Optimization of a Sonotrode Extraction Method and New Insight of Phenolic Composition of Fucus vesiculosus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fitting the Model
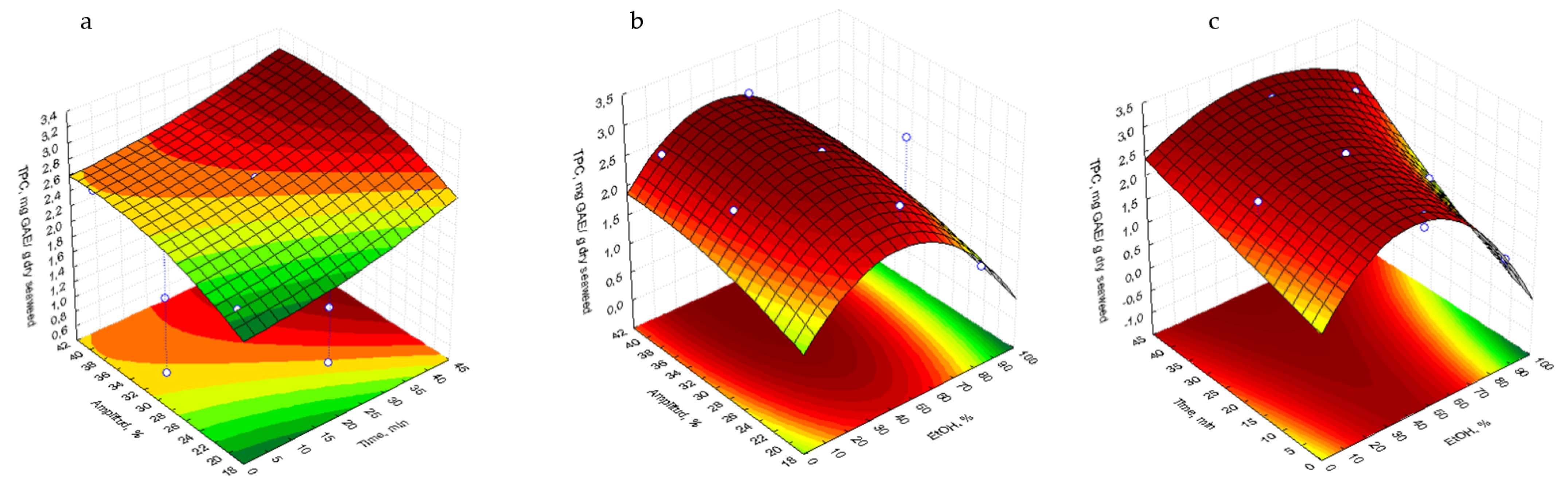
2.2. Analysis of Response Surfaces
2.3. Optimization of Sonotrode Parameters
2.4. HPLC-ESI-TOF-MS Tentative Profiling of Phenolic Compounds in the Optimized F. vesiculosus Extract
2.4.1. Phenolic Acids
2.4.2. Phlorotannins
2.4.3. Flavonoids
2.4.4. Halophenols
2.5. Antioxidant Activity of F. vesiculosus Extract
3. Materials and Methods
3.1. Reagents and Plant Material
3.2. Extraction of Phenolic Compounds from F. vesiculosus Algae Using Sonotrode
3.3. Experimental Design
3.4. Total Phenolic Content Assay (TPC)
3.5. HPLC-ESI-TOF-MS Analysis
3.6. Antioxidant Capacity Assays
3.6.1. Ferric-Reducing Antioxidant Power
3.6.2. DPPH Assay
3.6.3. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, H.; Lee, J.; Park, Y.-K.; Lee, J.-Y. Exploring the Health Benefits and Concerns of Brown Seaweed Consumption: A Comprehensive Review of Bioactive Compounds in Brown Seaweed and Its Potential Therapeutic Effects. J. Agric. Food Res. 2024, 17, 101215. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus Vesiculosus. Mar. Drugs 2022, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Jorge, G.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medina, M.A.; Sansón, M.; González-Rodríguez, Á.M. Comparative Study of Antioxidant Potential in Air-Dried Brown Macroalgae of the Canary Islands. LWT 2024, 206, 116549. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Ferreira, S.S.; Silva, A.M.S.; Coimbra, M.A.; Cardoso, S.M. Fucus Vesiculosus-Rich Extracts as Potential Functional Food Ingredients: A Holistic Extraction Approach. Foods 2024, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Yu, Q.; Fan, L. Comparative Extraction and Antioxidant Potential of Bioactive Compounds from Fucus Vesiculosus: Kinetic Modeling and UPLC-Q-TOF-MS Phenolic Profiling. Food Biosci. 2024, 57, 103575. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Ribeiro, A.R.; Patinha, C.; Silva, A.M.S.; Cardoso, S.M.; Costa, R. Water Extraction Kinetics of Bioactive Compounds of Fucus Vesiculosus. Molecules 2019, 24, 3408. [Google Scholar] [CrossRef]
- Kurth, C.; Welling, M.; Pohnert, G. Sulfated Phenolic Acids from Dasycladales Siphonous Green Algae. Phytochemistry 2015, 117, 417–423. [Google Scholar] [CrossRef]
- Li, Y.; Rárová, L.; Scarpato, S.; Çiçek, S.S.; Jordheim, M.; Štenclová, T.; Strnad, M.; Mangoni, A.; Zidorn, C. Seasonal Variation of Phenolic Compounds in Zostera Marina (Zosteraceae) from the Baltic Sea. Phytochemistry 2022, 196, 113099. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M. Emerging Sulfated Flavonoids and Other Polyphenols as Drugs: Nature as an Inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef] [PubMed]
- Wekre, M.E.; Holmelid, B.; Underhaug, J.; Pedersen, B.; Kopplin, G.; Jordheim, M. Characterization of High Value Products in the Side-Stream of Laminaria Hyperborea Alginate Production—Targeting the Phenolic Content. Algal Res. 2023, 72, 103109. [Google Scholar] [CrossRef]
- Supikova, K.; Kosinova, A.; Vavrusa, M.; Koplikova, L.; François, A.; Pospisil, J.; Zatloukal, M.; Wever, R.; Hartog, A.; Gruz, J. Sulfated Phenolic Acids in Plants. Planta 2022, 255, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Ma, W.C.J.; Ang, P.O.; Kim, J.S.; Chen, F. Seasonal Variations of Bromophenols in Brown Algae (Padina Arboroscens, Sargassum Siliquastrum, and Lobophora Variegata) Collected in Hong Kong. J. Agric. Food Chem. 2003, 51, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Hartmann, A.; Orfanoudaki, M.; Ngoc, H.N.; Nagl, M.; Karsten, U.; Heesch, S.; Ganzera, M. Development and Validation of an HPLC Method for the Quantitative Analysis of Bromophenolic Compounds in the Red Alga Vertebrata Lanosa. Mar. Drugs 2019, 17, 675. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Fu, Q.; Xiao, J. Free and Bound Phenolic Profiles and Antioxidant Ability of Eleven Marine Macroalgae from the South China Sea. Front. Nutr. 2024, 11, 1459757. [Google Scholar] [CrossRef]
- Coelho, M.; Duarte, A.P.; Pinto, S.; Botelho, H.M.; Reis, C.P.; Serralheiro, M.L.; Pacheco, R. Edible Seaweeds Extracts: Characterization and Functional Properties for Health Conditions. Antioxidants 2023, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Sivagnanam, S.P.; Rameshkumar, S.; Pednekar, M.; Fitzpatrick, S.; Rai, D.K.; Padamati, R.B.; O’Donnell, C.; Tiwari, B.K. Sequential Extraction of Fucoidan, Laminarin, Mannitol, Alginate and Protein from Brown Macroalgae Ascophyllum Nodosum and Fucus Vesiculosus. Int. J. Biol. Macromol. 2024, 256, 128195. [Google Scholar] [CrossRef] [PubMed]
- Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe. Mar. Drugs 2018, 16, 265. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Mamatmyrodov, K.; Popov, N. Recovery of Bioactive Complex from Brown Algae by Alternative Extraction Approaches. Bioresour. Technol. Rep. 2024, 26, 101810. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’doherty, J.; Jaiswal, A.K.; Tiwari, B.K.; Rajauria, G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bonet, D.; García-Oms, S.; Belda-Antolí, M.; Padrón-Sanz, C.; Lloris-Carsi, J.M.; Cejalvo-Lapeña, D. RP-HPLC-DAD Determination of the Differences in the Polyphenol Content of Fucus Vesiculosus Extracts with Similar Antioxidant Activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1184, 122978. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Kamal, A.; Yu, Q.; Fan, L. Optimizing Phlorotannins Extraction from Fucus Vesiculosus Using Box-Behnken Design: Unveiling Techniques for Enhanced Antioxidant Activity and Metabolic Enzyme Inhibition. Algal Res. 2024, 83, 103723. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus Vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’doherty, J. Seasonal Variation of the Proximate Composition, Mineral Content, Fatty Acid Profiles and Other Phytochemical Constituents of Selected Brown Macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, N.P.; Bruhn, A.; Cole, A.; Nielsen, M.O. Targeted and Untargeted Metabolic Profiling to Discover Bioactive Compounds in Seaweeds and Hemp Using Gas and Liquid Chromatography-Mass Spectrometry. Metabolites 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Kundisová, I.; Colom, H.; Juan, M.E.; Planas, J.M. Pharmacokinetics of Hydroxytyrosol and Its Sulfate and Glucuronide Metabolites after the Oral Administration of Table Olives to Sprague-Dawley Rats. J. Agric. Food Chem. 2024, 72, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling Phlorotannins from Fucus spp. of the Northern Portuguese Coastline: Chemical Approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure Dependent Antioxidant Capacity of Phlorotannins from Icelandic Fucus Vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Allwood, J.W.; Evans, H.; Austin, C.; McDougall, G.J. Extraction, Enrichment, and LC-MSn-Based Characterization of Phlorotannins and Related Phenolics from the Brown Seaweed, Ascophyllum Nodosum. Mar. Drugs 2020, 18, 448. [Google Scholar] [CrossRef]
- Dhanraj, V.; Karuppaiah, J.; Balakrishnan, R.; Elangovan, N. Myricetin Attenuates Neurodegeneration and Cognitive Impairment in Parkinsonism. Front. Biosci. Elit. 2018, 10, 481–494. [Google Scholar] [CrossRef]
- McLafferty, F.W. Mass Spectrometric Analysis: Aliphatic Halogenated Compounds. Anal. Chem. 1962, 34, 2–15. [Google Scholar] [CrossRef]
- Holdsworth, D.K. Mass Spectral Analysis of Halogen Compounds. J. Chem. Educ. 1983, 60, 103–104. [Google Scholar] [CrossRef]
- Vetter, W. Marine Halogenated Natural Products of Environmental Relevance. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2006; Volume 188, pp. 1–57. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two New Bromophenols from the Red Alga Odonthalia Corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Popplewell, W.L.; Northcote, P.T. Colensolide A: A New Nitrogenous Bromophenol from the New Zealand Marine Red Alga Osmundaria Colensoi. Tetrahedron Lett. 2009, 50, 6814–6817. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-Vitro Cytotoxic Activities of the Major Bromophenols of the Red Alga Polysiphonia Lanosa and Some Novel Synthetic Isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Jacobtorweihen, J.; Schmitt, M.; Spiegler, V. Amino Acid-Coupled Bromophenols and a Sulfated Dimethylsulfonium Lanosol from the Red Alga Vertebrata Lanosa. Mar. Drugs 2022, 20, 420. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Subbiah, V.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Evaluation of the Antioxidant Potential of Brown Seaweeds Extracted by Different Solvents and Characterization of Their Phenolic Compounds by LC–ESI-QTOF–MS/MS. J. Food Sci. 2023, 88, 3737–3757. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate Composition, Phenolic Content and in Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum Nodosum, Bifurcaria Bifurcata and Fucus Vesiculosus. Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil Unde. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus Vesiculosus by Pressurized Liquid Extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Aznar-Ramos, M.J.; Verardo, V.; Gómez-Caravaca, A.M. Development of an Effective Sonotrode Based Extraction Technique for the Recovery of Phenolic Compounds with Antioxidant Activities in Cherimoya Leaves. Plants 2022, 11, 2034. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]




| Independent Factors | Dependent Factor | |||
|---|---|---|---|---|
| No | X1 | X2 | X3 | TPC (mg GAE/g Dry Seaweed) |
| 1 | 10 (−1) | 2 (−1) | 30 (0) | 1.70 ± 0.12 |
| 2 | 10 (−1) | 21 (0) | 20 (−1) | 1.68 ± 0.07 |
| 3 | 10 (−1) | 21 (0) | 40 (1) | 2.52 ± 0.13 |
| 4 | 10 (−1) | 40 (1) | 30 (0) | 2.39 ± 0.15 |
| 5 | 50 (0) | 2 (−1) | 20 (−1) | 2.20 ± 0.09 |
| 6 | 50 (0) | 2 (−1) | 40 (1) | 2.46 ± 0.20 |
| 7 | 50 (0) | 21 (0) | 30 (0) | 2.63 ± 0.14 |
| 8 | 50 (0) | 21 (0) | 30 (0) | 2.72 ± 0.16 |
| 9 | 50 (0) | 21 (0) | 30 (0) | 2.70 ± 0.13 |
| 10 | 50 (0) | 40 (1) | 20 (−1) | 2.62 ± 0.17 |
| 11 | 50 (0) | 40 (1) | 40 (1) | 2.93 ± 0.09 |
| 12 | 90 (1) | 2 (−1) | 30 (0) | 0.71 ± 0.06 |
| 13 | 90 (1) | 21 (0) | 20 (−1) | 0.95 ± 0.11 |
| 14 | 90 (1) | 21 (0) | 40 (1) | 1.43 ± 0.17 |
| 15 | 90 (1) | 40 (1) | 30 (0) | 2.34 ± 0.18 |
| Regression Coefficients | TPC (mg GAE/g d.w.) | |
|---|---|---|
| Effect | p-Value | |
| β0 | 1.9933 | 0.0000 * |
| Linear | ||
| β1 | −0.6492 | 0.0033 * |
| β2 | 0.9211 | 0.0016 * |
| β3 | 0.5372 | 0.0048 * |
| Crossed | ||
| β12 | 0.4711 | 0.0112 * |
| β13 | −0.1781 | 0.0710 |
| β23 | 0.0276 | 0.6377 |
| Quadratic | ||
| β11 | 0.9069 | 0.0008 * |
| β22 | −0.0005 | 0.9863 |
| β33 | 0.1402 | 0.0328 * |
| R2 | 0.9942 | |
| p model | 0.0025 * | |
| p lack of fit | 0.1945 | |
| Parameter | Optimal Conditions |
|---|---|
| Ethanol (%) | 40 |
| Time (min) | 38 |
| Amplitude (%) | 36 |
| Results | |
| TPC predicted value (mg GAE/g d.w.) | 3.01 ± 0.14 |
| TPC empirical value (mg GAE/g d.w.) | 2.89 ± 0.24 |
| Coefficient of variation (%) | 6.19 |
| Yield of extraction (mg dry extract/g dry algae) | 245.2 ± 9.1 |
| Peak No. | Retention Time (min) | m/z Exp. [M-H]− | m/z Calc. [M-H]− | Molecular Formula | Error (ppm) | Score | Fragments | Proposed Compound |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids and derivatives | ||||||||
| 1 | 1.101 | 246.9944 | 246.9912 | C8H8O7S | 13 | 92.23 | 203.0029 (-CO2), 123.0428 (-CO2, -SO3), 121.0294, 108.0213 | Vanillic acid 4-sulfate |
| 3 | 2.206 | 233.0119 | 233.0120 | C8H10O6S | −0.4 | 85.44 | 153.0521 (-SO3), 123.0462 | Hydroxy tyrosol sulfate |
| Phlorotannins | ||||||||
| 2 | 1.73 | 497.072 | 497.072 | C24H18O12 | −1.1 | 99.99 | 479.0722 (-H2O), 353.0290(-1PGU, -H2O, -1), 339.0494, 230.0214 (-2PGU, -H2O, +1), 139.0032, 124.0742 | Fucodiphloroethol |
| 4 | 3.212 | 745.1027 | 745.1041 | C36H26O18 | −1.9 | 86.13 | 727.0961 (-H2O), 709.0927 (-2H2O), 585.0652, 477.0439 (-2 PGU, -16/-2 PGU, -H2O, +2), 453.0212, 353.0500, 267.0306, 229.0145, 165.0181 | Fucophlorethol hexamer I |
| 5 | 3.402 | 745.1021 | 745.1041 | C36H26O18 | −2.7 | 97.96 | 727.0924 (-H2O), 709.0813 (-2H2O), 621.0874 (-1PGU, +2), 603.0735 (-1PGU, -H2O. +2), 537.0646, 453.0399, 411.0307, 353.0197, 245.0097, 165.0190, 139.0041 | Fucophlorethol hexamer II |
| 6 | 3.5 | 621.0878 | 621.088 | C30H22O15 | −0.3 | 99.01 | 603.0768 (-H2O), 585.0658 (-2H2O), 559.0663, 477 (-1PGU, -H2O), 353.0272, 335.0200, 229.0137 (-3PGU, -H2O), 139.0058 | Fucotriphlorethol isomer I |
| 7 | 3.84 | 621.0879 | 621.088 | C30H22O15 | −0.2 | 99.96 | 603.0775 (-H2O), 585.0636 (-2H2O), 477.0457 (-1PGU, -H2O), 339.0500, 245.0087, 229.0143 (-3PGU, -H20, +4), 139.0042 | Fucotriphlorethol isomer II |
| 8 | 5.401 | 745.1033 | 745.1041 | C36H26O18 | −1.1 | 82.9 | 727.0924 (-H2O), 601.0630 (-1PGU, -H2O), 583.0519 (-1PGU, -2H2O), 477.0485 (-2PGU, -16/-2PGU, -H2O, +2), 461.0493, 353.0289, 335.0193, 245.0081, 229.0141, 139.0038 | Fucophlorethol hexamer III |
| 9 | 5.645 | 745.1020 | 745.1041 | C36H26O18 | −2.8 | 95.71 | 727.0910 (-H2O), 709.0802 (-2H2O), 601.0621 (-1PGU, -H2O), 583.0531, 477.0448 (-2PGU, -16/-2PGU, -H2O, +2), 339.0496, 229.0128 | Fucophlorethol hexamer IV |
| 10 | 6.948 | 869.1188 | 869.1201 | C42H30O21 | −1.5 | 99.99 | 851.1092 (-H2O), 833.1029 (-2H2O), 725.0798 (-1PGU, -H2O), 707.0670 (-1PGU, -2H2O), 619.0766 (-2PGU, +2), 601.0629 (-2PGU, -16/-2PGU, -H2O, +2), 461.0506, 353.0299, 335.0214, 245.0108, 229.0146, 139.0029 | Fucophlorethol heptamer I |
| 11 | 7.233 | 869.1198 | 869.1201 | C42H30O21 | −0.3 | 81.55 | 851.1093 (-H2O), 833.0983 (-2H2O),725.0778 (-1PGU, -H2O), 707.0662 (-1PGU, -2H2O), 477.0472, 427.9290, 367.0085, 339.0498, 245.0094, 229.0132 | Fucophlorethol heptamer I |
| 12 | 8.057 | 993.1396 | 993.1362 | C48H34O24 | 2.2 | 93.17 | 975.1302 (-H2O), 869.1177 (-PGU), 851.1154 (-PGU, -H2O), 603.0748, 461.0552, 229.0147 | Fucophloretol octamer I |
| 13 | 8.409 | 993.1406 | 993.1362 | C48H34O24 | 4.4 | 81.64 | 975.1277 (-H2O), 849.0968 (-1PGU, -H2O), 635.0671, 601.0621, 461.0542, 353.0300, 247.0234, 229.0155 | Fucophlorethol octamer II |
| 14 | 8.607 | 933.1369 | 993.1362 | C48H34O24 | 0.7 | 99.87 | 975.1270 (-H2O), 849.0989 (-1PGU, -H2O), 831.0840, 745.1002, 711.1008, 603.0814, 477.0471, 353.0313, 245.0103, 229.0139 | Fucophlorethol octamer III |
| 15 | 9.385 | 591.0070 | 591.0081 | C24H16O16S | −1.9 | 100 | 511.0480 (-SO3), 385.0163 (-1PGU, -SO3, +1), 245.0124, 229.0139, 139.0042 | Diphlorethohydroxycarmalol sulphate |
| 16 | 9.509 | 1241.1735 | 1241.1683 | C60H42O30 | 1.8 | 87.32 | 1223.1594 (-H2O), 1117.1472 (-1PGU, +2), 1099.1378 (-1PGU, -H2O, +2), 487.1113, 392.1327, 353.0309, 257.0666, 229.0140 | Fucophlorethol decamer |
| 17 | 9.745 | 1117.1542 | 1117.1522 | C54H38O27 | 1.8 | 100 | 1099.1375 (-H2O), 975.1223 (-PGU, -H2O), 835.1108, 601.0684, 353.0303, 245.0073, 229.0148 | Fucophlorethol nonamer |
| Flavonoids | ||||||||
| 19 | 10.883 | 317.0298 | 317.0297 | C15H10O8 | 0.3 | 100 | 178.9658 | Myricetin |
| Halophenols | ||||||||
| 18 | 10.618 | 376.8174 | 376.8330 | C7H6O6SBr2 | - | - | 296.8598 (-SO3), 245.0122, 96.9615 | Lanosol sulfate |
| 20 | 10.883 | 476.7614 | - | - | - | - | 396.8675 (-SO3) | Dibromophenol sulfate |
| 21 | 10.883 | 454.8676 | - | - | - | - | 374.8629 (-SO3) | Dibromophenol sulfate |
| 22 | 10.932 | 154.9906 | 154.9900 | C7H5O2Cl | 3.9 | 100 | - | 2-Chlorobenzoic acid |
| 23 | 10.957 | 332.9717 | 332.9721 | C16H8O4Cl2 | −1.2 | 99.57 | 154.9906, 111.0001 | Dichlorophenol |
| 24 | 10.957 | 392.9803 | - | - | - | - | - | Trichlorophenol |
| 25 | 10.957 | 488.9576 | - | - | - | - | - | Tetrachlorophenol |
| Antioxidant Assay | Results |
|---|---|
| FRAP (mmol TE/g d.w.) | 143.7 ± 5.8 |
| DPPH (EC50 μg d.w./mL) | 105.6 ± 3.2 |
| TEAC (mmol Trolox/g d.w.) | 189.1 ± 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martínez, L.; Santos-Mejías, A.; De la Torre-Ramírez, J.M.; Baños, A.; Verardo, V.; Gómez-Caravaca, A.M. Optimization of a Sonotrode Extraction Method and New Insight of Phenolic Composition of Fucus vesiculosus. Mar. Drugs 2025, 23, 40. https://doi.org/10.3390/md23010040
Gil-Martínez L, Santos-Mejías A, De la Torre-Ramírez JM, Baños A, Verardo V, Gómez-Caravaca AM. Optimization of a Sonotrode Extraction Method and New Insight of Phenolic Composition of Fucus vesiculosus. Marine Drugs. 2025; 23(1):40. https://doi.org/10.3390/md23010040
Chicago/Turabian StyleGil-Martínez, Lidia, Alejandro Santos-Mejías, José Manuel De la Torre-Ramírez, Alberto Baños, Vito Verardo, and Ana M. Gómez-Caravaca. 2025. "Optimization of a Sonotrode Extraction Method and New Insight of Phenolic Composition of Fucus vesiculosus" Marine Drugs 23, no. 1: 40. https://doi.org/10.3390/md23010040
APA StyleGil-Martínez, L., Santos-Mejías, A., De la Torre-Ramírez, J. M., Baños, A., Verardo, V., & Gómez-Caravaca, A. M. (2025). Optimization of a Sonotrode Extraction Method and New Insight of Phenolic Composition of Fucus vesiculosus. Marine Drugs, 23(1), 40. https://doi.org/10.3390/md23010040








