Regulation of Safracin Biosynthesis and Transport in Pseudomonas poae PMA22
Abstract
:1. Introduction
2. Results
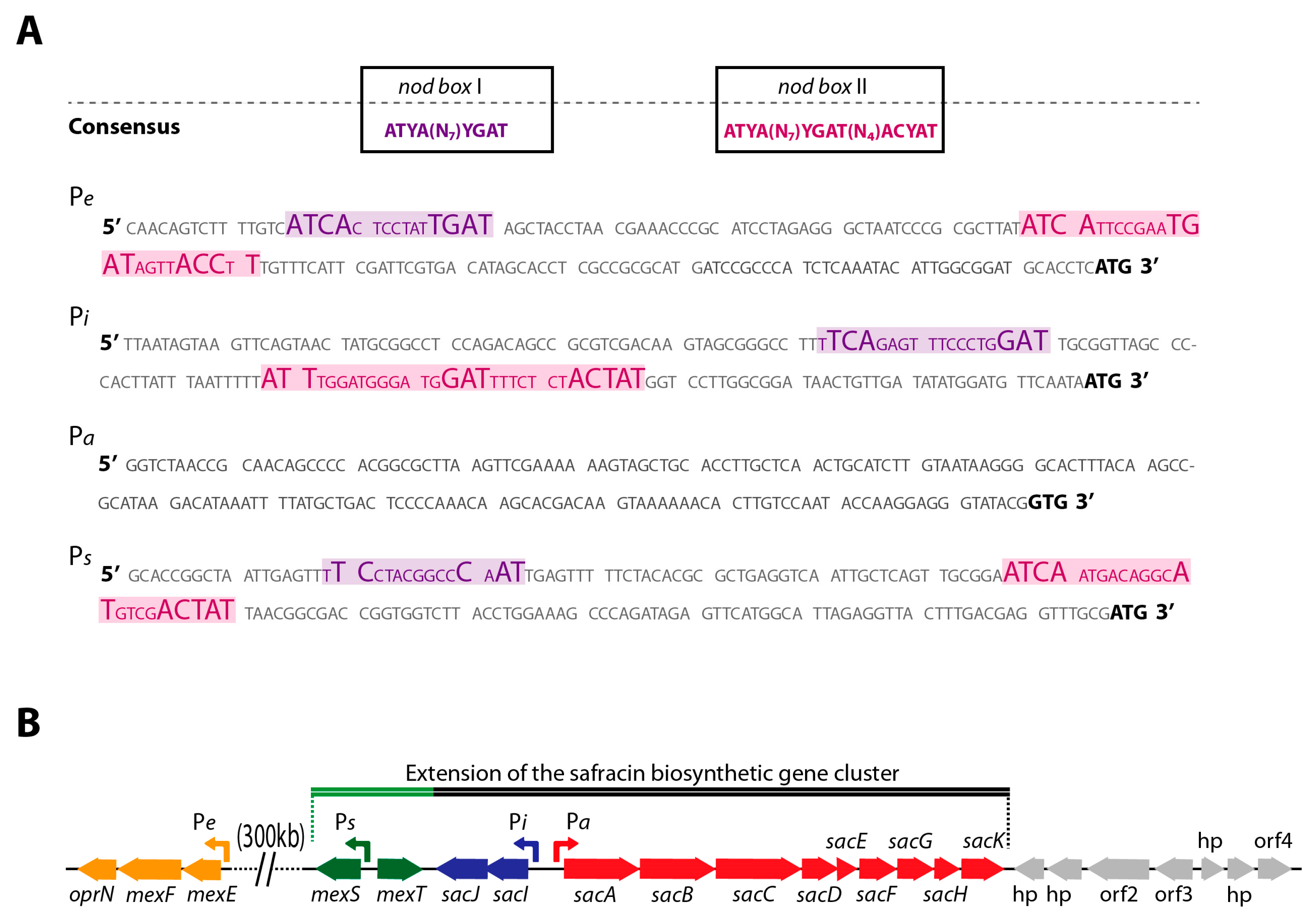
2.1. Co-Localization of mexT and BGC Sac in P. poae PMA22 Genome
2.2. MexT and MexS Are Functional in P. poae PMA22
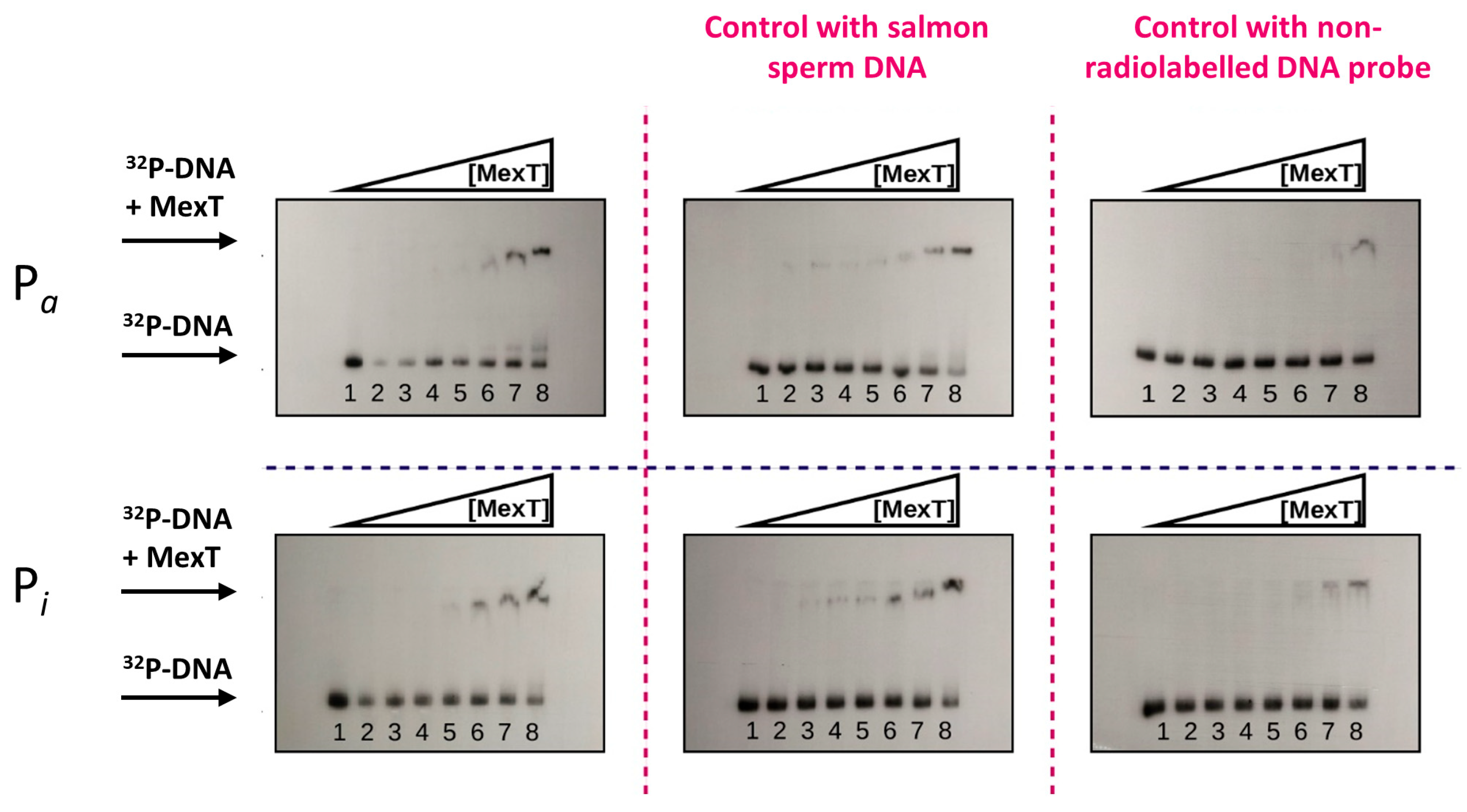
2.3. In Vitro Interaction of MexT with Pa and Pi Promoters
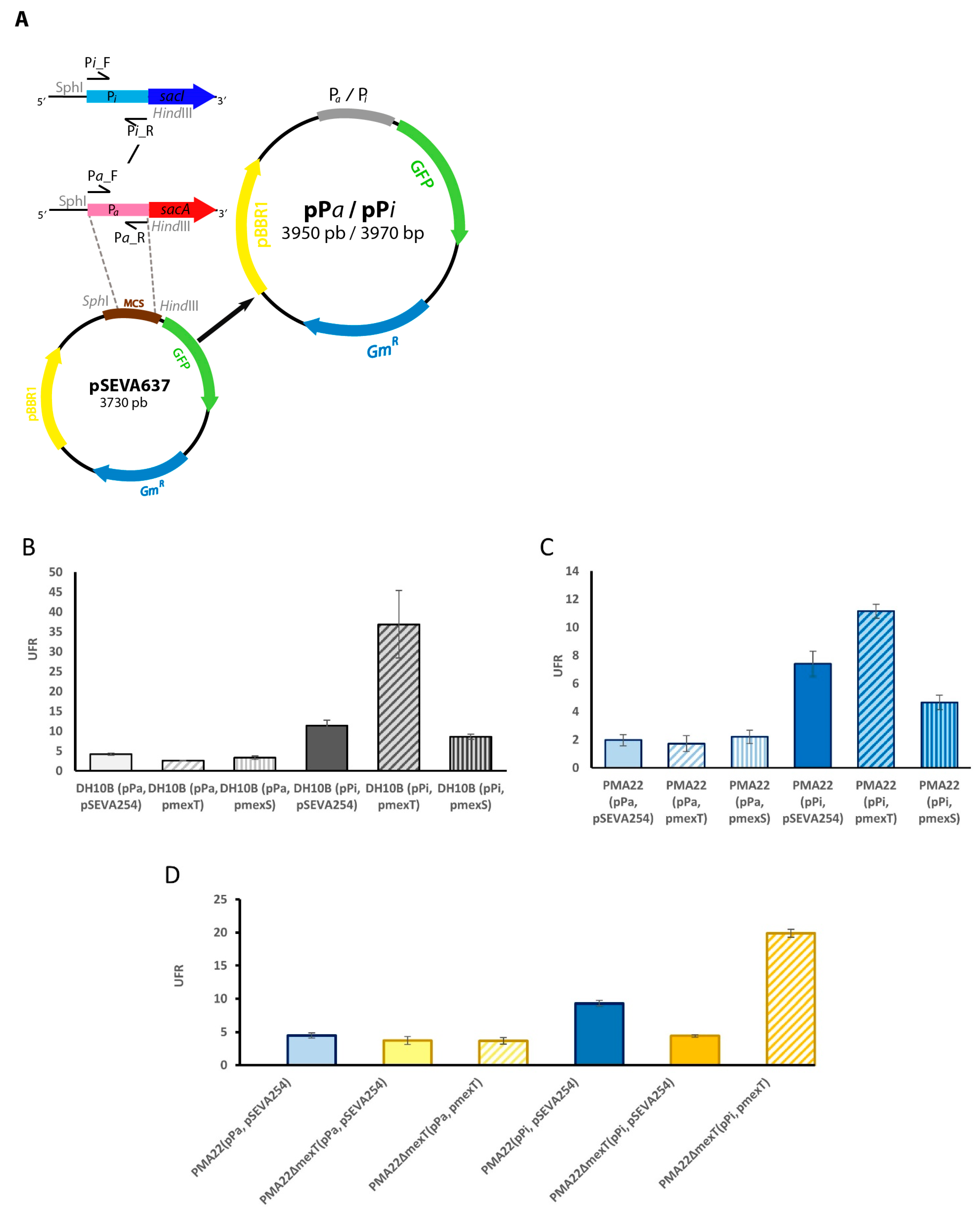
2.4. Role of MexT in the Expression of BGC Sac
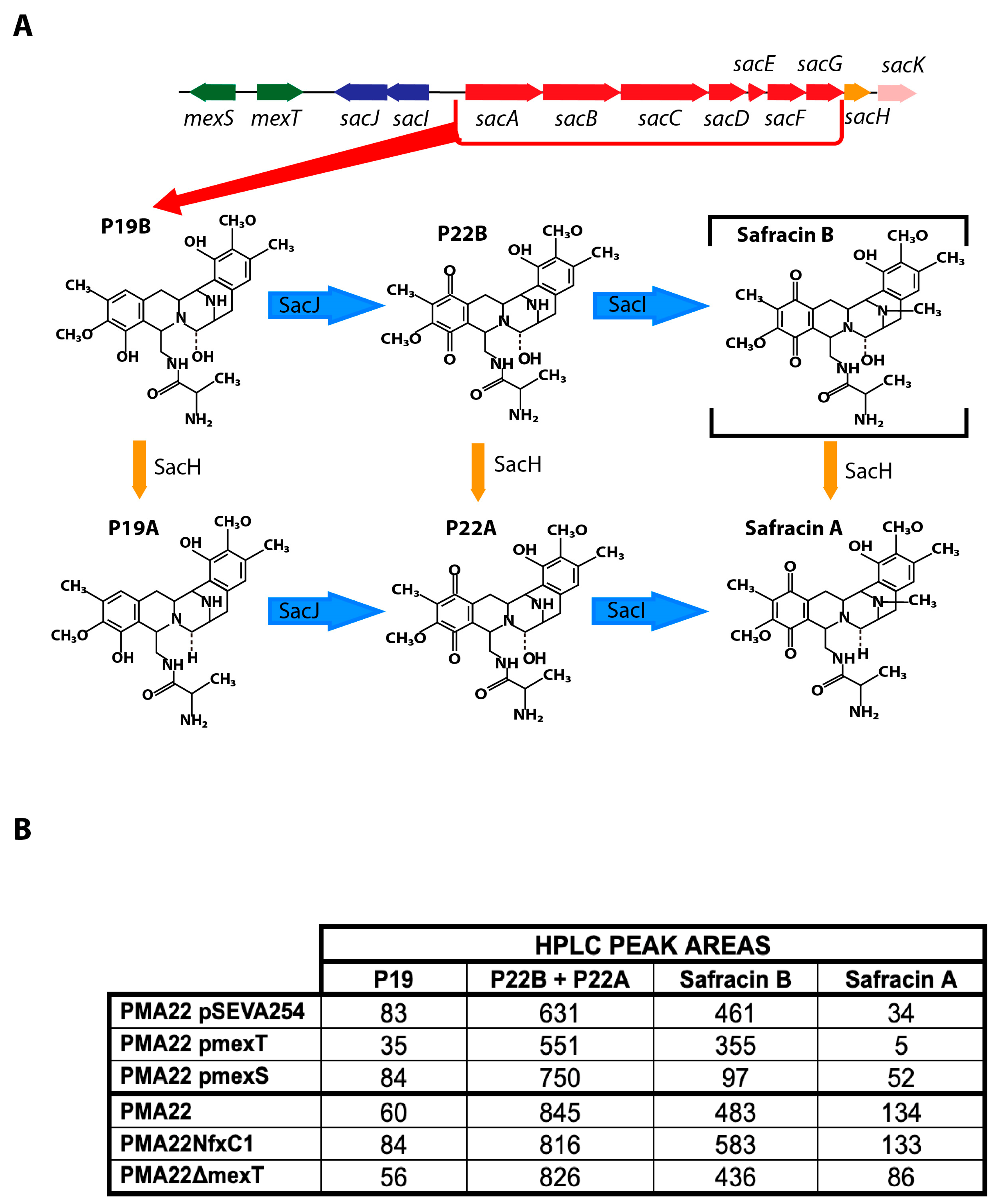
2.5. Effect of MexT and MexS on Safracin Production
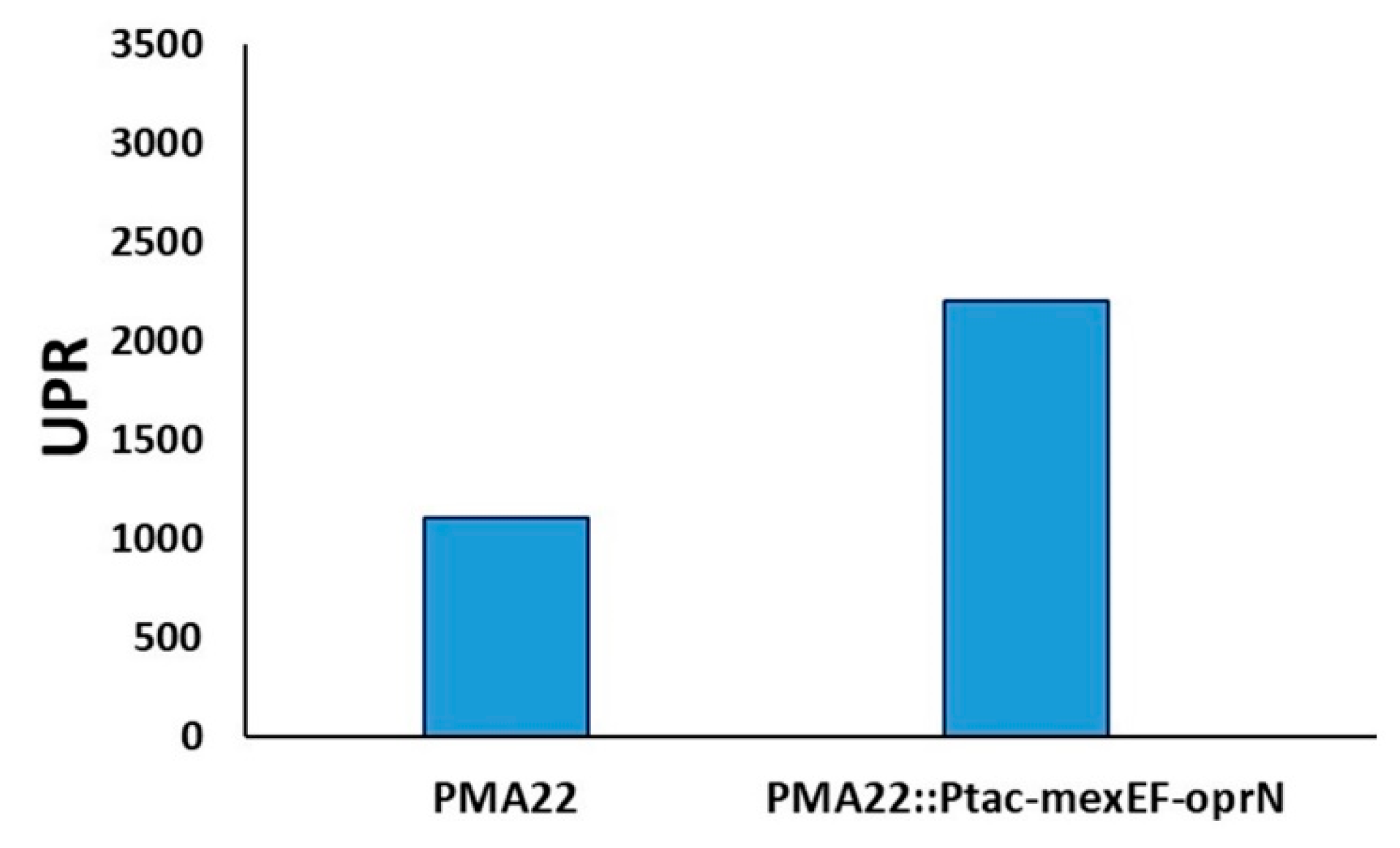
2.6. Implication of MexEF OprN Efflux Pump in the Transport of Safracin
3. Materials and Methods
3.1. Bacterial Strains, Plasmids, Primers, Media, and Growth Conditions
3.2. DNA Manipulation
3.3. MexT Overproduction and Purification
3.4. Electro-Mobility Shift Assays (EMSA)
3.5. Construction of P. poae PMA22 Mutant Strains
3.6. Complementation of P. poae PAM22 Knockout Strains
3.7. Production and Extraction of Safracins
3.8. Analysis of Safracin Production
3.9. Fluorescence Measurements
3.10. Bioinformatic Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marahiel, M.A.; Essen, L.O. Chapter 13 Nonribosomal Peptide Synthetases. Mechanistic and Structural Aspects of Essential Domains. Methods Enzymol. 2009, 458, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Duban, M.; Cociancich, S.; Leclère, V. Nonribosomal peptide synthesis definitely working out of the rules. Microorganisms 2022, 10, 577. [Google Scholar] [CrossRef]
- Amiri Moghaddam, J.; Jautzus, T.; Alanjary, M.; Beemelmanns, C. Recent highlights of biosynthetic studies on marine natural products. Org. Biomol. Chem. 2021, 19, 123–140. [Google Scholar] [CrossRef]
- Méndez, C.; Salas, J.A. The role of ABC transporters in antibiotic-producing organisms: Drug secretion and resistance mechanisms. Res. Microbiol. 2001, 152, 341–350. [Google Scholar] [CrossRef]
- Martín, J.F.; Casqueiro, J.; Liras, P. Secretion systems for secondary metabolites: How producer cells send out messages of intercellular communication. Curr. Opin. Microbiol. 2005, 8, 282–293. [Google Scholar] [CrossRef]
- Severi, E.; Thomas, G.H. Antibiotic export: Transporters involved in the final step of natural product production. Microbiology 2019, 165, 805–817. [Google Scholar] [CrossRef]
- Ullán, R.V.; Casqueiro, J.; Bañuelos, O.; Fernández, F.J.; Gutiérrez, S.; Martín, J.F. A Novel epimerization system in fungal secondary metabolism involved in the conversion of isopenicillin N into penicillin N in Acremonium chrysogenum. J. Biol. Chem. 2002, 277, 46216–46225. [Google Scholar] [CrossRef]
- Teijeira, F.; Ullán, R.V.; Guerra, S.M.; García-Estrada, C.; Vaca, I.; Martín, J.F. The transporter CefM involved in translocation of biosynthetic intermediates is essential for cephalosporin production. Biochem. J. 2009, 418, 113–124. [Google Scholar] [CrossRef]
- Ullán, R.V.; Teijeira, F.; Guerra, S.M.; Vaca, I.; Martín, J.F. Characterization of a novel peroxisome membrane protein essential for conversion of isopenicillin N into cephalosporin C. Biochem. J. 2010, 432, 227–236. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Bhattacharya, N.; Olm, M.R.; Song, Y.S.; Banfield, J.F. Transporter genes in biosynthetic gene clusters predict metabolite characteristics and siderophore activity. Genome Res. 2021, 31, 239–250. [Google Scholar] [CrossRef]
- Ikeda, Y.; Shimada, Y.; Honjo, K.; Okumoto, T.; Munakata, T. Safracins, new antitumor antibiotics III. Biological activity. J. Antibiot. 1983, 36, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Idemoto, H.; Hirayama, F.; Yamamoto, K.; Iwao, K.; Asao, T.; Munakata, T. Safracins, new antitumor antibiotics I. Producing organism, fermentation and isolation. J. Antibiot. 1983, 36, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Acebo, P.; Gomez, A.; Schleissner, C.; Rodríguez, P.; Aparicio, T.; Conde, S.; Muñoz, R.; de la Calle, F.; Garcia, J.L.; et al. Molecular characterization of the safracin biosynthetic pathway from Pseudomonas fluorescens A2-2: Designing new cytotoxic compounds. Mol. Microbiol. 2005, 56, 144–154. [Google Scholar] [CrossRef]
- Cuevas, C.; Pérez, M.; Martín, M.J.; Chicharro, J.L.; Fernández-Rivas, C.; Flores, M.; Francesch, A.; Gallego, P.; Zarzuelo, M.; de La Calle, F.; et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org. Lett. 2000, 2, 2545–2548. [Google Scholar] [CrossRef]
- Pospiech, A.; Cluzel, B.; Bietenhader, J.; Schupp, T. A new Myxococcus xanthus gene cluster for the biosynthesis of the antibiotic saframycin Mx1 encoding a peptide synthetase. Microbiology 1995, 141, 1793–1803. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shao, N.; Wen, W.H.; Tang, G.L. A cryptic palmitoyl chain involved in safracin biosynthesis facilitates post-NRPS modifications. Org. Lett. 2022, 24, 127–131. [Google Scholar] [CrossRef]
- Köhler, T.; Epp, S.F.; Curty, L.K.; Pechère, J.C. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 1999, 181, 6300–6305. [Google Scholar] [CrossRef]
- Köhler, T.; Michéa-Hamzehpour, M.; Henze, U.; Gotoh, N.; Curty, L.K.; Pechère, J.C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 1997, 23, 345–354. [Google Scholar] [CrossRef]
- Tian, Z.; Fargier, E.; Adams, C. Transcriptome profiling defines a novel regulon modulated by the LysR-type transcriptional regulator MexT in Pseudomonas aeruginosa. Nucleic Acids Res. 2009, 37, 7546–7559. [Google Scholar] [CrossRef]
- Tian, Z.X.; Mac Aogáin, M.; O’Connor, H.F.; Fargier, E.; Mooij, M.J.; Adams, C.; Wang, Y.P.; O’Gara, F. MexT modulates virulence determinants in Pseudomonas aeruginosa independent of the MexEF-OprN efflux pump. Microb. Pathog. 2009, 47, 237–241. [Google Scholar] [CrossRef]
- Maseda, H.; Uwate, M.; Nakae, T. Transcriptional regulation of the mexEF-oprN multidrug efflux pump operon by MexT and an unidentified repressor in nfxC-type mutant of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010, 311, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Richardot, C.; Juarez, P.; Jeannot, K.; Patry, I.; Plésiat, P.; Llanes, C. Amino acid substitutions account for most mexS alterations in clinical nfxC mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- LoVullo, E.D.; Schweizer, H.P. Pseudomonas aeruginosa mexT is an indicator of PAO1 strain integrity. J. Med. Microbiol. 2020, 69, 139–145. [Google Scholar] [CrossRef]
- Maseda, H.; Saito, K.; Nakajima, A.; Nakae, T. Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2000, 192, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Luong, P.M.; Shogan, B.D.; Zaborin, A.; Belogortseva, N.; Shrout, J.D.; Zaborina, O.; Alverdy, J.C. Emergence of the P2 phenotype in Pseudomonas aeruginosa PAO1 strains involves various mutations in mexT or mexF. J. Bacteriol. 2014, 196, 504–513. [Google Scholar] [CrossRef]
- Sobel, M.L.; Neshat, S.; Poole, K. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1246–1253. [Google Scholar] [CrossRef]
- Jornvall, H.; Bengt, P.; Jeffery, J. Characteristics of alcohol/polyol dehydrogenases: The zinc-containing long-chain alcohol dehydrogenases. Eur. J. Biochem. 1987, 167, 195–201. [Google Scholar] [CrossRef]
- Sun, H.W.; Plapp, B.V. Progressive sequence alignment and molecular evolution of the Zn-containing alcohol dehydrogenase family. J. Mol. Evol. 1992, 34, 522–535. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Wirth, R.; Friesenegger, A.; Fiedler, S. Transformation of various species of gram-negative bacteria belonging to 11 different genera by electroporation. Mol. Gen. Genet. 1989, 216, 175–177. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Sharma, S.B.; Signer, E.R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 1990, 4, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T.; Clardy, J. The evolution of gene collectives: How natural selection drives chemical innovation. Proc. Natl. Acad. Sci. USA 2008, 105, 4601–4608. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.P.; Metsä-Ketelä, M. A pharmaceutical model for the molecular evolution of microbial natural products. FEBS J. 2020, 287, 1429–1449. [Google Scholar] [CrossRef]
- Malik, V.S. Genetics and biochemistry of secondary metabolism. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 1982; Volume 28, pp. 27–115. [Google Scholar]
- Spížek, J.; Tichý, P. Some aspects of overproduction of secondary metabolites. Folia Microbiol. 1995, 40, 43–50. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Merighi, M.; Smith, R.; Narasimhan, G.; Lory, S.; Mathee, K. The regulatory repertoire of Pseudomonas aeruginosa AmpC ß-lactamase regulator AmpR includes virulence genes. PLoS ONE 2012, 7, e34067. [Google Scholar] [CrossRef]
- Westfall, L.W.; Carty, N.L.; Layland, N.; Kuan, P.; Colmer-Hamood, J.A.; Hamood, A.N. mvaT mutation modifies the expression of the Pseudomonas aeruginosa multidrug efflux operon mexEF-oprN. FEMS Microbiol. Lett. 2006, 255, 247–254. [Google Scholar] [CrossRef]
- Zaoui, C.; Overhage, J.; Löns, D.; Zimmermann, A.; Müsken, M.; Bielecki, P.; Pustelny, C.; Becker, T.; Nimtz, M.; Häussler, S. An orphan sensor kinase controls quinolone signal production via MexT in Pseudomonas aeruginosa. Mol. Microbiol. 2012, 83, 536–547. [Google Scholar] [CrossRef]
- Juarez, P.; Jeannot, K.; Plésiat, P.; Llanes, C. Toxic electrophiles induce expression of the multidrug efflux pump MexEF-OprN in Pseudomonas aeruginosa through a novel transcriptional regulator, CmrA. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Liao, J.; Schurr, M.J.; Sauer, K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2013, 195, 3352–3363. [Google Scholar] [CrossRef]
- Wang, D.; Seeve, C.; Pierson, L.S.; Pierson, E.A. Transcriptome profiling reveals links between ParS/ParR, MexEF-OprN, and quorum sensing in the regulation of adaptation and virulence in Pseudomonas aeruginosa. BMC Genom. 2013, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Asif, A.; Manzoor, A.; Azeem, M.; Sarwar, G.; Rashid, N.; Qaisar, U. Mutation in pvcABCD operon of Pseudomonas aeruginosa modulates MexEF-OprN efflux system and hence resistance to chloramphenicol and ciprofloxacin. Microb. Pathog. 2020, 149, 104491. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Cox, J.S. Interaction between polyketide synthase and transporter suggests coupled synthesis and export of virulence lipid in M. tuberculosis. PLoS Pathog. 2005, 1, e2. [Google Scholar] [CrossRef]
- Ostash, I.; Rebets, Y.; Ostash, B.; Kobylyanskyy, A.; Myronovskyy, M.; Nakamura, T.; Walker, S.; Fedorenko, V. An ABC transporter encoding gene lndW confers resistance to landomycin E. Arch. Microbiol. 2008, 190, 105–109. [Google Scholar] [CrossRef]
- Velasco, A.; de la Calle, F.; Aparicio, T.; Schleissner, C.; Acebo, P.; Rodríguez, P.; Reyes, F.; Henríquez, R. Gene Cluster Involved in Safracin Biosynthesis and Its Uses for Genetic Engineering. U.S. Patent US7723068B2, 25 May 2010. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Delgado, J.G.; Acedos, M.G.; de la Calle, F.; Rodríguez, P.; García, J.L.; Galán, B. Regulation of Safracin Biosynthesis and Transport in Pseudomonas poae PMA22. Mar. Drugs 2024, 22, 418. https://doi.org/10.3390/md22090418
Hernández Delgado JG, Acedos MG, de la Calle F, Rodríguez P, García JL, Galán B. Regulation of Safracin Biosynthesis and Transport in Pseudomonas poae PMA22. Marine Drugs. 2024; 22(9):418. https://doi.org/10.3390/md22090418
Chicago/Turabian StyleHernández Delgado, J. Gerardo, Miguel G. Acedos, Fernando de la Calle, Pilar Rodríguez, José Luis García, and Beatriz Galán. 2024. "Regulation of Safracin Biosynthesis and Transport in Pseudomonas poae PMA22" Marine Drugs 22, no. 9: 418. https://doi.org/10.3390/md22090418







