In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii
Abstract
1. Introduction
2. Results and Discussion
2.1. Inhibition of Tyrosinase and Antioxidant Activity of NP-HCH
2.2. Identification and Molecular Docking
2.3. Molecular Dynamics Simulation
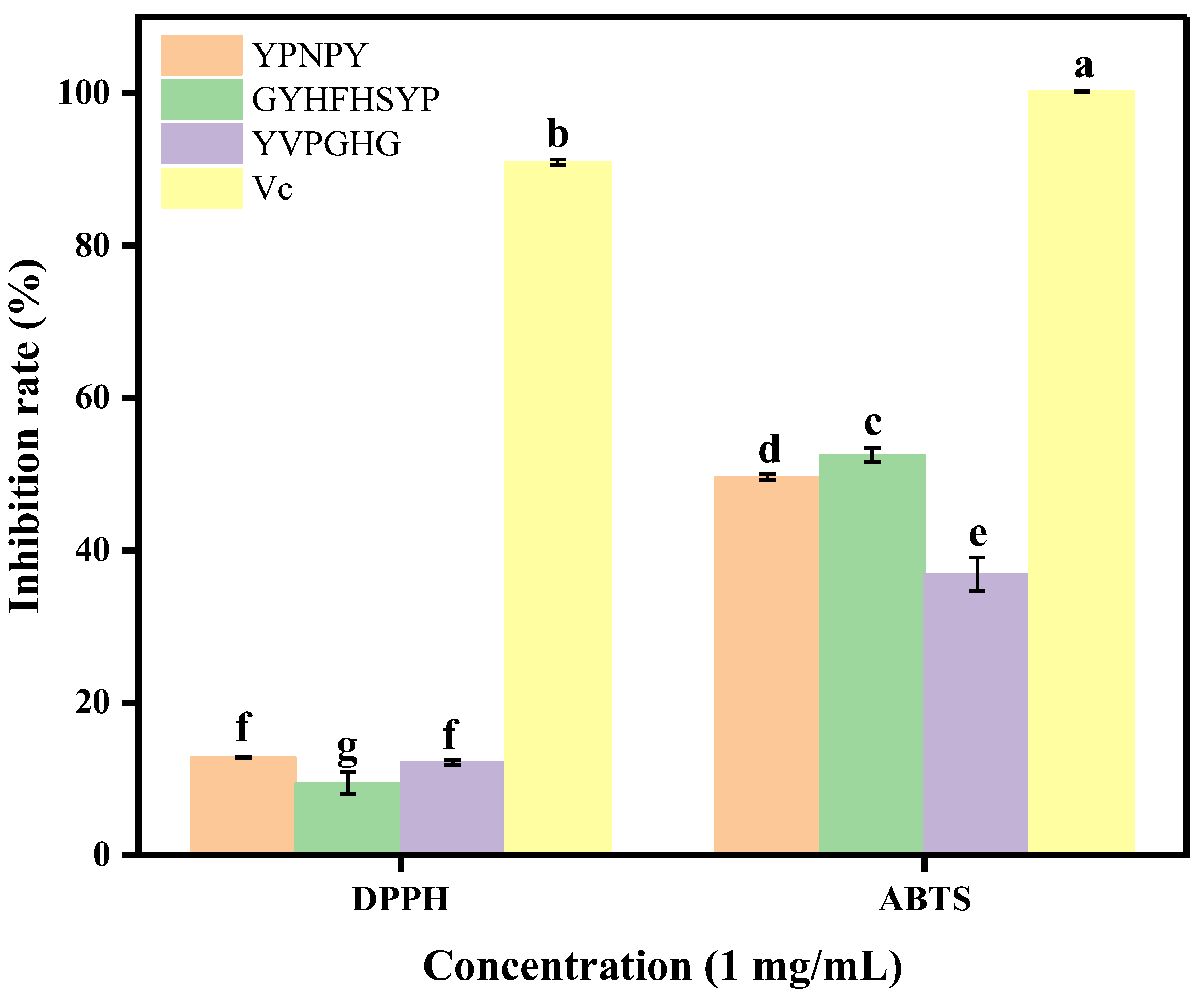
2.4. Tyrosinase Inhibitory Activity and Antioxidant Capacity of Synthetic Peptides
3. Materials and Methods
3.1. Materials
3.2. Preparation of Enzymatic Digests of Nacre Peptides
3.3. Tyrosinase Inhibitory Activity and Antioxidant Activity Assay of Enzyme Digests
3.4. Protein Sequence Identification
3.5. Molecular Docking Studies
3.6. Molecular Dynamics Simulations
3.7. Peptides Synthesis
3.8. Enzyme Inhibition Kinetic Assay
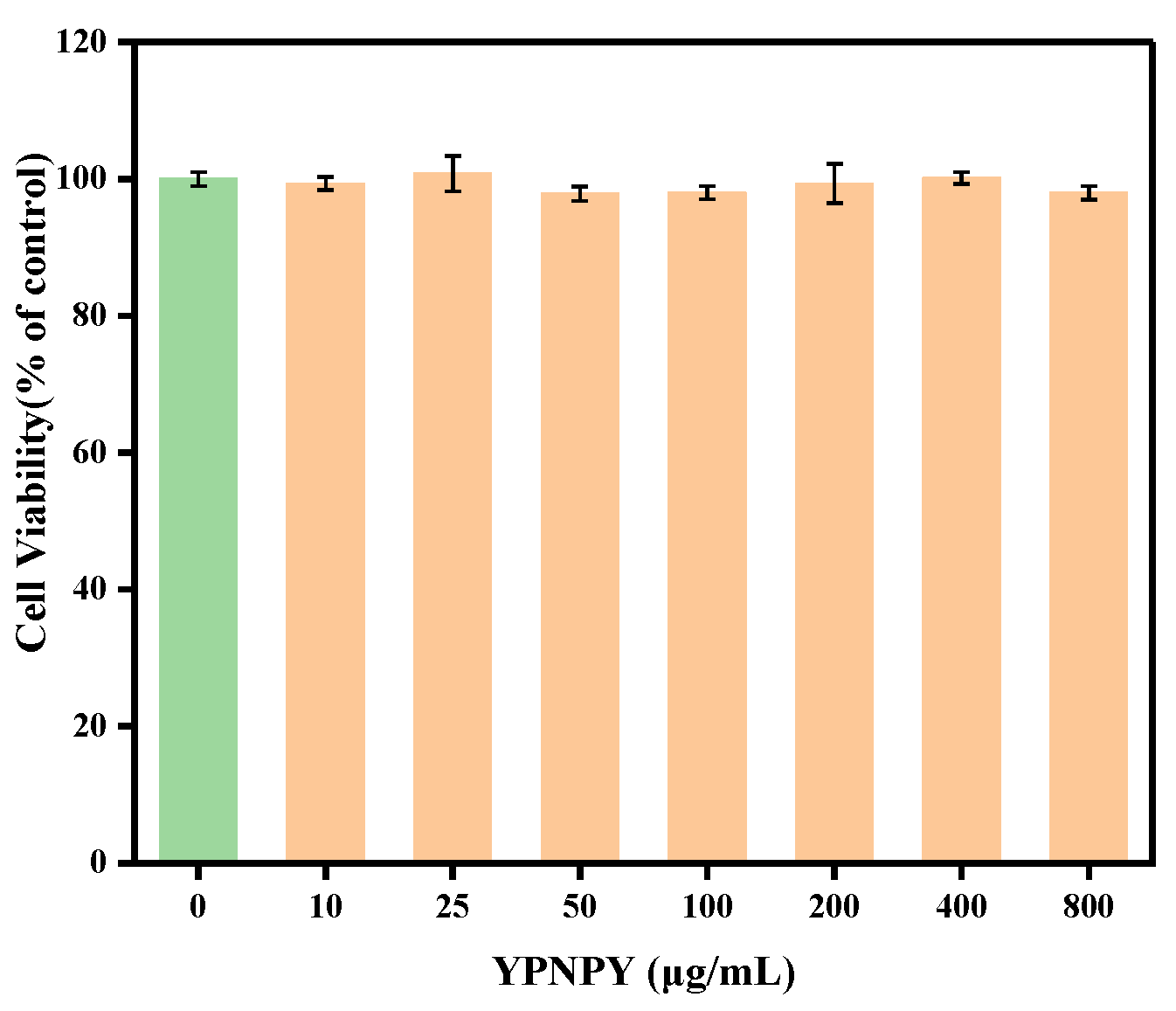
3.9. Cytotoxicity of YPNPY to B16F10 Cells
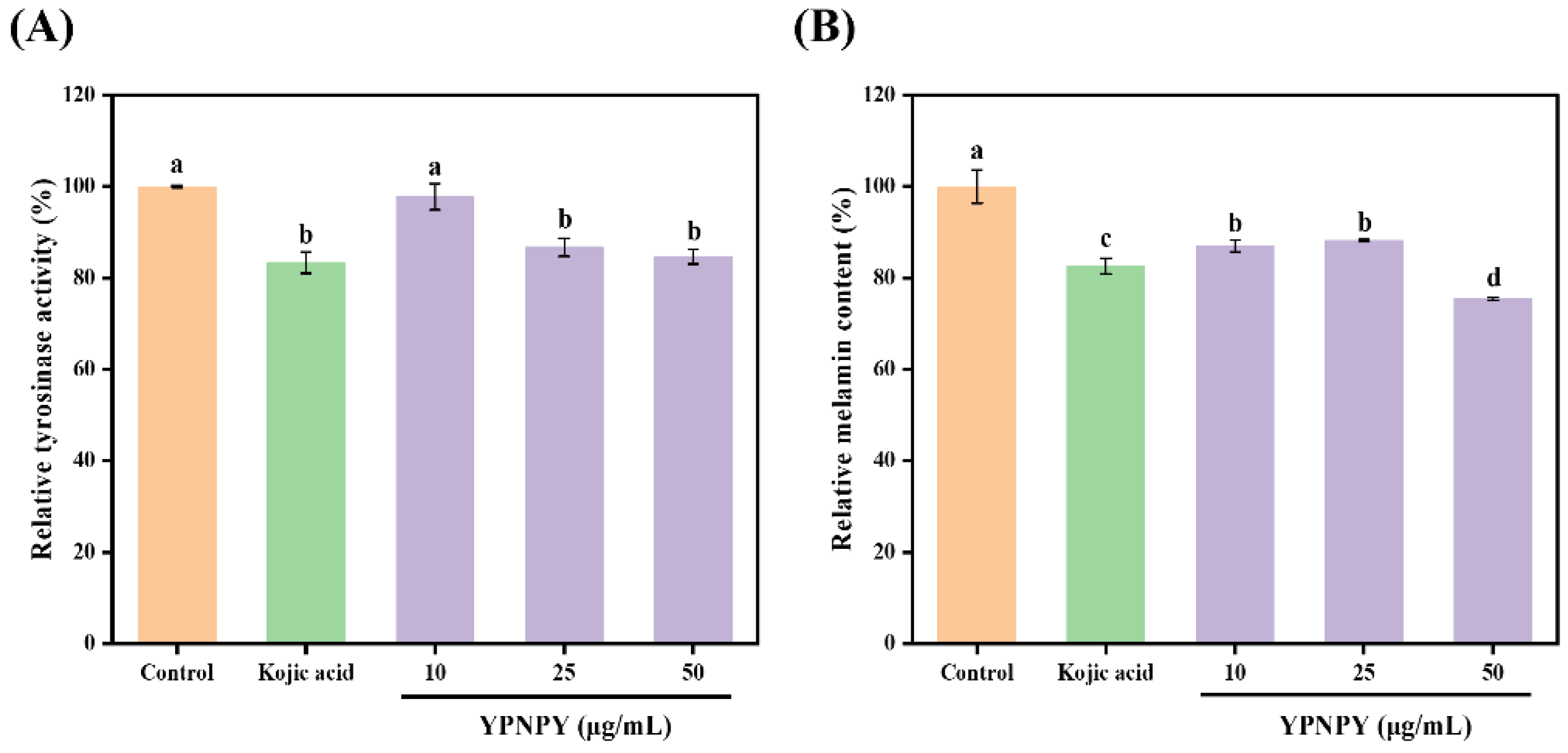
3.10. Effect of YPNPY on Melanin and Tyrosinase Synthesis in B16F10 Melanoma Cells
3.11. Effect of YPNPY on Antioxidant Enzyme Activity in B16F10 Cells
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Zhang, M.; Bai, Z.; He, J.; Li, J. The mantle exosome proteins of Hyriopsis cumingii participate in shell and nacre color formation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100844. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, H.; Hu, H.; Li, J.; Bai, Z. Estimation of non-nucleated pearl quality traits from donor and host mussel-derived genetic parameters in the golden strain of Hyriopsis cumingii. Aquaculture 2022, 560, 738460. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayale, A.; Zanella-Cleon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Miao, Y.; Zhu, X.; Duan, S.; Wang, Y.; Wang, X.; Wu, C.; Wang, G. Expression of bone morphogenetic protein 10 and its role in biomineralization in Hyriopsis cumingii. Int. J. Biol. Macromol. 2023, 253, 127245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Li, S.-R.; Yao, S.; Bi, Q.-R.; Hou, J.-J.; Cai, L.-Y.; Han, S.-M.; Wu, W.-Y.; Guo, D.-A. Anticonvulsant and sedative-hypnotic activity screening of pearl and nacre (mother of pearl). J. Ethnopharmacol. 2016, 181, 229–235. [Google Scholar] [CrossRef]
- Jin, C.; Li, J.-L.; Liu, X.-J. Teosin, a novel basic shell matrix protein from Hyriopsis cumingii induces calcium carbonate polycrystal formation. Int. J. Biol. Macromol. 2020, 150, 1229–1237. [Google Scholar] [CrossRef]
- Zhu, M.; Su, F.; Leng, J.; Jian, S.; Yi, P.; Wen, C.; Hu, B. Two NF-κB subunits are associated with antimicrobial immunity in Hyriopsis cumingii. Dev. Comp. Immunol. 2022, 129, 104336. [Google Scholar] [CrossRef]
- Wei, M.; Qiu, H.; Zhou, J.; Yang, C.; Chen, Y.; You, L. The Anti-Photoaging Activity of Peptides from Pinctada martensii Meat. Mar. Drugs 2022, 20, 770. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, M.; You, L. Protective Effect of Peptides from Pinctada Martensii Meat on the H2O2-Induced Oxidative Injured HepG2 Cells. Antioxidants 2023, 12, 535. [Google Scholar] [CrossRef]
- Cordero, R.J.B.; Casadevall, A. Melanin. Curr. Biol. 2020, 30, R142–R143. [Google Scholar] [CrossRef]
- Loh, X.J.; Young, D.J.; Guo, H.; Tang, L.; Wu, Y.; Zhang, G.; Tang, C.; Ruan, H. Pearl Powder—An Emerging Material for Biomedical Applications: A Review. Materials 2021, 14, 2797. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The metabolism of melanin synthesis—From melanocytes to melanoma. Pigment Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wu, Z.; Zheng, R.; Yin, N.; Han, F.; Zhao, Z.; Dai, M.; Han, D.; Wang, W.; Niu, L. Inhibition mechanism of melanin formation based on antioxidant scavenging of reactive oxygen species. Analyst 2022, 147, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef]
- Zilles, J.C.; dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef]
- Masub, N.; Khachemoune, A. Cosmetic skin lightening use and side effects. J. Dermatol. Treat. 2022, 33, 1287–1292. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Hasegawa, Y. Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice. Pharmaceuticals 2024, 17, 713. [Google Scholar] [CrossRef]
- Qiao, D.; Ke, C.; Hu, B.; Luo, J.; Ye, H.; Sun, Y.; Yan, X.; Zeng, X. Antioxidant activities of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2009, 78, 199–204. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Hui, K.-M.; Zhang, Y.-H.; Liu, Y.; Wang, Y.-Q.; Zhao, L.-J.; Lin, L.; Chai, L.-Q.; Wei, S.; Lan, J.-F. Three STATs are involved in the regulation of the expression of antimicrobial peptides in the triangle sail mussel, Hyriopsis cumingii. Fish Shellfish Immunol. 2017, 63, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Jiang, X.; Zhang, S.; Gao, X.; Paudel, Y.N.; Zhang, P.; Wang, R.; Liu, K.; Jin, M. Neuroprotective effect of YIAEDAER peptide against Parkinson’s disease like pathology in zebrafish. Biomed. Pharmacother. 2022, 147, 112629. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Miao, J.; Liao, W.; Huang, C.; Chen, B.; Li, Y.; Wang, X.; Yu, Y.; Liang, X.; Zhao, H.; et al. Rapid screening of novel tyrosinase inhibitory peptides from a pearl shell meat hydrolysate by molecular docking and the anti-melanin mechanism. Food Funct. 2023, 14, 1446–1458. [Google Scholar] [CrossRef]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The Art and Science of Molecular Docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef]
- Zeng, H.-j.; Liu, Z.; Hu, G.-z.; Qu, L.-b.; Yang, R. Investigation on the binding of aloe-emodin with tyrosinase by spectral analysis and molecular docking. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 211, 79–85. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Hsiao, N.-W.; Tseng, T.-S.; Chen, W.-C.; Lin, H.-H.; Leu, S.-J.; Yang, E.-W.; Tsai, K.-C. Phage Display-Mediated Discovery of Novel Tyrosinase-Targeting Tetrapeptide Inhibitors Reveals the Significance of N-Terminal Preference of Cysteine Residues and Their Functional Sulfur Atom. Mol. Pharmacol. 2015, 87, 218–230. [Google Scholar] [CrossRef]
- Wang, W.; Lin, H.; Shen, W.; Qin, X.; Gao, J.; Cao, W.; Zheng, H.; Chen, Z.; Zhang, Z. Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism. Foods 2023, 12, 3884. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, L.; Zhang, Q.; Chen, F.; Yu, Z. In silico identification and mechanistic evaluation of novel tyrosinase inhibitory peptides derived from coconut proteins. Food Biosci. 2024, 61, 104595. [Google Scholar] [CrossRef]
- Yu, Z.; Fu, L.; Zhang, Q.; Zhao, W. In silico identification and molecular mechanism of novel egg white-derived tyrosinase inhibitory peptides. Food Biosci. 2024, 57, 103567. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Lian, J.; Gao, F.; Khan, A.J.; Wang, T.; Zhang, F. Molecular Simulation Study on the Interaction between Tyrosinase and Flavonoids from Sea Buckthorn. Acs Omega 2021, 6, 21579–21585. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fan, L.; Ding, Z. The inhibition mechanisms between asparagus polyphenols after hydrothermal treatment and tyrosinase: A circular dichroism spectrum, fluorescence, and molecular docking study. Food Biosci. 2022, 48, 101790. [Google Scholar] [CrossRef]
- Yoshino, M.; Murakami, K. A graphical method for determining inhibition constants. J. Enzym. Inhib. Med. Chem. 2009, 24, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kim, J.H.; Su, X.; Kim, J.A. The Luteolinidin and Petunidin 3-O-Glucoside: A Competitive Inhibitor of Tyrosinase. Molecules 2022, 27, 5703. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Xu, K.; Wang, X.; Wen, Y.-T.; Wang, L.-J.; Huang, D.-Q.; Chen, X.-X.; Chai, W.-M. Punicalagin as a novel tyrosinase and melanin inhibitor: Inhibitory activity and mechanism. Lwt-Food Sci. Technol. 2022, 161, 113318. [Google Scholar] [CrossRef]
- Hu, Z.; Sha, X.; Zhang, L.; Huang, S.; Tu, Z. Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties. Foods 2022, 11, 391. [Google Scholar] [CrossRef]
- Yu, S.; He, M.; Zhai, Y.; Xie, Z.; Xu, S.; Yu, S.; Xiao, H.; Song, Y. Inhibitory activity and mechanism of trilobatin on tyrosinase: Kinetics, interaction mechanism and molecular docking. Food Funct. 2021, 12, 2569–2579. [Google Scholar] [CrossRef]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Wang, W.; Zhang, J.; Yin, J.; Le, T.; Xue, J.; Engelhardt, U.H.; Jiang, H. Kojic Acid Showed Consistent Inhibitory Activity on Tyrosinase from Mushroom and in Cultured B16F10 Cells Compared with Arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J.-a. Anti-melanogenic effects of epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG) and gallocatechin-3-gallate (GCG) via down-regulation of cAMP/CREB/MITF signaling pathway in B16F10 melanoma cells. Fitoterapia 2020, 145, 104634. [Google Scholar] [CrossRef]
- Lin, H.; Shen, W.; Jiang, Y.; Wu, Q.; Gao, J.; Cao, W.; Zheng, H.; Chen, Z.; Zhong, S.; Qin, X. Anti-Inflammatory Activity of Peptides from Ruditapes philippinarum in Lipopolysaccharide-Induced RAW264.7 Cells and Mice. Foods 2024, 13, 883. [Google Scholar] [CrossRef]





| No. | Peptide Sequence | Score | Molecular Weight | Length | Binding Energy /(kcal/mol) | GRAVY |
|---|---|---|---|---|---|---|
| 1 | YVPGHGW | 35.00 | 814.4 | 7 | −10.7 | −0.51 |
| 2 | GYHFHSYP | 24.6 | 1006.4 | 8 | −10.7 | −1.13 |
| 3 | YPNPY | 24.27 | 652.3 | 5 | −10.4 | −1.86 |
| 4 | YVTFTP | 28.59 | 726.4 | 6 | −9.5 | 0.45 |
| 5 | YVPGHG | 44.80 | 628.3 | 6 | −9.4 | −0.45 |
| 6 | YFGGY | 23.22 | 605.2 | 5 | −9.3 | −0.12 |
| 7 | PVGGYY | 21.99 | 654.3 | 6 | −9.3 | −0.13 |
| 8 | EQYVPGH | 36.93 | 828.4 | 7 | −9.2 | −1.33 |
| 9 | GFPYGPG | 22.72 | 693.3 | 7 | −9.1 | −0.41 |
| 10 | EQYVPGHG | 35.90 | 885.4 | 8 | −9.0 | −1.21 |
| 11 | TVYTP | 26.34 | 579.3 | 5 | −9.0 | −0.02 |
| 12 | GHAVSTPRG | 65.51 | 880.5 | 9 | −8.9 | −0.62 |
| 13 | FDAQPDT | 23.94 | 792.3 | 7 | −8.9 | −1.17 |
| Peptides | Hydrogen Bonds | Hydrophobic Interaction | Electrostatic Interaction |
|---|---|---|---|
| GYHFHSYP | HIS85 ALA246 GLY245 SER282 HIS244 | PRO277 PRO284 | - |
| YPNPY | HIS263 ASN260 ASN81 | VAL283 ALA286 | - |
| YVPGHG | ASN81 ALA323 ASN260 HIS244 VAL283 | - | - |
| Kojic acid | HIS259 HIS263 MET280 | - |
| Substances | Peptide Sequence | Tyrosinase Inhibitory Activity IC50/(mM) |
|---|---|---|
| peptides | YPNPY | 0.545 ± 0.028 |
| GYHFHSYP | >5 | |
| YVPGHG | >50 | |
| Kojic acid | — | 0.010 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Li, F.; Kang, J.; Xie, S.; Qin, X.; Gao, J.; Chen, Z.; Cao, W.; Zheng, H.; Song, W. In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii. Mar. Drugs 2024, 22, 420. https://doi.org/10.3390/md22090420
Lin H, Li F, Kang J, Xie S, Qin X, Gao J, Chen Z, Cao W, Zheng H, Song W. In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii. Marine Drugs. 2024; 22(9):420. https://doi.org/10.3390/md22090420
Chicago/Turabian StyleLin, Haisheng, Fei Li, Jiaao Kang, Shaohe Xie, Xiaoming Qin, Jialong Gao, Zhongqin Chen, Wenhong Cao, Huina Zheng, and Wenkui Song. 2024. "In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii" Marine Drugs 22, no. 9: 420. https://doi.org/10.3390/md22090420
APA StyleLin, H., Li, F., Kang, J., Xie, S., Qin, X., Gao, J., Chen, Z., Cao, W., Zheng, H., & Song, W. (2024). In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii. Marine Drugs, 22(9), 420. https://doi.org/10.3390/md22090420








