Optimizing Antioxidant Potential: Factorial Design-Based Formulation of Fucoidan and Gallic Acid-Conjugated Dextran Blends
Abstract
:1. Introduction
2. Results
2.1. Formulation of Polysaccharide Blends Incorporating FucA and Dex-Gal
Conjugation of Gallic Acid with Dextran
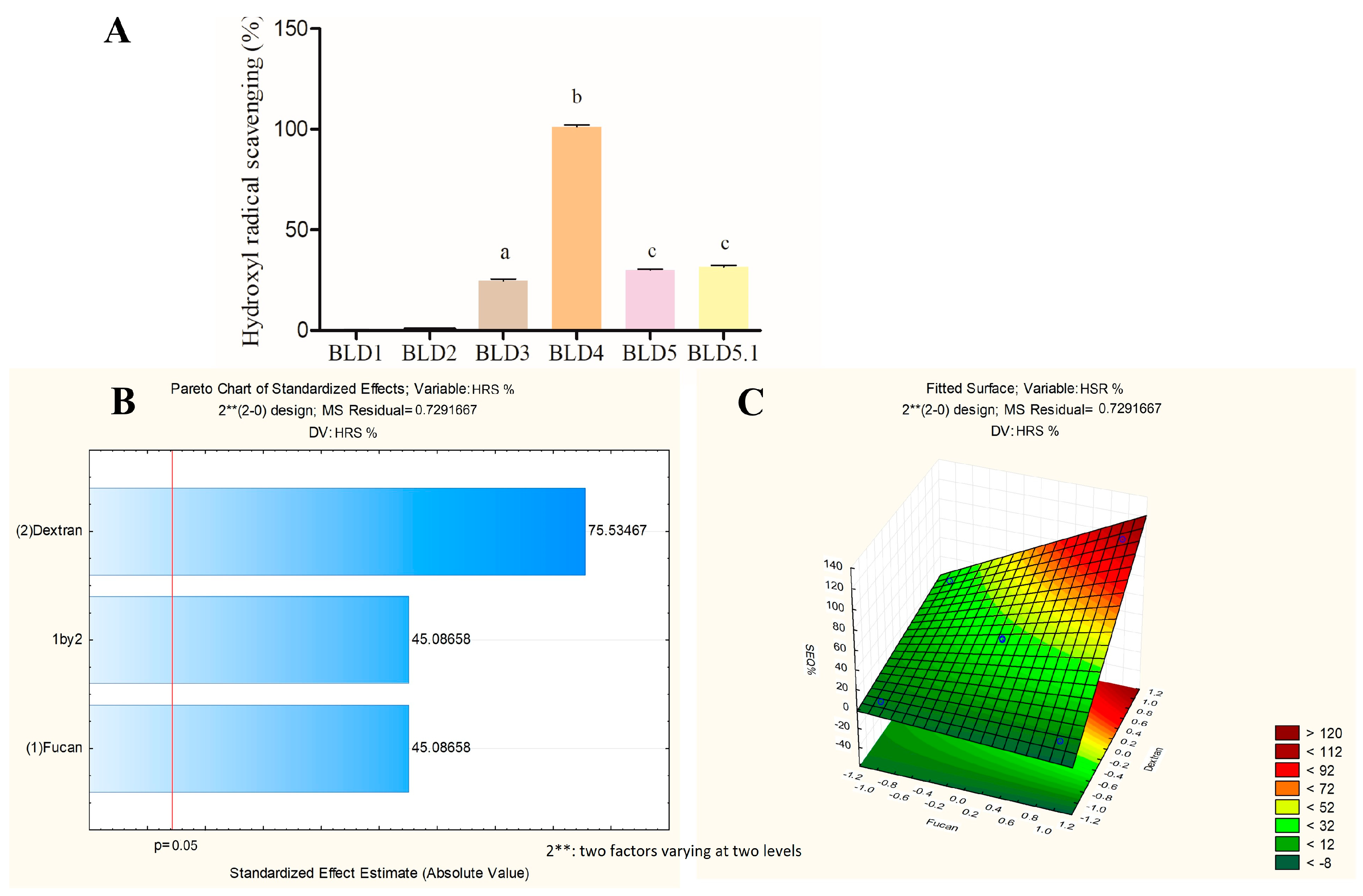
2.2. An Assessment of the Influence of the Amount of FucA and Dex-Gal on the Antioxidant Activity of the Blends Obtained
2.3. Assessment of BLD4 Cytotoxicity in 3T3 Cells
2.4. Effect of BLD4 on H2O2-Induced Oxidative Stress in 3T3 Cells
3. Materials and Methods
3.1. Materials
3.2. Conjugation of Dextran with Gallic Acid (GA)
3.3. The Determination of the GA Percentage
3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.5. Formulation of Blends Containing FucA and Dex-Gal Using Factorial Design
3.6. In Vitro Antioxidant Tests
3.6.1. Total Antioxidant Capacity (TAC)
3.6.2. Reducing Power Assay
3.6.3. Hydroxyl Radical Scavenging Activity Assay
3.7. Antioxidant Tests on 3T3 Cell Lines
3.7.1. Assessment of Cellular Cytotoxicity
3.7.2. Assessment of Oxidative Stress Induced by Hydrogen Peroxide (H2O2)
Determination of Oxidative Stress Condition
Assessment of Concomitant Effect
Assessment of Preventive Effect
3.8. Statistical Analyzes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic exogenous and endogenous antioxidants. Free Radic. Biol. Med. 2019, 1, 11–22. [Google Scholar] [CrossRef]
- Pasini, A.M.; Cominacini, L. Potential Benefits of Antioxidant Phytochemicals on Endogenous Antioxidants Defences in Chronic Diseases. Antioxidants 2023, 12, 890. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef]
- Arunkumar, K.; Raja, R.; Kumar, V.B.S.; Joseph, A.; Shilpa, T.; Carvalho, I.S. Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. Food Meas. 2021, 15, 567–576. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxid. Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Silva, M.M.C.L.; Dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Fu, Y.; Jiao, H.; Sun, J.; Okoye, C.O.; Zhang, H.; Li, Y.; Lu, X.; Wang, Q.; Liu, J. Structure-activity relationships of bioactive polysaccharides extracted from macroalgae towards biomedical application: A review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef]
- Rocha, H.A.; Moraes, F.A.; Trindade, E.S.; Franco, C.R.; Torquato, R.J.; Veiga, S.S.; Valente, A.P.; Mourão, P.A.; Leite, E.L.; Nader, H.B.; et al. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schröederi. An ideal antithrombotic agent? J. Biol. Chem. 2005, 280, 41278–41288. [Google Scholar] [CrossRef]
- Rocha, H.A.; Bezerra, L.C.; de Albuquerque, I.R.; Costa, L.S.; Guerra, C.M.; de Abreu, L.D.; Nader, H.B.; Leite, E.L. A xylogalactofucan from the brown seaweed Spatoglossum schröederi stimulates the synthesis of an antithrombotic heparan sulfate from endothelial cells. Planta Med. 2005, 71, 379–381. [Google Scholar] [CrossRef]
- Leite, E.D.; Medeiros, M.G.L.; Rocha, H.A.O.; Farias, G.G.M.; Silva, L.F.; Chavante, S.F.; Abreu, L.R.D.; Dietrich, C.P.; Nader, H.B. Structure and pharmacological activities of sulfated xylofucoglucuronan from the alga Spatoglossum schröederi. Plant Sci. 1998, 132, 215–228. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Melo-Silveira, R.F.; Silva, F.V.; Felipe, M.B.; Medeiros, S.R.; Leite, E.L.; Rocha, H.A. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef]
- Dantas-Santos, N.; Almeida-Lima, J.; Vidal, A.A.J.; Lopes Gomes, D.; Medeiros Oliveira, R.; Santos Pedrosa, S.; Pereira, P.; Gama, F.M.; Oliveira Rocha, H.A. Antiproliferative activity of fucan nanogel. Mar. Drugs 2012, 10, 2002–2022. [Google Scholar] [CrossRef]
- Souza, A.O.; Oliveira, J.W.d.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity. Nanomaterials 2022, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Souza, I.; Pessatti, J.B.K.; da Silva, L.R.; de Lima Bellan, D.; de Souza, I.R.; Cestari, M.M.; de Assis, H.C.S.; Rocha, H.A.O.; Simas, F.F.; da Silva Trindade, E.; et al. Protective potencial of sulfated polysaccharides from tropical seaweeds against alkylating- and oxidizing-induced genotoxicity. Int. J. Biol. Macromol. 2022, 211, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Souza, C.R.M.; Silva, H.M.D.; Jales, J.T.; Gomez, L.A.S.; da Silveira, E.J.D.; Rocha, H.A.O.; Souto, J.T. Anti-Inflammatory Activity of Fucan from Spatoglossum schröederi in a Murine Model of Generalized Inflammation Induced by Zymosan. Mar. Drugs 2023, 21, 557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic Acid-Dextran Conjugate: Green Synthesis of a Novel Antioxidant. Antioxidants 2019, 8, 478. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 98, 865–872. [Google Scholar] [PubMed]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Abas, F.; Lai, O.M.; Ho, C.W.; Yin, J.; Hedegaard, R.V.; Skibsted, L.H.; Tan, C.P. Antioxidant synergism between ethanolic Centella asiatica extracts and α-tocopherol in model systems. Food Chem. 2013, 138, 1215–1219. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Man, Y.; Li, N.; Zhou, Y.U. Effects of vitamins E and C combined with β-carotene on cognitive function in the elderly. Exp. Ther. Med. 2015, 9, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Campanelli-Morais, Y.; Silva, C.H.F.; Dantas, M.R.d.N.; Sabry, D.A.; Sassaki, G.L.; Moreira, S.M.G.; Rocha, H.A.O. A Blend Consisting of Agaran from Seaweed Gracilaria birdiae and Chromium Picolinate Is a Better Antioxidant Agent than These Two Compounds Alone. Mar. Drugs 2023, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Tokiwa, Y. Polymerization of vinyl sugar ester using ascorbic acid and hydrogen peroxide as a redox reagent. Carbohydr. Polym. 2006, 64, 218–223. [Google Scholar] [CrossRef]
- Yi, J.; Huang, H.; Wen, Z.; Fan, Y. Fabrication of chitosan-gallic acid conjugate for improvement of physicochemical stability of β-carotene nanoemulsion: Impact of Mw of chitosan. Food Chem. 2021, 362, 130218. [Google Scholar] [CrossRef]
- Fernandes-Negreiros, M.M.; Batista, L.A.N.C.; Viana, R.L.S.; Sabry, D.A.; Paiva, A.A.O.; Paiva, W.S.; Machado, R.I.A.; Sousa Junior, F.L.; Lima, D.L.; Vitoriano, J.O.; et al. Gallic Acid-Laminarin Conjugate Is a Better Antioxidant than Sulfated or Carboxylated Laminarin. Antioxidants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Paiva, W.S.; Queiroz, M.F.; Araujo Sabry, D.; Santiago, A.L.C.M.A.; Sassaki, G.L.; Batista, A.C.L.; Rocha, H.A.O. Preparation, Structural Characterization, and Property Investigation of Gallic Acid-Grafted Fungal Chitosan Conjugate. J. Fungi 2021, 7, 812. [Google Scholar] [CrossRef]
- Melo, K.; Lisboa, L.S.; Queiroz, M.F.; Paiva, W.S.; Luchiari, A.C.; Camara, R.B.G.; Costa, L.S.; Rocha, H.A.O. Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method. Mar. Drugs 2022, 20, 490. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Nan, Z.; Li, Y.; Ma, J.; Ding, J.; Lv, Y.; Yang, J. Detection of dextran, maltodextrin and soluble starch in the adulterated Lycium barbarum polysaccharides (LBPs) using Fourier-transform infrared spectroscopy (FTIR) and machine learning models. Heliyon 2023, 9, e17115. [Google Scholar] [CrossRef]
- Hernández, C.A.; Pérez-Bernal, M.; Abreu, D.; Valdivia, O.; Delgado, M.; Dorta, D.; Domínguez, A.G.; Pérez, E.R.; Sánchez-Ríos, J.M. Step-by-step full factorial design to optimize a quantitative sandwich ELISA. Anal. Biochem. 2023, 674, 115195. [Google Scholar] [CrossRef]
- Ashfaq, R.; Sisa, B.; Kovács, A.; Berkó, S.; Szécsényi, M.; Burián, K.; Vályi, P.; Budai-Szűcs, M. Factorial design of in situ gelling two-compartment systems containing chlorhexidine for the treatment of periodontitis. Eur. J. Pharm. Sci. 2023, 191, 106607. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wires Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 3, 171–185. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and Antioxidant Activity of the Complex of Tea Polyphenols and Oat β-Glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Z.; Ma, H.; Wang, S.; Xie, W.; Chen, Y.; Xie, C.; Guo, A.; Wang, C.; Zheng, M. Characterisation and antioxidant activity of polysaccharide iron (III) complex in Qingzhuan Dark Tea. Food Sci. Technol. 2022, 42, e119421. [Google Scholar] [CrossRef]
- Fuso, A.; Dejonghe, W.; Cauwenberghs, L.; Rosso, G.; Rosso, F.; Manera, I.; Caligiani, A. DPPH radical scavenging activity of xylo-oligosaccharides mixtures of controlled composition: A step forward in understanding structure–activity relationship. J. Funct. Foods 2023, 101, 105417. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 14, 129. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Padilha, C.E.; Fortunato Dantas, P.V.; de Sousa, F.C.J.; de Santana Souza, D.F.; de Oliveira, J.A.; de Macedo, G.R.; dos Santos, E.S. Mathematical modeling of the whole expanded bed adsorption process to recover and purify chitosanases from the unclarified fermentation broth of Paenibacillus ehimensis. J. Chromatogr. B 2016, 1039, 44–50. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [PubMed]







| Group | O-H | C-H | C=O (GA Ester) | C-C | C-O | α-(1→6) | C-O-C | α-D-Glucose |
|---|---|---|---|---|---|---|---|---|
| Dex | 3394 | 2924 | - | - | 1461 | 1022 | 1129 | 869 |
| Dex-Gal | 3392 | 2927 | 1743 | 1543 | 1460 | 1020 | 1127 | 866 |
| Response Variables | |||
|---|---|---|---|
| Samples | TAC 1 | Hydroxyl Radical Scavenging (%) | Reducing Power (%) |
| BLD1 | 0.0 | 0.0 | 0.0 |
| BLD2 | 12.1 | 0.0 | 17.0 |
| BLD3 | 16.7 | 26.0 | 12.0 |
| BLD4 | 23.1 | 103.0 | 19.7 |
| BLD5 | 12.7 | 31.0 | 14.0 |
| BLD5.1 | 11.6 | 32.5 | 13.0 |
| Factor | Levels | ||
|---|---|---|---|
| (−1) | 0 | (+1) | |
| FucA | 0% | 50% | 100% |
| Dex-Gal | 0% | 50% | 100% |
| Sample | FucA Level | Dex-Gal Level |
|---|---|---|
| BLD1 | −1 | −1 |
| BLD2 | +1 | −1 |
| BLD3 | −1 | +1 |
| BLD4 | +1 | +1 |
| BLD5 | 0 | 0 |
| BLD5.1 | 0 | 0 |
| FucA | Dex-Gal | |||
|---|---|---|---|---|
| Sample | Proportion | Concentration (mM) | Proportion | Concentration (mM) |
| BLD1 | 0.0 | 0.0 | 0.0 | 0.0 |
| BLD2 | 100% | 0.5 | 0.0 | 0.0 |
| BLD3 | 0.0 | 0.0 | 100% | 0.5 |
| BLD4 | 100% | 0.5 | 100% | 0.5 |
| BLD5 | 50% | 0.25 | 50% | 0.25 |
| BLD5.1 | 50% | 0.25 | 50% | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.H.F.; Silva, M.M.C.L.; Paiva, W.S.; de Medeiros, M.J.C.; Queiroz, M.F.; Matta, L.D.M.; dos Santos, E.S.; Rocha, H.A.O. Optimizing Antioxidant Potential: Factorial Design-Based Formulation of Fucoidan and Gallic Acid-Conjugated Dextran Blends. Mar. Drugs 2024, 22, 417. https://doi.org/10.3390/md22090417
Silva CHF, Silva MMCL, Paiva WS, de Medeiros MJC, Queiroz MF, Matta LDM, dos Santos ES, Rocha HAO. Optimizing Antioxidant Potential: Factorial Design-Based Formulation of Fucoidan and Gallic Acid-Conjugated Dextran Blends. Marine Drugs. 2024; 22(9):417. https://doi.org/10.3390/md22090417
Chicago/Turabian StyleSilva, Cynthia Haynara Ferreira, Maylla Maria Correia Leite Silva, Weslley Souza Paiva, Mayara Jane Campos de Medeiros, Moacir Fernandes Queiroz, Luciana Duarte Martins Matta, Everaldo Silvino dos Santos, and Hugo Alexandre Oliveira Rocha. 2024. "Optimizing Antioxidant Potential: Factorial Design-Based Formulation of Fucoidan and Gallic Acid-Conjugated Dextran Blends" Marine Drugs 22, no. 9: 417. https://doi.org/10.3390/md22090417







