflammatory Potential of Seaweed Extracts as Functional Ingredients. Hydrobiology 2022, 1, 469–482. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Packer, M.A.; Hayes, M. Angiotensin-I-Converting Enzyme Inhibitory Activity of Protein Hydrolysates Generated from the Macroalga Laminaria Digitata (Hudson) JV Lamouroux 1813. Foods 2022, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Appl. Sci. 2021, 11, 11261. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lu, Y.-A.; Kim, M.-Y.; Jeon, Y.-J.; Lee, S.-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J.; Gonçalves, A.M. Seaweed Proteins: A Step towards Sustainability? Nutrients 2024, 16, 1123. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Hajfathalian, M.; Jacobsen, C. Effect of Post-Extraction Ultrasonication on Compositional Features and Antioxidant Activities of Enzymatic/Alkaline Extracts of Palmaria Palmata. Mar. Drugs 2024, 22, 179. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gayen, K.; Bhowmick, T.K. Green Extraction of Biomolecules from Algae Using Subcritical and Supercritical Fluids. Biomass Convers. Biorefin 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus Vesiculosus by Pressurized Liquid Extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Gisbert, M.; Barcala, M.; Rosell, C.M.; Sineiro, J.; Moreira, R. Aqueous Extracts Characteristics Obtained by Ultrasound-Assisted Extraction from Ascophyllum Nodosum Seaweeds: Effect of Operation Conditions. J. Appl. Phycol. 2021, 33, 3297–3308. [Google Scholar] [CrossRef]
- Lee, Z.J.; Xie, C.; Duan, X.; Ng, K.; Suleria, H.A.R. Optimization of Ultrasonic Extraction Parameters for the Recovery of Phenolic Compounds in Brown Seaweed: Comparison with Conventional Techniques. Antioxidants 2024, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous Microwave-Assisted Step-by-Step Extraction of Bioactive Water-Soluble Materials and Fucoidan from Brown Seaweed Undaria Pinnatifida Waste. Biomass Convers. Biorefin 2024, 14, 7673–7682. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-Assisted Extraction of Proteins from the Seaweeds Macrocystis Pyrifera and Chondracanthus Chamissoi: Characterization of the Extracts and Their Bioactive Potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-Assisted Extraction and Characterization of Protein from Red Seaweed Palmaria Palmata. Algal Res. 2020, 47, 101849. [Google Scholar] [CrossRef]
- Veide Vilg, J.; Undeland, I. PH-Driven Solubilization and Isoelectric Precipitation of Proteins from the Brown Seaweed Saccharina Latissima—Effects of Osmotic Shock, Water Volume and Temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef]
- Sun, Z.; Chi, Q.; Sun, L.; Liu, Y. Protein Extraction from Microalgae Residue and Nutritional Assessment. Bioprocess. Biosyst. Eng. 2022, 45, 1879–1888. [Google Scholar] [CrossRef]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise Review of the Red Macroalga Dulse, Palmaria palmata (L.) Weber & Mohr. J. Appl. Phycol. 2023, 35, 523–550. [Google Scholar] [CrossRef]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.Ó.; Friðjónsson, Ó.H.; Jónsdóttir, R. Palmaria palmata as an Alternative Protein Source: Enzymatic Protein Extraction, Amino Acid Composition, and Nitrogen-to-Protein Conversion Factor. J. Appl. Phycol. 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Reza, T.; Jaczynski, J. Isoelectric Solubilization/Precipitation as a Means to Recover Protein and Lipids from Seafood By-Products. In Seafood Processing by-Products: Trends and Applications; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 101–123. ISBN 978-1-4614-9590-1. [Google Scholar]
- Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids. In The Complex World of Polysaccharides; Karunaratne, D.N., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Zheng, J.; Van der Meeren, P.; Sun, W. New Insights into Protein–Polysaccharide Complex Coacervation: Dynamics, Molecular Parameters, and Applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and Precipitation in Polysaccharide–Protein Systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Li, S.; Song, C.; Zhang, S.; Ren, J.; Udenigwe, C.C. Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications. Gels 2022, 8, 135. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on Plant Protein–Polysaccharide Complex Coacervation, and the Functionality and Applicability of Formed Complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Soussi Hachfi, R.; Hamon, P.; Rousseau, F.; Famelart, M.H.; Bouhallab, S. Ionic Strength Dependence of the Complex Coacervation between Lactoferrin and β-Lactoglobulin. Foods 2023, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yu, S.; Zhai, K.; Bao, N.; Rashed, M.M.A.; Wu, X. Fabrication and Characterization of Complex Coacervation: The Integration of Sesame Protein Isolate-Polysaccharides. Foods 2023, 12, 3696. [Google Scholar] [CrossRef]
- Chiu, T.-H.; Chen, M.-L.; Chang, H.-A. Comparisons of Emulsifying Properties of Maillard Reaction Products Conjugated by Green, Red Seaweeds and Various Commercial Proteins. Food Hydrocoll. 2009, 23, 2270–2277. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, G.; Wilson, K.R. Elucidating the Mechanism for the Reaction of O-Phthalaldehyde with Primary Amines in the Presence of Thiols. J. Phys. Chem. B 2023, 127, 3257–3265. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, X. Ortho-Phthalaldehyde (OPA)-Based Chemoselective Protein Bioconjugation and Peptide Cyclization. Methods Enzym. Enzymol. 2020, 639, 237–261. [Google Scholar] [CrossRef]
- Cruz-Solis, I.; Ibarra-Herrera, C.C.; Rocha-Pizaña, M.D.R.; Luna-Vital, D. Alkaline Extraction—Isoelectric Precipitation of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Hernández-Álvarez, A.J., Mondor, M., Nosworthy, M.G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–29. ISBN 978-3-031-16968-7. [Google Scholar] [CrossRef]
- Ying, Y.; Li, H. Recent Progress in the Analysis of Protein Deamidation Using Mass Spectrometry. Methods 2022, 200, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced Alkaline Extraction Techniques for Isolating and Modifying Plant-Based Proteins. Food Hydrocoll. 2023, 145, 109132. [Google Scholar] [CrossRef]
- Lewis, C.; Hughes, B.H.; Vasquez, M.; Wall, A.M.; Northrup, V.L.; Witzleb, T.J.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Effect of PH on the Binding of Sodium, Lysine, and Arginine Counterions to l-Undecyl Leucinate Micelles. J. Surfactants Deterg. 2016, 19, 1175–1188. [Google Scholar] [CrossRef]
- Kuang, J.; Tao, Y.; Song, Y.; Chemmalil, L.; Mussa, N.; Ding, J.; Li, Z.J. Understanding the Pathway and Kinetics of Aspartic Acid Isomerization in Peptide Mapping Methods for Monoclonal Antibodies. Anal. Bioanal. Chem. 2021, 413, 2113–2123. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, K.; Ji, W.; Kumar, V.B.; Rencus-Lazar, S.; Gazit, E. Histidine as a Key Modulator of Molecular Self-Assembly: Peptide-Based Supramolecular Materials Inspired by Biological Systems. Mater. Today 2022, 60, 106–127. [Google Scholar] [CrossRef]
- Liao, S.M.; Du, Q.S.; Meng, J.Z.; Pang, Z.W.; Huang, R.B. The Multiple Roles of Histidine in Protein Interactions. Chem. Cent. J. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Mirko, B.; Schendel, R.R. Determination of (Total) Phenolics and Antioxidant Capacity in Food and Ingredients. In Food Analysis; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 455–468. ISBN 978-3-319-45776-5. [Google Scholar]
- Bock, A.; Kieserling, H.; Steinhäuser, U.; Rohn, S. Impact of Phenolic Acid Derivatives on the Oxidative Stability of β-Lactoglobulin-Stabilized Emulsions. Antioxidants 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Maksoud, A.A.; Abd El-Ghany, I.H.; El-Beltagi, H.S.; Anankanbil, S.; Banerijee, C.; Petersen, S.V.; Pérez, B.; Guo, Z. Adding Functionality to Milk-Based Protein: Preparation, and Physico-Chemical Characterization of β-Lactoglobulin-Phenolic Conjugates. Food Chem. 2018, 241, 281–289. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’grady, M.N.; O’sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef]
- Aluko, R.E. 5-Amino Acids, Peptides, and Proteins as Antioxidants for Food Preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 105–140. ISBN 978-1-78242-089-7. [Google Scholar]
- Sheng, Y.; Wang, W.Y.; Wu, M.F.; Wang, Y.M.; Zhu, W.Y.; Chi, C.F.; Wang, B. Eighteen Novel Bioactive Peptides from Monkfish (Lophius litulon) Swim Bladders: Production, Identification, Antioxidant Activity, and Stability. Mar. Drugs 2023, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, X.-M.; Chi, Y.-M.; Wang, C.-F.; Kondracki, B.-; Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, PH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenserbaerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhao, Y.Q.; Wang, Y.M.; Zhao, W.H.; Wang, P.; Chi, C.F.; Wang, B. Antioxidant Peptides from Antarctic Krill (Euphausia superba) Hydrolysate: Preparation, Identification and Cytoprotection on H2O2-Induced Oxidative Stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Maleki, N.; Roomiani, L.; Tadayoni, M. Microwave-Assisted Extraction Optimization, Antimicrobial and Antioxidant Properties of Carrageenan from Red Algae (Gracilaria acerosa). J. Food Meas. Charact. 2023, 17, 1156–1166. [Google Scholar] [CrossRef]
- Ali, K.A.; Wahba, M.I.; Abou-Zeid, R.E.; Kamel, S. Development of Carrageenan Modified with Nanocellulose-Based Materials in Removing of Cu2+, Pb2+, Ca2+, Mg2+, and Fe2+. Int. J. Environ. Sci. Technol. 2019, 16, 5569–5576. [Google Scholar] [CrossRef]
- Lawson, M.K.; Valko, M.; Cronin, M.T.D.; Jomová, K. Chelators in Iron and Copper Toxicity. Curr. Pharmacol. Rep. 2016, 2, 271–280. [Google Scholar] [CrossRef]
- Milach, O.A.; Mel’sitova, I.V.; Yurkova, I.L. Pro(Anti)Oxidant Properties of Amino Acids and Their Derivatives in The Presence of Fe2+ and Cu2+ Ions. Russ. J. Gen. Chem. 2020, 90, 987–993. [Google Scholar] [CrossRef]
- Hau, E.H.; Teh, S.S.; Yeo, S.K.; Chua, B.L.; Owatworakit, A.; Xiao, J.; Mah, S.H. Physicochemical and Functional Properties of Flavourzyme-Extracted Protein Hydrolysate from Oil Palm Leaves. Biomass Convers. Biorefin 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Bjørlie, M.; Hartmann, J.C.; Rasmussen, L.H.; Yesiltas, B.; Sørensen, A.D.M.; Gregersen Echers, S.; Jacobsen, C. Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics. Antioxidants 2024, 13, 346. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
| Aqueous Extraction on Seaweed | Enzymatic Extraction on Solid Fractions from Aqueous Extraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liquid Fraction (LA) | Solid Fraction (SA) | Liquid Fraction (LE) | Solid Fraction (SE) | |||||||||
| pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | pH 3 | pH 6 | pH 9 | |
| Phenylalanine * | 0.40 ± 0.01 c | 0.63 ± 0.04 c | 0.63 ± 0.03 c | 6.21 ± 0.59 b | 5.53 ± 0.31 b | 5.02 ± 0.52 b | 1.09 ± 0.14 c | 0.73 ± 0.18 c | 0.77 ± 0.46 c | 12.04 ± 1.13 a | 11.89 ± 1.43 a | 10.91 ± 0.72 a |
| Leucine * | 0.75 ± 0.04 c | 0.92 ± 0.11 c | 0.94 ± 0.09 c | 11.59 ± 1.30 b | 10.38 ± 0.50 b | 9.23 ± 1.01 b | 1.66 ± 0.23 c | 1.15 ± 0.32 c | 1.30 ± 0.52 c | 21.18 ± 2.28 a | 21.22 ± 2.58 a | 19.34 ± 1.62 a |
| Isoleucine * | 0.42 ± 0.03 c | 0.55 ± 0.06 c | 0.54 ± 0.06 c | 6.40 ± 0.69 b | 5.69 ± 0.37 b | 5.19 ± 0.62 b | 0.98 ± 0.16 c | 0.64 ± 0.18 c | 0.79 ± 0.34 c | 11.85 ± 1.14 a | 11.91 ± 1.68 a | 10.92 ± 0.92 a |
| Methionine * | 0.19 ± 0.03 c | 0.28 ± 0.06 c | 0.28 ± 0.03 c | 2.96 ± 0.35 b | 2.68 ± 0.19 b | 2.42 ± 0.31 b | 0.40 ± 0.04 c | 0.27 ± 0.04 c | 0.32 ± 0.12 c | 5.63 ± 0.55 a | 5.73 ± 0.68 a | 5.24 ± 0.39 a |
| Tyrosine * | 0.41 ± 0.04 c | 0.71 ± 0.27 c | 0.71 ± 0.04 c | 6.88 ± 0.70 b | 5.99 ± 0.31 b | 5.51 ± 0.55 b | 1.14 ± 0.08 c | 0.65 ± 0.18 c | 0.74 ± 0.40 c | 14.08 ± 0.32 a | 13.65 ± 0.73 a | 12.64 ± 0.70 a |
| Proline | 2.71 ± 0.05 c | 2.91 ± 0.29 c | 2.86 ± 0.10 c | 8.39 ± 0.85 b | 7.40 ± 0.29 b | 6.95 ± 0.77 b | 2.74 ± 0.11 c | 2.22 ± 0.28 c | 1.76 ± 0.55 c | 15.45 ± 1.48 a | 15.84 ± 1.66 a | 14.38 ± 0.86 a |
| Valine * | 1.04 ± 0.05 c | 1.13 ± 0.13 c | 1.12 ± 0.08 c | 12.35 ± 1.34 b | 10.95 ± 0.46 b | 10.11 ± 1.09 b | 2.22 ± 0.30 c | 1.88 ± 0.31 c | 1.95 ± 0.28 c | 24.00 ± 2.34 a | 23.88 ± 2.33 a | 21.83 ± 1.70 a |
| Alanine | 2.49 ± 0.15 c | 2.41 ± 0.32 c | 2.27 ± 0.28 c | 13.88 ± 1.28 b | 12.45 ± 0.67 b | 11.69 ± 1.30 b | 4.25 ± 0.62 c | 3.80 ± 0.29 c | 4.11 ± 0.24 c | 25.36 ± 2.32 a | 26.33 ± 2.94 a | 23.76 ± 1.53 a |
| Threonine * | 1.21 ± 0.07 c | 1.40 ± 0.23 c | 1.38 ± 0.11 c | 8.55 ± 1.05 b | 7.83 ± 0.39 b | 6.88 ± 1.43 b | 1.57 ± 0.22 c | 1.59 ± 0.25 c | 1.59 ± 0.36 c | 18.34 ± 1.65 a | 18.89 ± 1.97 a | 17.16 ± 1.09 a |
| Glycine | 2.39 ± 0.38 c | 3.00 ± 0.41 c | 2.93 ± 0.31 c | 13.02 ± 1.12 b | 11.80 ± 0.90 b | 10.92 ± 1.09 b | 3.56 ± 0.45 c | 2.76 ± 0.55 c | 2.75 ± 0.73 c | 22.82 ± 1.86 a | 23.23 ± 1.99 a | 21.33 ± 1.29 a |
| Serine | 3.12 ± 0.75 c | 2.62 ± 0.53 c | 2.59 ± 0.29 c | 13.20 ± 1.28 b | 12.32 ± 0.64 b | 11.18 ± 1.07 b | 3.11 ± 0.58 c | 3.35 ± 0.54 c | 2.91 ± 0.75 c | 22.58 ± 2.12 a | 22.74 ± 2.27 a | 21.32 ± 1.22 a |
| Arginine | 0.68 ± 0.24 e | 0.74 ± 0.24 e | 0.83 ± 0.18 e | 10.90 ± 0.94 c | 9.21 ± 0.45 cd | 8.24 ± 0.74 d | 1.03 ± 0.11 e | 0.99 ± 0.35 e | 1.06 ± 0.40 e | 20.39 ± 1.80 a | 20.37 ± 1.98 a | 18.10 ± 1.10 b |
| Histidine * | ND ** | ND | ND | 3.31 ± 0.64 b | 2.76 ± 0.29 bc | 2.32 ± 0.30 c | ND | ND | ND | 5.06 ± 0.59 a | 5.46 ± 0.70 a | 4.83 ± 0.39 a |
| Glutamic acid | 9.52 ± 0.24 def | 11.80 ± 0.89 d | 11.03 ± 0.44 de | 23.09 ± 1.98 b | 20.24 ± 0.82 bc | 18.86 ± 2.15 c | 7.85 ± 0.41 ef | 6.08 ± 0.86 f | 6.23 ± 1.44 f | 35.15 ± 2.72 a | 35.44 ± 3.66 a | 32.13 ± 2.31 a |
| Cystine * | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.09 ± 0.67 a | 1.41 ± 0.50 b | 1.27 ± 0.27 b |
| Aspartic acid | 8.62 ± 0.67 d | 8.43 ± 0.62 d | 9.22 ± 0.43 d | 24.19 ± 3.10 b | 21.68 ± 1.30 bc | 20.06 ± 2.21 c | 5.95 ± 0.51 de | 4.46 ± 0.67 e | 5.55 ± 1.34 de | 36.18 ± 2.79 a | 37.29 ± 3.41 a | 34.27 ± 1.77 a |
| TAA *** | 33.95 ± 1.57 d | 37.51 ± 2.97 d | 37.34 ± 1.27 d | 164.92 ± 16.40 b | 146.90 ± 6.90 bc | 134.58 ± 14.70 c | 37.54 ± 3.57 d | 30.56 ± 3.30 d | 31.84 ± 7.19 d | 292.20 ± 25.73 a | 295.29 ± 30.84 a | 269.45 ± 16.81 a |
| EAA | 0.42 ± 0.14 c | 5.61 ± 0.69 c | 5.60 ± 0.20 c | 58.26 ± 6.15 b | 51.80 ± 2.05 b | 46.67 ± 5.60 b | 9.05 ± 1.08 c | 6.91 ± 1.22 c | 7.46 ± 2.45 c | 114.26 ± 10.94 a | 114.04 ± 13.12 a | 104.15 ± 7.05 a |
| EAA/TAA | 0.130 ± 0.002 d | 0.149 ± 0.009 d | 0.150 ± 0.005 d | 0.353 ± 0.004 b | 0.353 ± 0.003 b | 0.346 ± 0.004 b | 0.241 ± 0.006 c | 0.224 ± 0.016 c | 0.230 ± 0.029 c | 0.391 ± 0.005 a | 0.386 ± 0.005 a | 0.386 ± 0.003 a |
| Extraction Method | pH Value | IC50 (mg.mL−1) for DPPH Radical Scavenging Activity | IC50 (mg.mL−1) for Fe2+ Chelating Activity | ||
|---|---|---|---|---|---|
| Liquid Fraction | Solid Fraction | Liquid Fraction | Solid Fraction | ||
| Aqueous extraction on seaweed (LA and SA) | 3 | 9.31 ± 0.19 c | 3.97 ± 0.07 a | NR * | 4.81 ± 0.05 bc |
| 6 | 9.03 ± 2.72 c | 2.85 ± 0.08 a | 5.06 ± 0.55 c | 11.84 ± 2.50 ef | |
| 9 | NR | 8.15 ± 0.02 bc | 1.26 ± 0.07 a | 8.97 ± 0.36 de | |
| Enzymatic extraction on solid fraction from aqueous extraction (LE and SE) | 3 | 10.41 ± 0.51 c | 2.29 ± 1.00 a | 5.52 ± 0.60 cd | 0.63 ± 0.04 a |
| 6 | 10.09 ± 0.57 c | 2.92 ± 0.02 a | 14.60 ± 0.15 f | 0.89 ± 0.07 a | |
| 9 | NR | 4.38 ± 0.17 ab | 8.92 ± 1.26 de | 1.35 ± 0.11 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghelichi, S.; Sørensen, A.-D.M.; Náthia-Neves, G.; Jacobsen, C. pH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria palmata: A Sequential Approach. Mar. Drugs 2024, 22, 413. https://doi.org/10.3390/md22090413
Ghelichi S, Sørensen A-DM, Náthia-Neves G, Jacobsen C. pH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria palmata: A Sequential Approach. Marine Drugs. 2024; 22(9):413. https://doi.org/10.3390/md22090413
Chicago/Turabian StyleGhelichi, Sakhi, Ann-Dorit Moltke Sørensen, Grazielle Náthia-Neves, and Charlotte Jacobsen. 2024. "pH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria palmata: A Sequential Approach" Marine Drugs 22, no. 9: 413. https://doi.org/10.3390/md22090413
APA StyleGhelichi, S., Sørensen, A.-D. M., Náthia-Neves, G., & Jacobsen, C. (2024). pH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria palmata: A Sequential Approach. Marine Drugs, 22(9), 413. https://doi.org/10.3390/md22090413









