Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Results
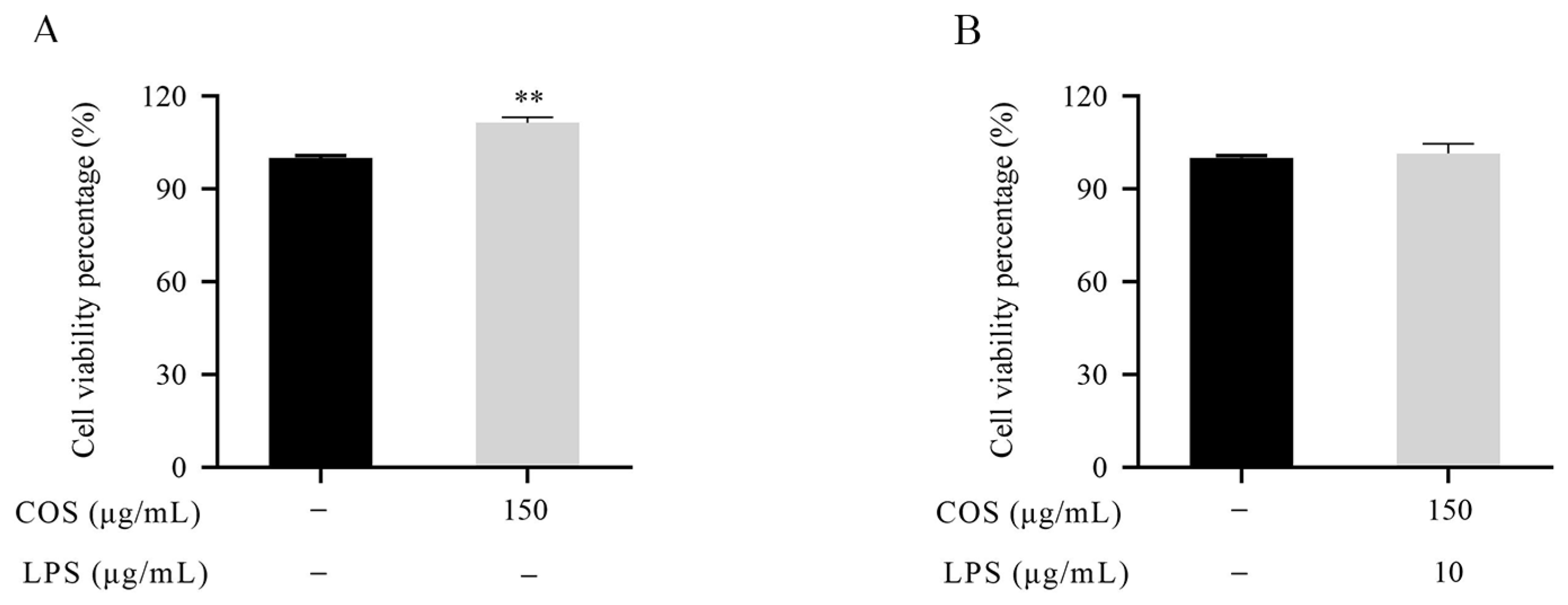
2.1. Effect of LPS and COS on BMEC Viability
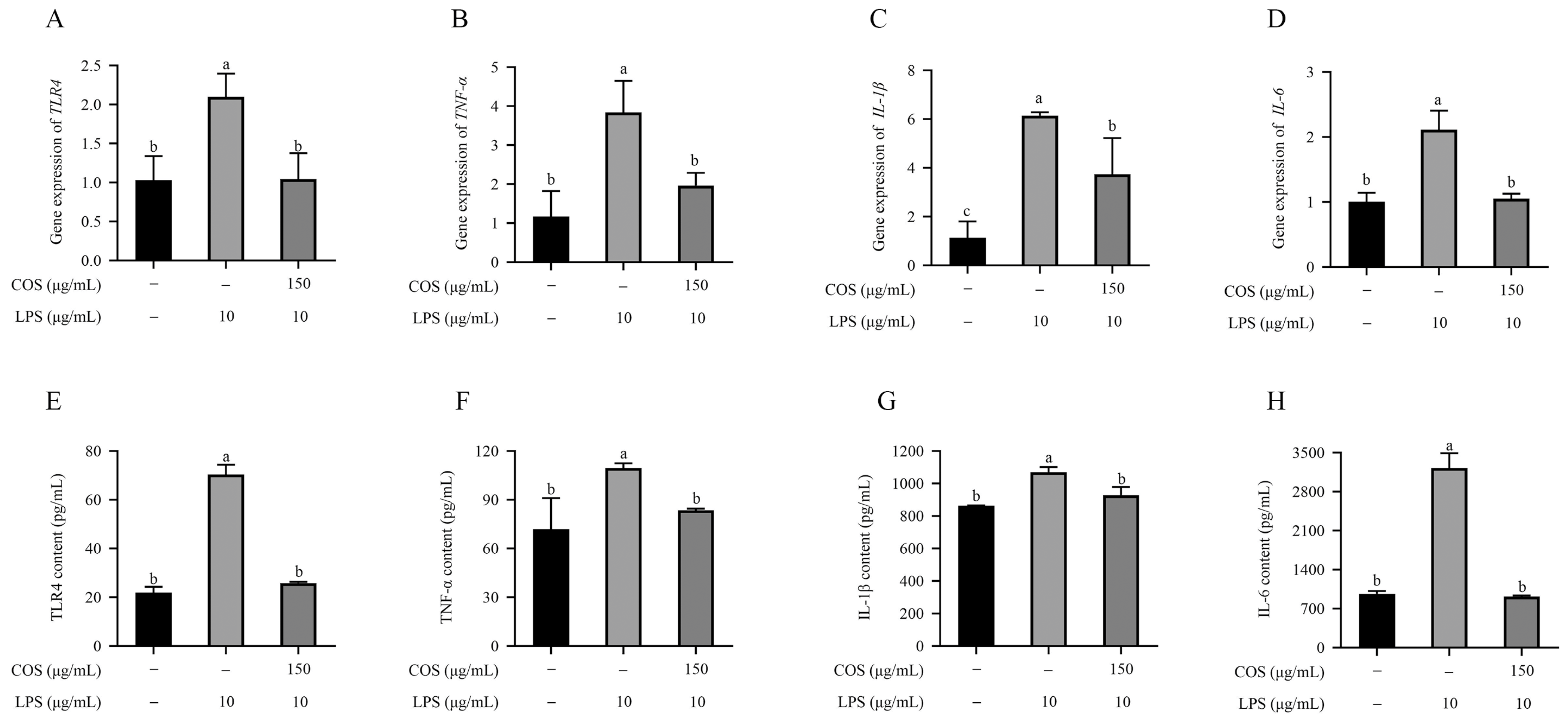
2.2. COS Alleviated Inflammatory Cytokine Secretion in LPS-Induced BMECs
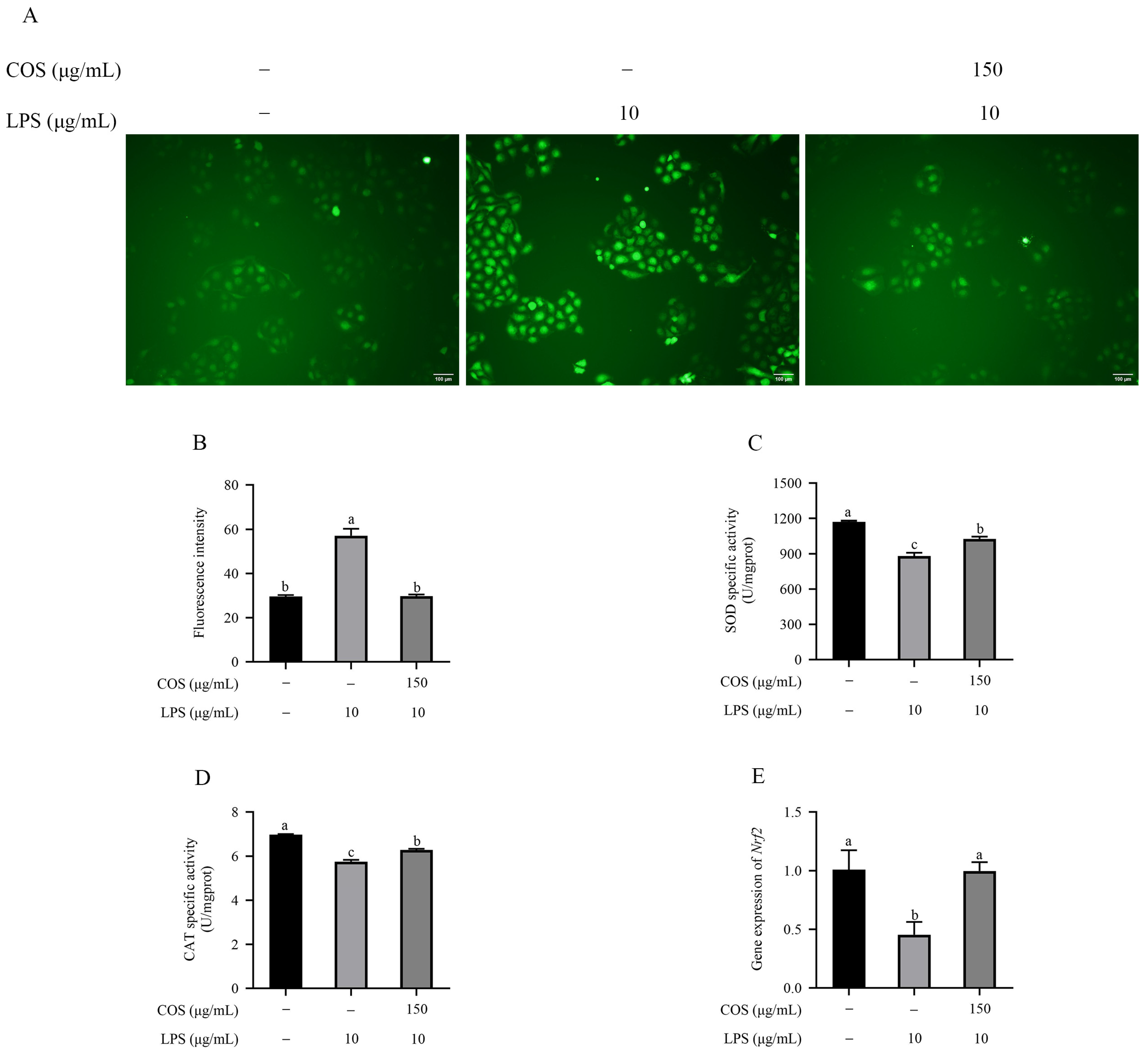
2.3. COS Mitigated Oxidative Stress in LPS Induced in BMECs
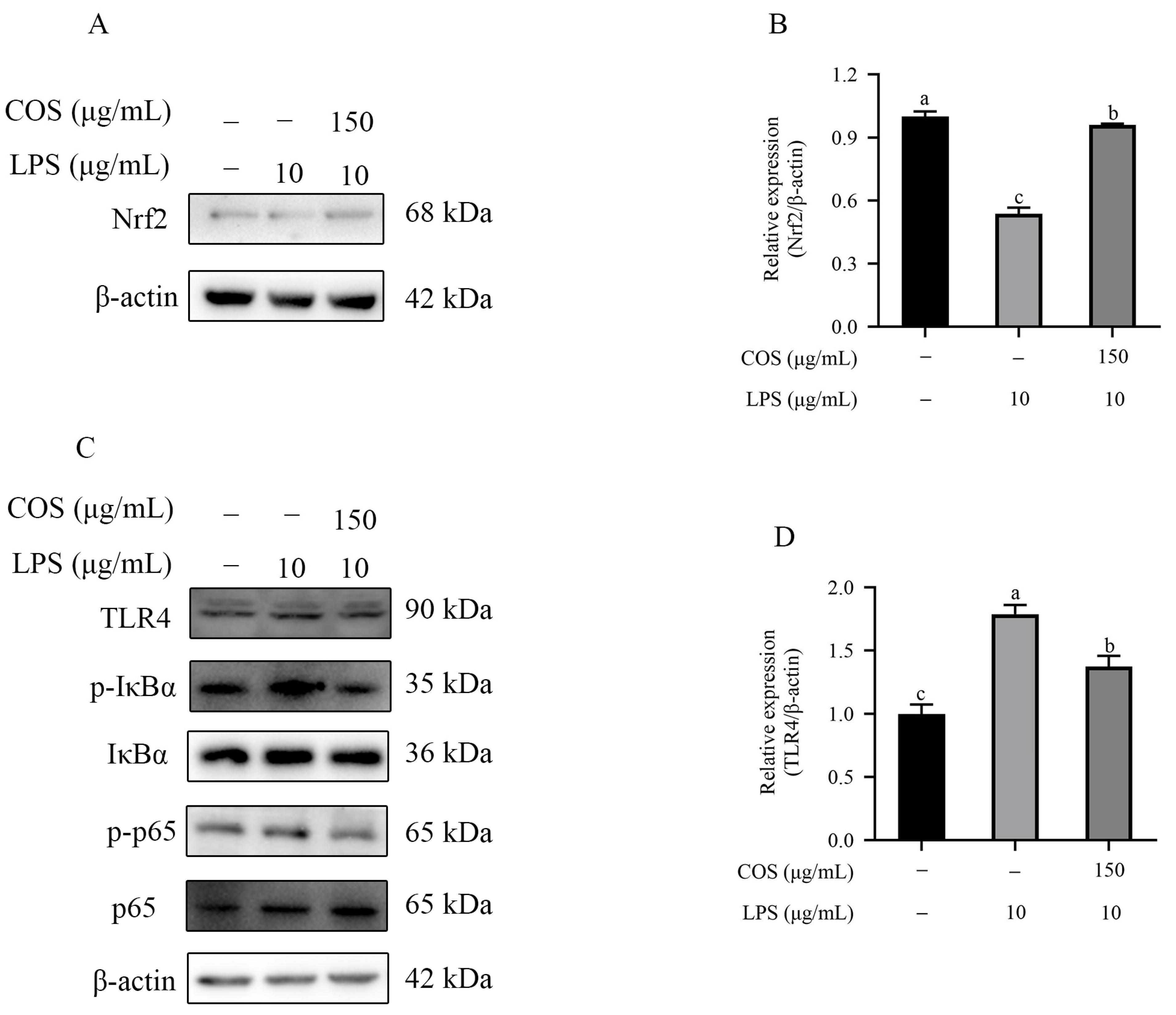
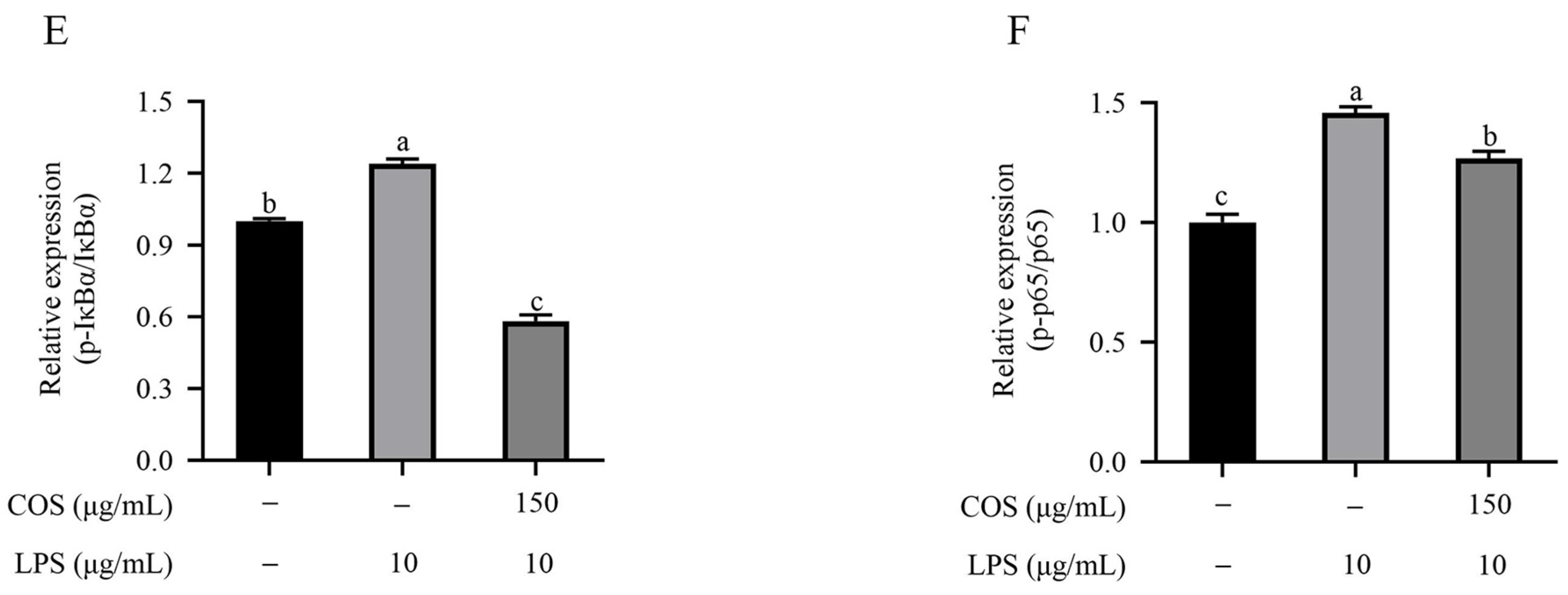
2.4. Effect of COS on Nrf2 and the TLR4/NF-κB Signaling Pathway in BMECs Stimulated with LPS
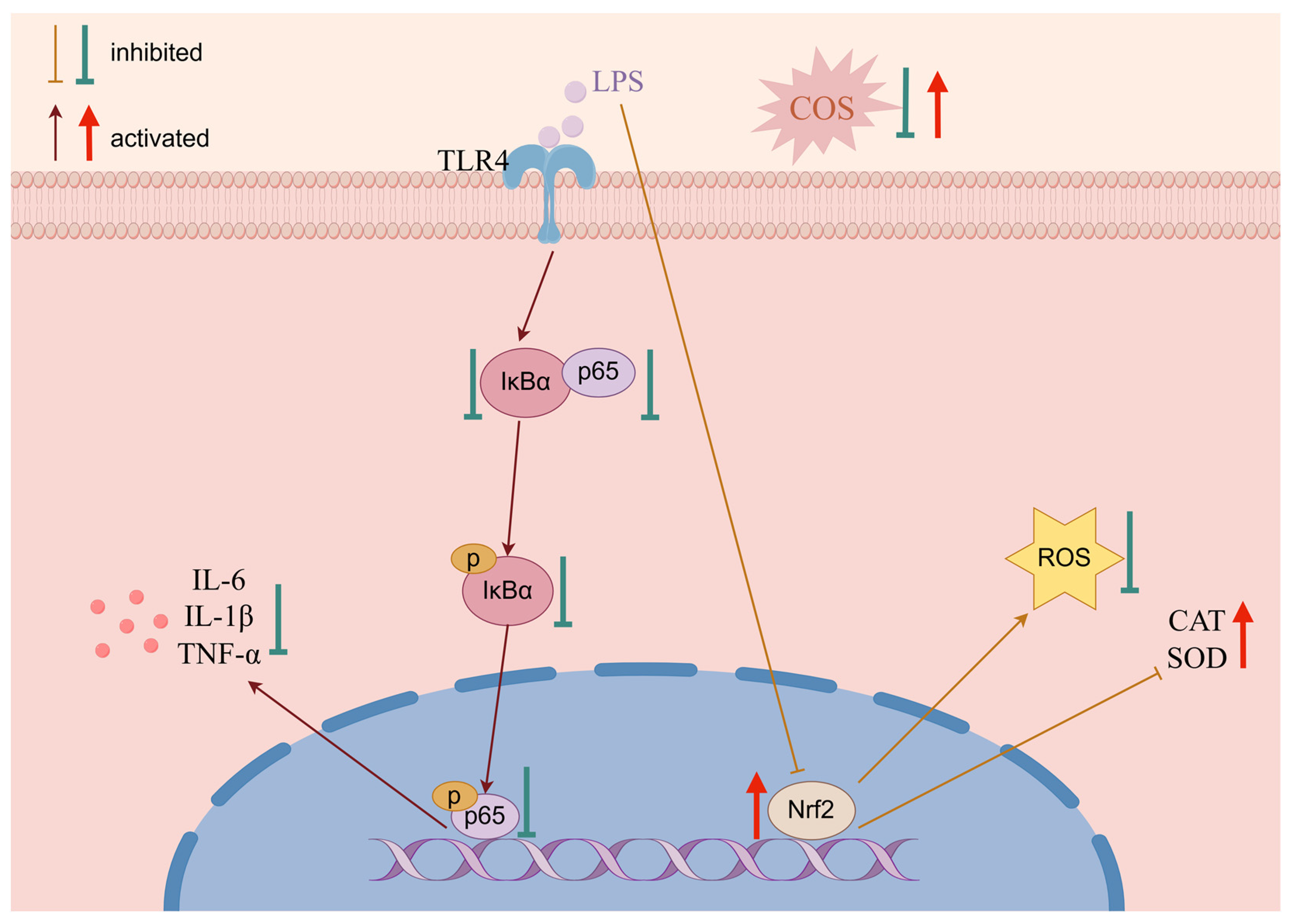
3. Discussion
4. Materials and Methods
4.1. BMECs Culture and Treatment
4.2. Assessment of Cell Viability
4.3. RT-qPCR
4.4. ROS Measurement
4.5. Antioxidant-Related Enzyme Activity Assays
4.6. ELISA
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Zhang, S.; Shang, X.; Wang, L.; Li, H.; Wang, X. Characteristics of Quinolone-Resistant Escherichia Coli Isolated from Bovine Mastitis in China. J. Dairy Sci. 2018, 101, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Thompson-Crispi, K.; Atalla, H.; Miglior, F.; Mallard, B.A. Bovine Mastitis: Frontiers in Immunogenetics. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Murinda, S.E. Antimicrobial Resistance of Mastitis Pathogens. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Qin, Y.; Xu, H.; Xie, J.; Hu, D.; Xue, B.; Hua, X. Antibacterial Activities and Preservative Effect of Chitosan Oligosaccharide Maillard Reaction Products on Penaeus Vannamei. Int. J. Biol. Macromol. 2017, 105, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by Chitosan Oligosaccharide in Intestinal Epithelial Cells: Mechanism of Action and Potential Applications in Intestinal Disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A Review on the Preparation of Chitosan Oligosaccharides and Application to Human Health, Animal Husbandry and Agricultural Production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan Oligosaccharide: Biological Activities and Potential Therapeutic Applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Yang, W.; Jiang, Q.; Loor, J.J.; Ouyang, L.; Tang, H.; Chang, R.; Peng, T.; Xu, C. Sirtuin 3 Relieves Inflammatory Responses Elicited by Lipopolysaccharide via the PGC1α-NFκB Pathway in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2023, 106, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan Inhibits Oxidative Stress and Alleviates LPS-Induced Inflammation and Apoptosis of BMECs by Activating the Nrf2 Signaling Pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Q.; Zhang, T.; Guo, W.; Qiao, W.; Deng, M. Protective Effects of Lixisenatide against Lipopolysaccharide-Induced Inflammation Response in MAC-T Bovine Mammary Epithelial Cells: A Therapeutic Implication in Mastitis. Chem. Res. Toxicol. 2020, 33, 982–987. [Google Scholar] [CrossRef]
- Ma, N.; Wei, G.; Zhang, H.; Dai, H.; Roy, A.C.; Shi, X.; Chang, G.; Shen, X. Cis-9, Trans-11 CLA Alleviates Lipopolysaccharide-Induced Depression of Fatty Acid Synthesis by Inhibiting Oxidative Stress and Autophagy in Bovine Mammary Epithelial Cells. Antioxidants 2021, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Chen, K.; Wang, Y.; Yang, F.; Wang, G. UFL1 Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF- κ B Pathway in Bovine Mammary Epithelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6505373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, Y.; Xu, H.; Li, L.; Hu, Y.; Liu, E.; Cui, J. Betaine Protects Bovine Mammary Epithelial Cells against LPS-Induced Inflammatory Response and Oxidative Damage via Modulating NF-κB and Nrf2 Signalling Pathway. Ital. J. Anim. Sci. 2022, 21, 859–869. [Google Scholar] [CrossRef]
- Yu, H.; Fan, J.; Zhang, Y.; Zhao, Z.; Lin, Z.; Jiang, P. Syndecan-3 Inhibits Lipopolysaccharide-Induced Inflammation of Bovine Mammary Epithelial Cells through the NF-κB Signal Transduction Pathway. J. Dairy Sci. 2024, 107, 11563–11575. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, C.; Basang, W.; Zhu, Y.; Wang, X.; Li, C.; Chen, L.; Zhou, X. Mechanisms by Which Mastitis Affects Reproduction in Dairy Cow: A Review. Reprod. Domest. Anim. 2021, 56, 1165–1175. [Google Scholar] [CrossRef]
- Rinaldi, M.; Li, R.W.; Capuco, A.V. Mastitis Associated Transcriptomic Disruptions in Cattle. Vet. Immunol. Immunopathol. 2010, 138, 267–279. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.-J.; Sipka, A.; et al. Host-Response Patterns of Intramammary Infections in Dairy Cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.-F.; Du, Y.-G. Chitosan Oligosaccharides Protect Mice from LPS Challenge by Attenuation of Inflammation and Oxidative Stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liao, M.; Zhu, Y.; Hu, X.; Wang, J. The Protective Role of Chitooligosaccharides against Chronic Ulcerative Colitis Induced by Dextran Sulfate Sodium in Mice. J. Funct. Foods 2021, 87, 104809. [Google Scholar] [CrossRef]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross Talk Between ER Stress, Oxidative Stress, and Inflammation in Health and Disease. In Stress Responses; Oslowski, C.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1292, pp. 205–214. ISBN 978-1-4939-2521-6. [Google Scholar]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in Characterisation and Biological Activities of Chitosan and Chitosan Oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Q.; Liang, Z.; Wang, M.; Wang, B.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Wang, J.; Ma, C.; Kang, W. Anti-Inflammatory and Antioxidant Effects of Chaetoglobosin Vb in LPS-Induced RAW264.7 Cells: Achieved via the MAPK and NF-κB Signaling Pathways. Food Chem. Toxicol. 2021, 147, 111915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xu, Y.; Lu, J.; Liu, M.; Dai, B.; Miao, J.; Yin, Y. Variant Innate Immune Responses of Mammary Epithelial Cells to Challenge by Staphylococcus aureus, Escherichia coli and the Regulating Effect of Taurine on These Bioprocesses. Free. Radic. Biol. Med. 2016, 96, 166–180. [Google Scholar] [CrossRef]
- Xu, W.; Huang, H.; Lin, C.; Jiang, Z.-F. Chitooligosaccharides Protect Rat Cortical Neurons against Copper Induced Damage by Attenuating Intracellular Level of Reactive Oxygen Species. Bioorg. Med. Chem. Lett. 2010, 20, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Wu, F.; Wang, Y.; Lin, Z.; Wang, H.; Zhang, J.; Zhao, Z. Chitosan Oligosaccharide Improves Intestinal Function by Promoting Intestinal Development, Alleviating Intestinal Inflammatory Response, and Enhancing Antioxidant Capacity in Broilers Aged d 1 to 14. Poult. Sci. 2024, 103, 103381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, S.; Gao, X.; Huang, H.; Lao, F.; Dai, X. Protective Effect of Chitosan Oligosaccharide against Hydrogen Peroxide-Mediated Oxidative Damage and Cell Apoptosis via Activating Nrf2/ARE Signaling Pathway. Neurotox. Res. 2021, 39, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ahmad, K.A.; Khan, F.U.; Yan, S.; Ihsan, A.U.; Ding, Q. Chitosan Oligosaccharides Prevent Doxorubicin-Induced Oxidative Stress and Cardiac Apoptosis through Activating P38 and JNK MAPK Mediated Nrf2/ARE Pathway. Chem.-Biol. Interact. 2019, 305, 54–65. [Google Scholar] [CrossRef]
- Che, H.-Y.; Zhou, C.-H.; Lyu, C.-C.; Meng, Y.; He, Y.-T.; Wang, H.-Q.; Wu, H.-Y.; Zhang, J.-B.; Yuan, B. Allicin Alleviated LPS-Induced Mastitis via the TLR4/NF-κB Signaling Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 3805. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Zhou, E.; Chen, L.; Kou, J.; Wang, J.; Yang, Z. Baicalein Attenuates Inflammatory Responses by Suppressing TLR4 Mediated NF-κB and MAPK Signaling Pathways in LPS-Induced Mastitis in Mice. Int. Immunopharmacol. 2015, 28, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S.; et al. Chitosan Oligosaccharide-Mediated Attenuation of LPS-Induced Inflammation in IPEC-J2 Cells Is Related to the TLR4/NF-κB Signaling Pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xu, Q.-S.; Du, Y.-G.; Xu, J. Chitosan Oligosaccharides Block LPS-Induced O-GlcNAcylation of NF-κB and Endothelial Inflammatory Response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan Oligosaccharide Reduces Intestinal Inflammation That Involves Calcium-Sensing Receptor (CaSR) Activation in Lipopolysaccharide (LPS)-Challenged Piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, L.; Peri, F. Recent Advances on Toll-like Receptor 4 Modulation: New Therapeutic Perspectives. Future Med. Chem. 2018, 10, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending Metabolism, Immunity, and Inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Qian, X.; Guo, Y.; Dong, W.; Yang, M.; Yang, H.; Huang, X.; Liang, X. Essential Oil from Chimonanthus Nitens Oliv. Leaves Ameliorate Inflammation and Oxidative Stress in LPS-Induced ALI through NF-κB and Nrf2 Signaling Pathways. J. Ethnopharmacol. 2024, 333, 118470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, Y.; Liang, X.; Xue, G.; Wu, H. Sanguinarine Enhances the Integrity of the Blood–Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis. Cells 2022, 11, 3658. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Abd El-Emam, M.M.; Mansour, M.F.; Behairy, A.; Khamis, T.; Mahmoud, S.M.; Alsemeh, A.E.; El Sayed, M.M.; Mady, F.M.; Qelliny, M.R. Berberine Hydrochloride-Loaded Lipid-Based Nanoparticles Ameliorate β-Cell Function by Targeting Nrf2/NF-κB Signaling Pathway in Alloxan-Induced Diabetes Using a Murine Model: Optimization through Full Factorial Design. J. Drug Deliv. Sci. Technol. 2024, 100, 106076. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Wu, C.; He, B.; Wang, X.; Zou, Y.; Ma, R.; Gu, Y.; Huang, J.; Li, S.; Wang, J.; Wang, J. Salvianolic Acid A Alleviates Cardiovascular Injury in Rats with Chronic Kidney Disease by Activating the PI3K/Akt/Nrf2/HO-1 and SIRT1/TLR4/NF-κB/P38 Signaling Pathways. J. Funct. Foods 2023, 110, 105861. [Google Scholar] [CrossRef]
- Luo, Z.; Dong, X.; Ke, Q.; Shen, L. Chitooligosaccharides Inhibit Ethanol-Induced Oxidative Stress via Activation of Nrf2 and Reduction of MAPK Phosphorylation. Oncol. Rep. 2014, 32, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Chanvorachote, P.; Reudhabibadh, R.; Singh, A.; Benjakul, S.; Roytrakul, S.; Hutamekalin, P. Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells. Foods 2023, 12, 2740. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Chen, J.W.; Gooneratne, R.; He, X.P.; Huang, J.Z.; Zhao, Z.H. Effects of Varied Molecular Weight of Chitosan Oligosaccharides on Growth Performance, Carcass Trait, Meat Quality, and Fat Metabolism in Indigenous Yellow-Feathered Chickens. J. Appl. Poult. Res. 2022, 31, 100221. [Google Scholar] [CrossRef]
- Fan, J.; Chen, J.; Wu, H.; Lu, X.; Fang, X.; Yin, F.; Zhao, Z.; Jiang, P.; Yu, H. Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway. Animals 2022, 12, 1692. [Google Scholar] [CrossRef]
| Genes | Sequence Number | Primer Sequences (from 5′ to 3′) |
|---|---|---|
| TLR4 | NM_174198.6 | F: GCAGGGAAAGTCAACTAAAC |
| R: ACATAAAGTGGAGGGGAATC | ||
| TNF-α | XM_005223596.4 | F: ACGGGCTTTACCTCATCTACTC |
| R: TGGCAGACAGGATGTTGACC | ||
| IL-1β | NM_174093.1 | F: GATGGCTTACTACAGTGACGA |
| R: AGATGAATGAAAGGATGCTC | ||
| IL-6 | NM_173923.2 | F: GATGCTTCCAATCTGGGTTCA |
| R: TCCTGATTTCCCTCATACTCG | ||
| Nrf2 | NM_001011678.2 | F: GTCTTCACTGCTCCTCCTCAG |
| R: CTCCCAAACTTGCTCAATGTC | ||
| β-actin | NM_173979.3 | F: AGAGCAAGAGAGGCATCC |
| R: TCGTTGTAGAAGGTGTGGT |
| Antibody | Catalog Number | Manufacturer |
|---|---|---|
| Nrf2 | AF0639 | Affinity Biosciences (Cincinnati, OH, USA) |
| TLR4 | bs-20595R | Bioss (Beijing, China) |
| p65 | 10745-1-AP | Proteintech Group, Inc. (Wuhan, China) |
| p-p65 | 3033T | Cell Signaling Technology, Inc. (Danvers, MA, USA) |
| p-IκBα | bs-2513R | Bioss (Beijing, China) |
| IκBα | 10268-1-AP | Proteintech Group, Inc. (Wuhan, China) |
| β-actin | AP0060 | Bioworld Biotech Co., Ltd. (Nanjing, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Zhou, Y.; Chen, R.; Tao, Q.; Lu, Q.; Xu, Q.; Yu, H.; Jiang, P.; Zhao, Z. Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells. Mar. Drugs 2025, 23, 31. https://doi.org/10.3390/md23010031
Lin Z, Zhou Y, Chen R, Tao Q, Lu Q, Xu Q, Yu H, Jiang P, Zhao Z. Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells. Marine Drugs. 2025; 23(1):31. https://doi.org/10.3390/md23010031
Chicago/Turabian StyleLin, Ziwei, Yanlong Zhou, Ruiwen Chen, Qiuyan Tao, Qiwen Lu, Qianchao Xu, Haibin Yu, Ping Jiang, and Zhihui Zhao. 2025. "Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells" Marine Drugs 23, no. 1: 31. https://doi.org/10.3390/md23010031
APA StyleLin, Z., Zhou, Y., Chen, R., Tao, Q., Lu, Q., Xu, Q., Yu, H., Jiang, P., & Zhao, Z. (2025). Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells. Marine Drugs, 23(1), 31. https://doi.org/10.3390/md23010031






