New Polyketides and a Ferroptosis Inhibitor from the Marine-Derived Fungus Diaporthe searlei CS-HF-1
Abstract
1. Introduction
2. Results and Discussion
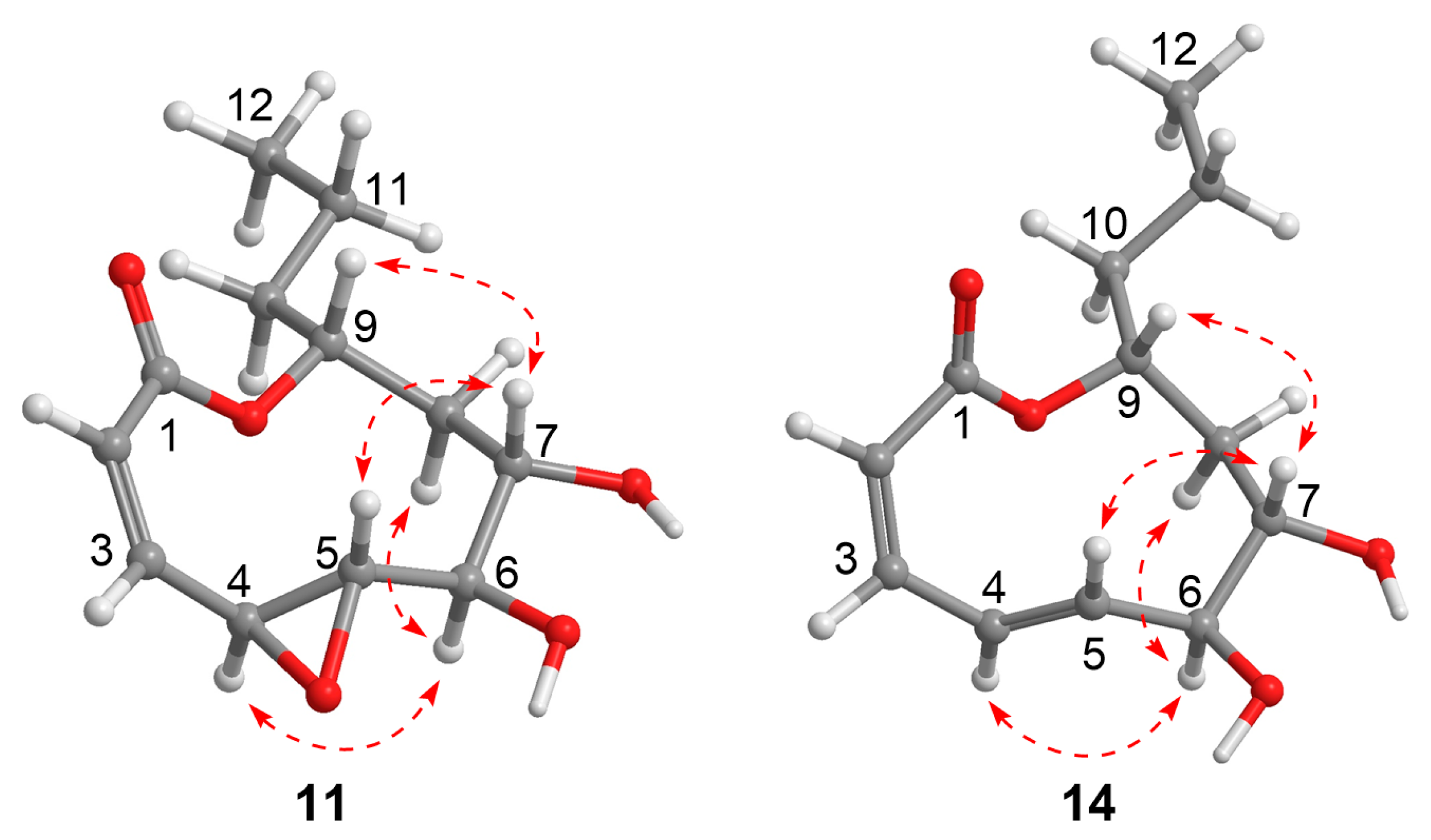
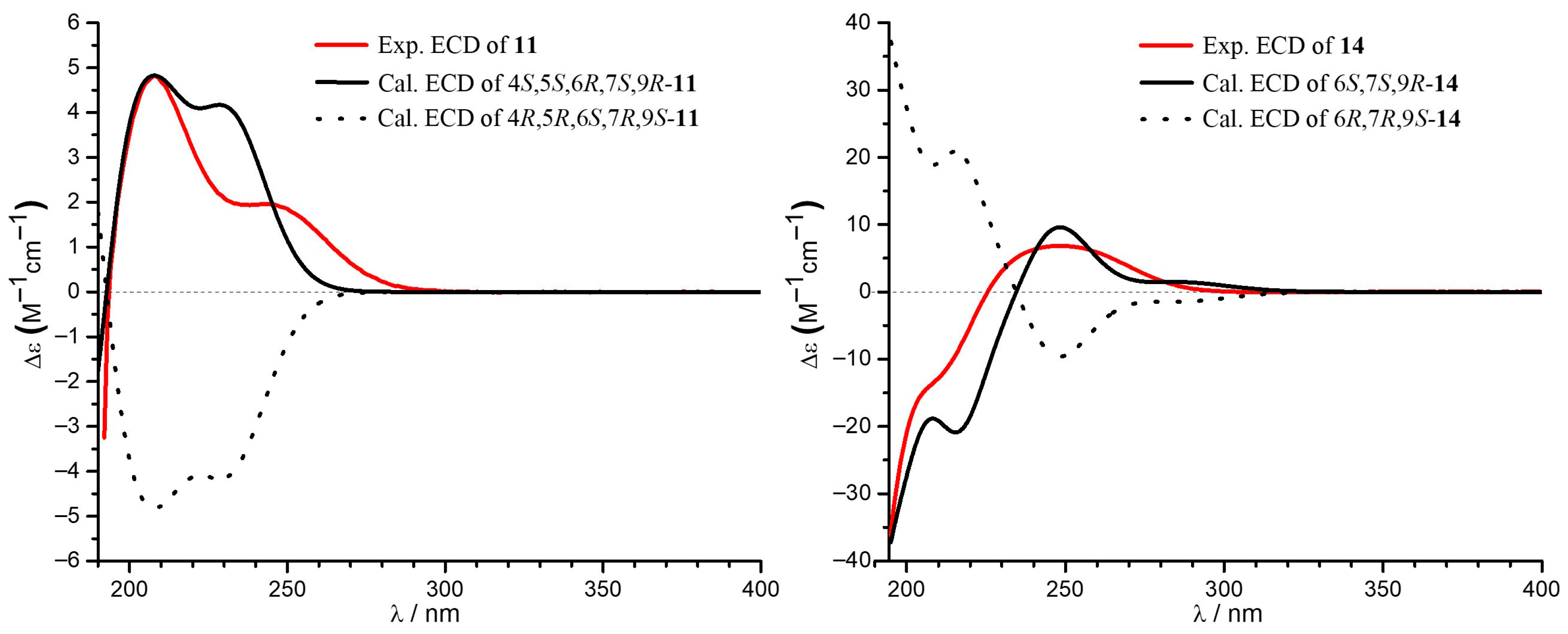
2.1. Structural Elucidation
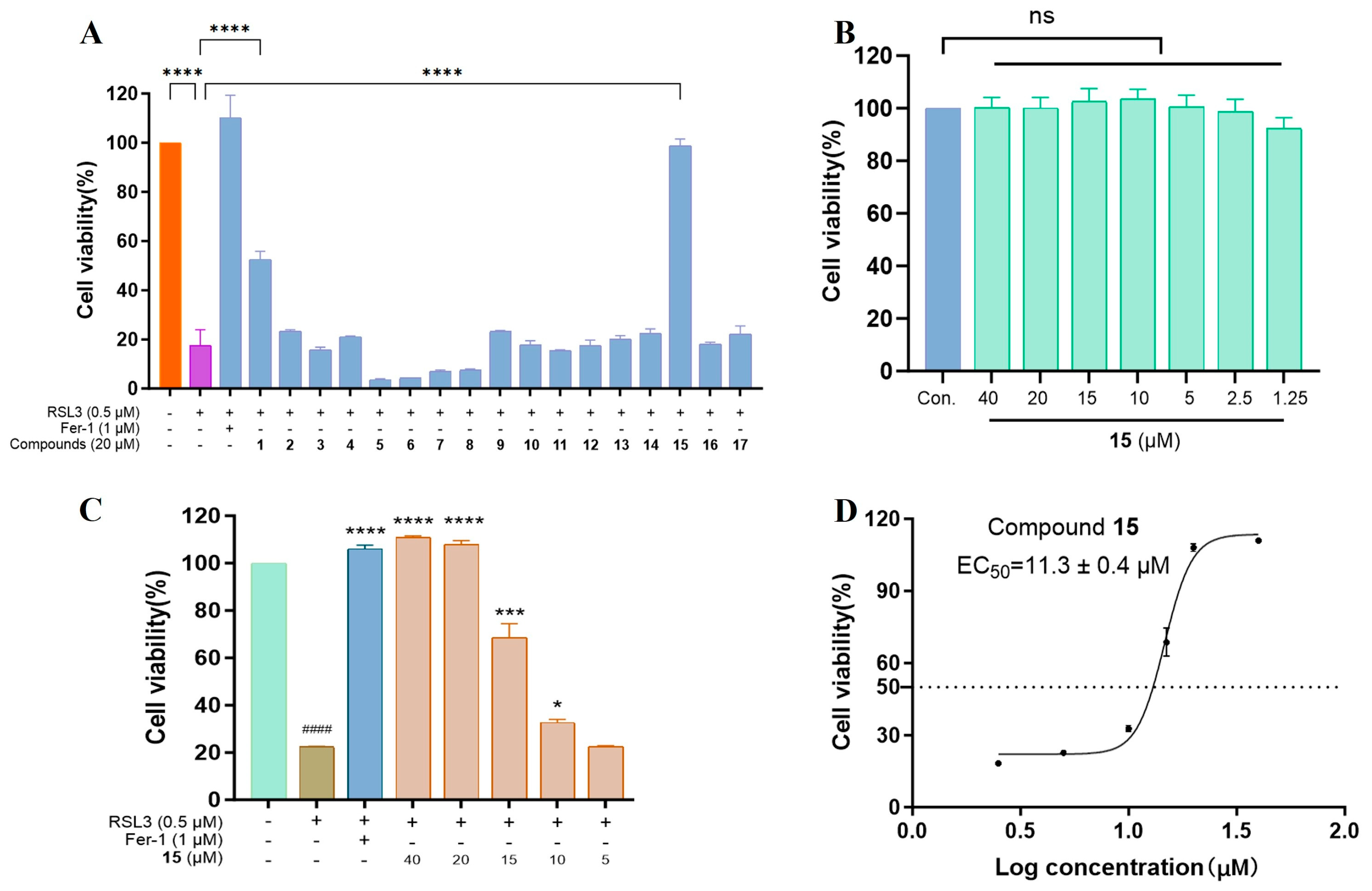
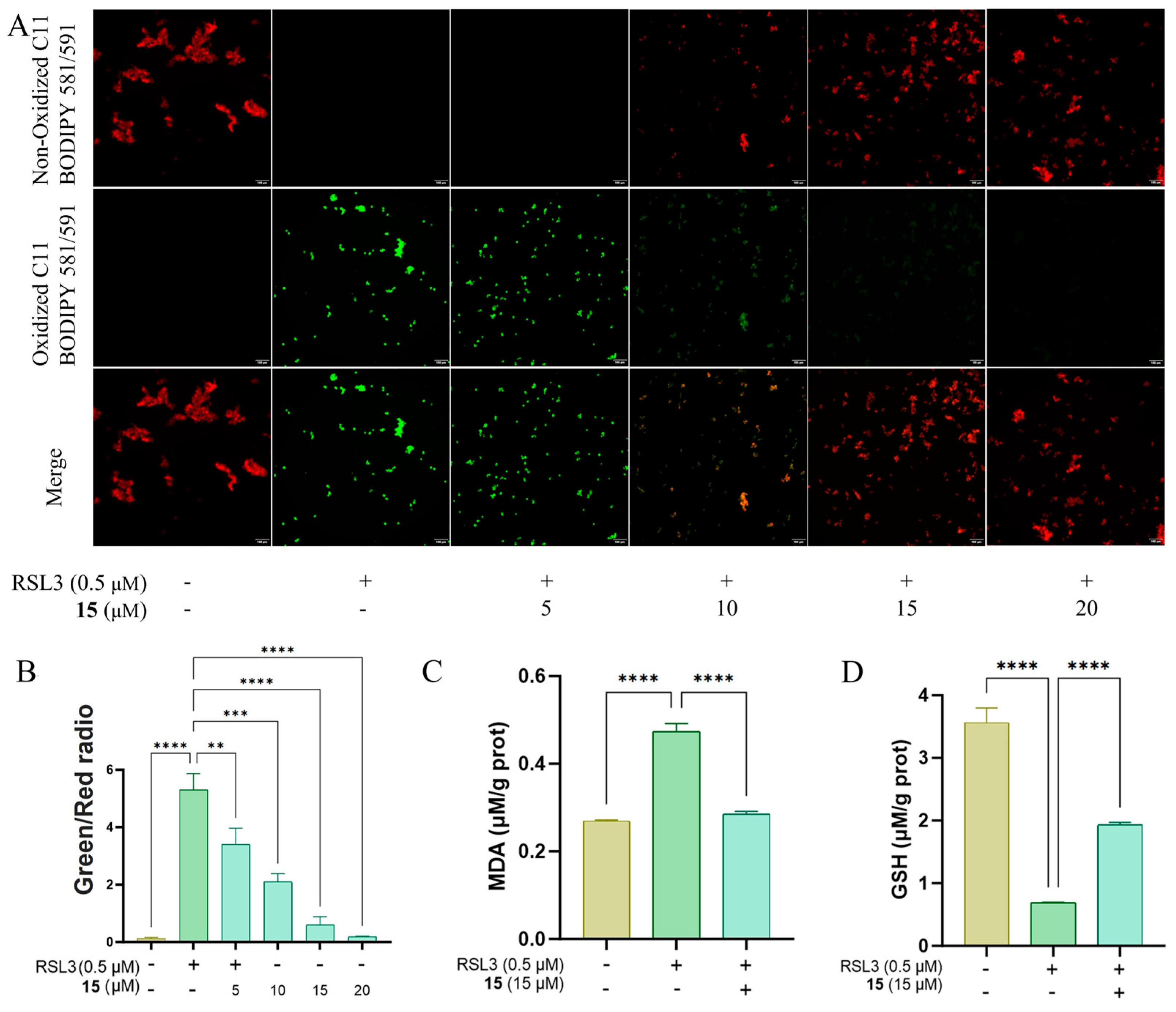
2.2. Biological Activity Assessment
2.2.1. Anti-Ferroptotic Activity in an RSL3-Induced Ferroptosis Model in HT22 Cells
2.2.2. Anti-Inflammatory Activity Against LPS-Activated NO Production in RAW264.7 Macrophages
3. Experimental Section
3.1. General Experimental Procedure
3.2. Fungal Strain and Identification
3.3. Fermentation, Extraction, and Isolation
3.4. ECD and NMR Calculation
3.5. Biological Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, S.; Ma, X.; Wu, H.; Zang, R.; Li, H.; Liu, M.; Li, Q.; Ma, Q.; Guo, Y.; Zhang, M. The six whole mitochondrial genomes for the Diaporthe species: Features, evolution and phylogeny. IMA Fungus 2025, 16, e140572. [Google Scholar] [CrossRef]
- Xu, T.-C.; Lu, Y.-H.; Wang, J.-F.; Song, Z.-Q.; Hou, Y.-G.; Liu, S.-S.; Liu, C.-S.; Wu, S.-H. Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms 2021, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Tong, W.-Y.; Leong, C.-R.; Tan, W.-N. Potential of endophytic Diaporthe sp. as a new source of bioactive compounds. J. Microbiol. Biotechnol. 2021, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhuang, S.; Qiao, Y.; Wu, Q.; Ma, Y.; Chen, S.; Liu, L.; Yan, Y.; Gao, Z. Metabolic shunting in marine-derived Diaporthe sp. SYSU-MS4722 yields diverse γ-butyrolactone derivatives with anti-inflammatory activity. J. Nat. Prod. 2025, 88, 1605–1615. [Google Scholar] [CrossRef]
- Yuan, S.; Zhuang, S.; Han, Y.; Wen, Y.; Chen, S.; Liu, L.; Lin, Q.; Yan, Y.; Gao, Z.; Wu, Q. Deletion of a polyketide synthase phoE induces monoterpenes production in ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Front. Microbiol. 2025, 16, 1593027. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yan, Y.; Zhou, Y.; Han, Y.; Yuan, S.; Chen, J.; Guo, H.; Lin, Z.; Lin, Q.; Chen, S.; et al. Genome mining of nonenzymatic ortho-quinone methide-based pseudonatural products from ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Bioorg. Chem. 2025, 154, 108081. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, T.; Sun, B.; Li, J.; She, Z.; Hu, G.; Wang, B. Oxygen-bridged cyclooctadienes and other polyketides from the mangrove endophytic fungus Diaporthe sp. ZJHJYZ-1. J. Mol. Struct. 2024, 1302, 137534. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, W.; Chen, T.; Tan, Q.; Zou, G.; Zang, Z.; Li, J.; Wang, B.; She, Z. Cytosporones W and X: Two mutually converting epimers from a mangrove endophytic fungus Diaporthe sp. ZJHJYZ-1. ACS Omega 2023, 8, 26628–26634. [Google Scholar] [CrossRef]
- Xing, D.-X.; Song, X.-S.; Pan, W.-C.; Cui, H.; Zhao, Z.-X. New chromone compounds from the marine derived fungus Diaporthe sp. XW12-1. Fitoterapia 2023, 164, 105384. [Google Scholar] [CrossRef]
- Zhai, G.; Chen, S.; Shen, H.; Guo, H.; Jiang, M.; Liu, L. Bioactive monoterpenes and polyketides from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 553. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Jiang, M.; Wu, Q.; Li, J.; Shen, H.; Liu, L. Mono- and dimeric xanthones with anti-glioma and anti-inflammatory activities from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 51. [Google Scholar] [CrossRef]
- Luo, X.-W.; Chen, C.-M.; Li, K.-L.; Lin, X.-P.; Gao, C.-H.; Zhou, X.-F.; Liu, Y.-H. Sesquiterpenoids and meroterpenoids from a mangrove derived fungus Diaporthe sp. SCSIO 41011. Nat. Prod. Res. 2021, 35, 282–288. [Google Scholar] [CrossRef]
- Hu, C.; Li, S.; Chen, Y.; Gao, X.; Liu, Z.; Zhang, W. Polyketides from the deep-sea-derived fungus Diaporthe phaseolorum FS459. Chin. J. Org. Chem. 2021, 41, 1591–1598. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, G.; Yang, W.; Zhao, Y.; Tan, Q.; Chen, L.; Wang, J.; Ma, C.; Kang, W.; She, Z. Metabolites with anti-Inflammatory activity from the mangrove endophytic fungus Diaporthe sp. QYM12. Mar. Drugs 2021, 19, 56. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Li, S.; Wang, Q.; Hu, C.; Liu, H.; Zhang, W. Bioactive metabolites from the deep-sea-derived fungus Diaporthe longicolla FS429. Mar. Drugs 2020, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Chen, Y.; Guo, H.; Li, S.-N.; Li, H.-H.; Liu, H.-X.; Liu, Z.; Zhang, W. Cytotoxic polyketides from a deep-sea sediment derived fungus Diaporthe phaseolorum FS431. Molecules 2019, 24, 3062. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Z.-M.; Chen, Y.-C.; Tan, H.-B.; Li, S.-N.; Li, H.-H.; Gao, X.-X.; Liu, H.-X.; Zhang, W.-M. Chromone-derived polyketides from the deep-sea fungus Diaporthe phaseolorum FS431. Mar. Drugs 2019, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, J.; Chen, F.; Lin, X.; Chen, C.; Zhou, X.; Liu, S.; Liu, Y. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza a virus activities. Front. Chem. 2018, 6, 282. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A-F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Chen, S.; Ding, M.; Huang, X.; Yuan, J.; She, Z. Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorg. Med. Chem. Lett. 2017, 27, 803–807. [Google Scholar] [CrossRef]
- Cui, H.; Ding, M.; Huang, D.; Zhang, Z.; Liu, H.; Huang, H.; She, Z. Chroman-4-one and pyrano [4,3-b] chromenone derivatives from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. RSC Adv. 2017, 7, 20128–20134. [Google Scholar] [CrossRef]
- Zang, L.Y.; Wei, W.; Guo, Y.; Wang, T.; Jiao, R.H.; Ng, S.W.; Tan, R.X.; Ge, H.M. Sesquiterpenoids from the mangrove-derived endophytic fungus Diaporthe sp. J. Nat. Prod. 2012, 75, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-X.; Hao, Y.; Yan, B.-Y.; Jin, Y.; Gao, Z.; Li, X.-W. Diverse secondary metabolites from the Ligia exotica-derived fungus Diaporthe melitensis HZ-1. Chem. Biodivers. 2025, 22, e202500919. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wei, X.; Xing, S.; Cheng, X.; Lu, J.; Yang, C.; Zhao, G.; Dai, Z.; Hou, S.; Liu, Y.; et al. Polyketide production in a mangrove-associated fungus Diaporthe goulteri, induced by chemical epigenetic modification. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Lu, H.; Feng, Y.; Lin, M.; Yu, X.; Liu, Y.; Tan, Y.; Luo, X. Discovery of polyketides and alkaloids from the mangrove endophytic fungus Diaporthe sp. and their anti-osteoclastogenic bioactivities. Chem. Biodivers. 2025, 22, e202500858. [Google Scholar] [CrossRef]
- Brown, A.R.; Hirschhorn, T.; Stockwell, B.R. Ferroptosis—Disease perils and therapeutic promise. Science 2024, 386, 848–849. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Bio. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Li, H.; Puopolo, T.; Seeram, N.P.; Liu, C.; Ma, H. Anti-ferroptotic effect of cannabidiol in human skin keratinocytes characterized by data-independent acquisition-based proteomics. J. Nat. Prod. 2024, 87, 1493–1499. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, Y.-S.; Li, J.-J.; Wan, Z.-Y.; Zhang, H. Unusual chaetoglobosins and a new type of ferroptosis inducer from an endophytic fungus Chaetomium sp. UJN-EF006. Bioorg. Chem. 2025, 158, 108342. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xiao, J.; Xu, W.; Liu, W.; Luo, Z.; Wu, P.; Yang, X.; Zhang, Z.; Cheng, Z. Agathic acid derivatives including a novel ferroptosis inhibitor from the fungus Penicillium thomii. Bioorg. Chem. 2025, 164, 108867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Wang, H.; Xu, W.; Wang, C.; Ma, H.; Zhong, F.; Ou, J.; Luo, Z.; Luo, H.-B.; et al. Ergone derivatives from the deep-sea-derived fungus Aspergillus terreus YPGA10 and 25,28-dihydroxyergone-induced apoptosis in human colon cancer SW620 Cells. J. Nat. Prod. 2024, 87, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ma, H.; Wu, Y.; Wang, C.; Li, Y.; Li, Q.; Cheng, Z. Andrastin-type meroterpenoids, α-pyrone polyketides, and sesquicarane derivatives from Penicillium sp., a fungus isolated from Pinus koraiensis seed. Phytochemistry 2024, 225, 114202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J.; Liu, R.; Liu, Y.; Liu, R.; Wu, Z.; Xu, W.; Ma, H.; Luo, H.-B.; Cheng, Z. Structure revisions of phenolic bisabolane sesquiterpenes and a ferroptosis inhibitor from the marine-derived fungus Aspergillus versicolor YPH93. J. Nat. Prod. 2023, 86, 830–841. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.; Fan, R.Z.; Zhang, J.; Li, Y.L.; Wang, X.W.; Han, S.Y.; Liu, W.; Pan, M.H.; Cheng, Z.B. Penithoketone and penithochromones A-L, polyketides from the deep-sea-derived fungus Penicillium thomii YPGA3. J. Nat. Prod. 2020, 83, 2679–2685. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Li, Y.L.; Xu, W.; Liu, W.; Liu, L.J.; Zhu, D.G.; Kang, Y.; Luo, Z.H.; Li, Q. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorg. Chem. 2019, 91, 103129. [Google Scholar] [CrossRef]
- Dominique, S.; Alex, P.G.; Christiane, E.Y.; Dodehe, Y.; Adèle, K.N. Diversity of endophytic fungi isolated from the bark of Ceiba pentandra (L.) Gaertn., (Bombacaceae) and antibacterial potential of secalonic acid A produced by Diaporthe searlei EC 321. Chem. Biodivers. 2023, 20, e202301010. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Wang, M.-Z.; Huang, Y.-J.; Shen, Y.-M. Secondary metabolites from Phomopsis sp. A123. Mycology 2010, 1, 254–261. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S.; Ogilvie, K.K.; Watson, A.K. Phytotoxic metabolites of Phomopsis convolvulus, a host-specific pathogen of field bindweed. Can. J. Chem. 1992, 70, 2276. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Hu, Z.-Y.; Shen, Y.-M. Two new cyclopeptides and one new nonenolide from Xylaria sp. 101. Nat. Prod. Commun. 2011, 6, 1843–1846. [Google Scholar] [CrossRef]
- Mopuri, S.R.; Palakodety, R.K. Total synthesis of the proposed structure of xylarolide. Tetrahedron Lett. 2019, 60, 150944. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, L.; Qin, X.; Huang, J.; Yang, R.; Li, J.; Huang, X. A new lactone from mangrove endophytic fungus Aspergillus sp. GXNU-A9. Nat. Prod. Res. 2023, 37, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Sawamura, S.; Kawakami, Y.; Sakamura, S. Dihydrogladiolic acid, another phytotoxin from Phoma asparagi Sacc. Agric. Biol. Chem. 1985, 49, 1891. [Google Scholar]
- Fujita, M.; Yamada, M.; Nakajima, S.; Kawai, K.; Nagai, M. O-Methylation effect on the carbon-13 nuclear magnetic resonance signals of ortho-disubstituted phenols and its application to structure determination of new phthalides from Aspergillus silvaticus. Chem. Pharm. Bull. 1984, 32, 2622. [Google Scholar] [CrossRef]
- Waugh, T.M.; Masters, J.; Aliev, A.E.; Marson, C.M. Monocyclic quinone structure-activity patterns: Synthesis of catalytic inhibitors of topoisomerase ii with potent antiproliferative activity. ChemMedChem 2020, 15, 114–124. [Google Scholar] [CrossRef]
- Sharma, A.; Slaughter, A.; Jena, N.; Feng, L.; Kessl, J.J.; Fadel, H.J.; Malani, N.; Male, F.; Wu, L.; Poeschla, E.; et al. A new class of multimerization selective inhibitors of HIV-1 integrase. PLoS Pathog. 2014, 10, e1004171. [Google Scholar] [CrossRef]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Zhao, J.J.; Liu, D.; Proksch, P.; Zhao, Z.M.; Lin, W.H. Eremophilane-type sesquiterpenoids from an Acremonium sp. fungus isolated from deep-sea sediments. J. Nat. Prod. 2016, 79, 1035–1047. [Google Scholar] [CrossRef]
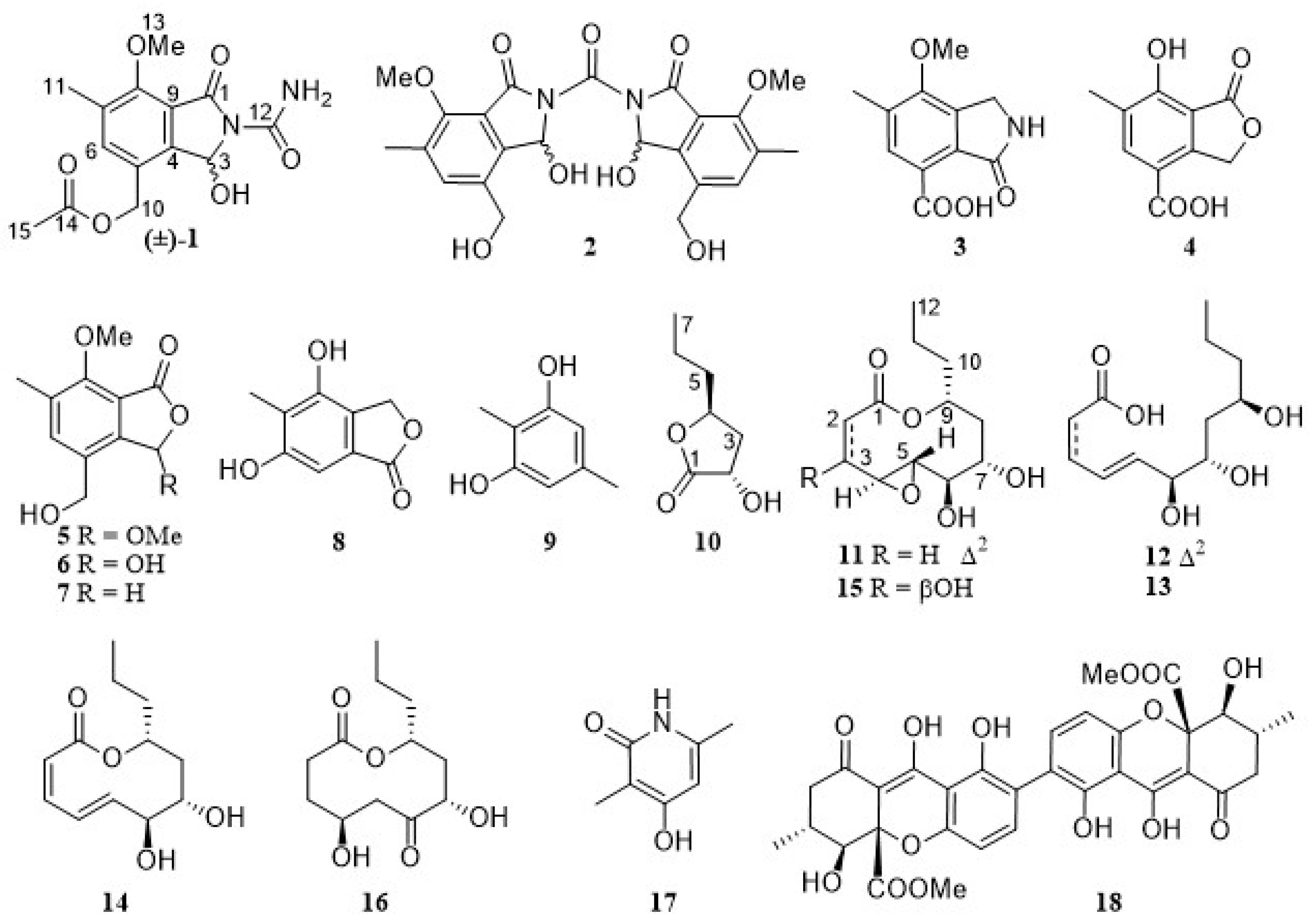
 ) and HMBC (
) and HMBC (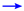 ) correlations of 1–4 and 10–13.
) correlations of 1–4 and 10–13.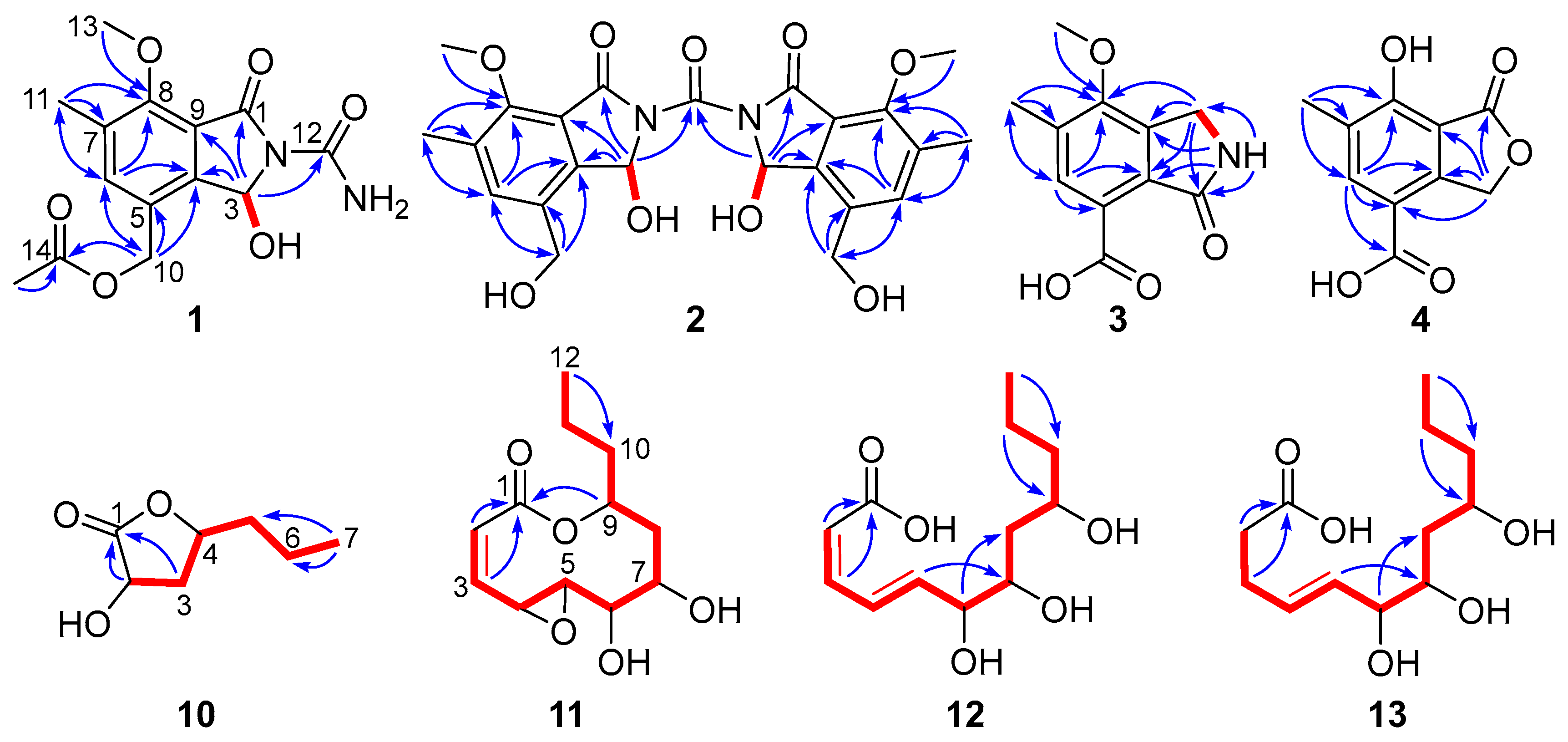
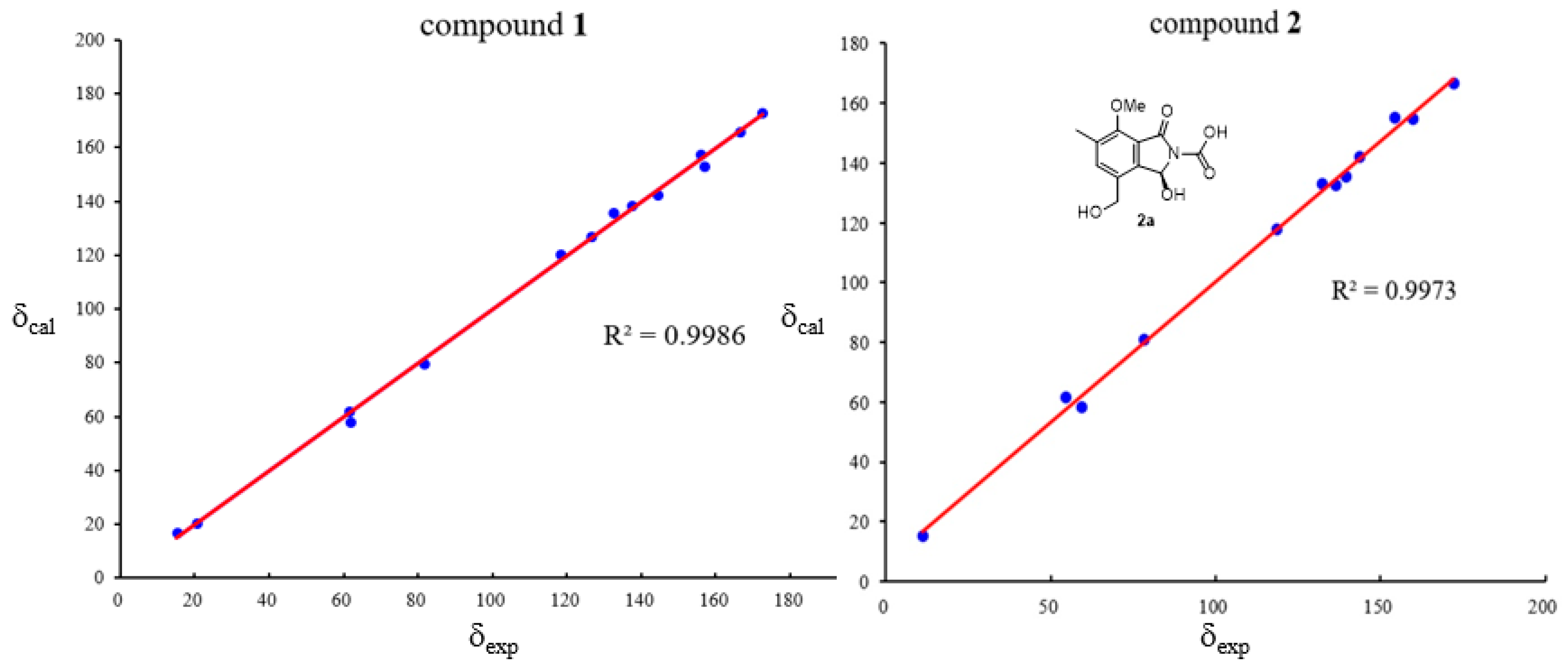

| No. | 1 a | 2 a | 3 a | 4 a | 5 b | 6 b | 7 b | 8 b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δH | δH | δH | δH | |
| 1 | 166.3, C | 166.6, C | 4.39, br s | 45.4, CH2 | 171.3, C | 5.20, s | ||||||
| 2 | 8.51, br s | |||||||||||
| 3 | 6.94, d (10.0) | 81.5, CH | 6.93, d (9.3) | 80.9, CH | 168.9, C | 5.40, s | 70.4, CH2 | 6.40, s | 6.60, s | 5.28, s | ||
| 4 | 144.3, C | 142.2, C | 143.5, C | 148.8, C | ||||||||
| 5 | 126.4, C | 132.8, C | 121.8, C | 115.1, C | 6.78, s | |||||||
| 6 | 7.61, s | 137.4, CH | 7.62, s | 135.5, CH | 7.66, s | 127.6, CH | 7.75, s | 130.2, CH | 7.60, s | 7.61, s | 7.58, s | |
| 7 | 132.6, C | 132.4, C | 132.3, C | 128.1, C | ||||||||
| 8 | 156.0, C | 154.8, C | 160.1, C | 165.9, C | ||||||||
| 9 | 118.3, C | 117.8, C | 128.1, C | 108.0, C | ||||||||
| 10 | 5.08, d (12.7) 4.98, d (12.7) | 61.5, CH2 | 4.51, br s | 58.4, CH2 | 166.4, C | 170.1, C | 4.68, s | |||||
| 11 | 2.28, s | 15.2, CH3 | 2.28, s | 15.2, CH3 | 2.32, s | 16.1, CH3 | 2.23, s | 15.6, CH3 | 2.33, s | 2.33, s | 2.31, s | 2.16, s |
| 12 | 156.9, C | 155.2, C | ||||||||||
| 13 | 3.93, s | 61.7, CH3 | 3.91, s | 61.6, CH3 | 3.80, s | 61.3, CH3 | 4.00, s | 4.00, s | 4.02, s | |||
| 14 | 170.2, C | |||||||||||
| 15 | 2.06, s | 20.5, CH3 | ||||||||||
| NH2 | 5.98, s | |||||||||||
| 3-OH | 7.40, d (10.0) | 7.71, d (9.3) | ||||||||||
| 3-OMe | 3.58, s | |||||||||||
| 10-OH | 5.30, br s | |||||||||||
| No. | 11 | 12 | 13 | 14 | 15 | 16 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δC | δC | |
| 1 | 166.6, C | 169.7, C | 176.2, C | 170.3, C | 171.3, C | 174.2, C | ||||
| 2 | 5.96, d (11.0, 1.5) | 126.0, CH | 5.65, d (11.4) | 118.9, CH | 2.43, m | 34.4, CH2 | 5.91, d (10.6) | 126.3, CH | 40.8, CH2 | 28.4, CH2 |
| 3 | 6.52, d (11.0) | 142.6, CH | 6.68, dd (11.5, 11.4) | 145.4, CH | 2.36, m | 29.0, CH2 | 6.70, d (10.6) | 141.0, CH | 64.9, CH | 28.9, CH2 |
| 4 | 3.75, br s | 57.5, CH | 7.54, dd (15.4, 11.5) | 128.8, CH | 5.73, m | 132.7, CH | 6.22, d (15.3) | 130.4, CH | 62.1, CH | 66.2, CH |
| 5 | 2.60, dd (8.7, 2.0) | 62.6, CH | 6.13, dd (15.4, 6.4) | 144.2, CH | 5.54, dd (14.9, 6.0) | 132.0, CH | 5.36, dd (15.3, 10.1) | 135.3, CH | 55.3, CH | 45.7, CH2 |
| 6 | 3.03, dd (8.7, 8.4) | 79.3, CH | 4.06, dd (6.4, 5.8) | 76.7, CH | 3.83, m | 77.3, CH | 3.78, dd (10.1, 9.1) | 79.3, CH | 78.8, CH | 211.1, C |
| 7 | 3.58, dd (8.4, 8.4) | 73.7, CH | 3.80, m | 72.3, CH | 3.70, m | 72.5, CH | 3.38, dd (9.1, 8.6) | 78.0, CH | 74.1, CH | 75.3, CH |
| 8 | 2.03, m | 41.6, CH2 | 1.51, m | 41.2, CH2 | 1.47, m | 41.2, CH2 | 1.88, dd (17.0, 8.6) 1.79 (17.0) | 42.0, CH2 | 41.6, CH2 | 39.6, CH2 |
| 9 | 4.88, m | 76.3, CH | 3.82, m | 68.8, CH | 3.81, m | 68.9, CH | 4.86, m | 77.1, CH | 74.6, CH | 73.8, CH |
| 10 | 1.69, m; 1.61, m | 38.5, CH2 | 1.43, m | 41.5, CH2 | 1.44, m | 41.4, CH2 | 1.54, m | 40.0, CH2 | 39.0, CH2 | 37.1, CH2 |
| 11 | 1.39, m | 19.3, CH2 | 1.44, m; 1.37, m | 19.9, CH2 | 1.44, m; 1.38, m | 19.9, CH2 | 1.34, m | 19.5, CH2 | 19.2, CH2 | 20.0, CH2 |
| 12 | 0.95, t (7.2) | 14.3, CH3 | 0.93, t (6.9) | 14.4, CH3 | 0.93, t (6.6) | 14.4, CH3 | 0.92, t (7.4) | 14.2, CH3 | 14.3, CH3 | 14.1, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Wu, P.; Zhang, Y.; Lv, Q.; Chi, Y.; Xu, W.; Lin, W.; Cheng, Z. New Polyketides and a Ferroptosis Inhibitor from the Marine-Derived Fungus Diaporthe searlei CS-HF-1. Mar. Drugs 2025, 23, 402. https://doi.org/10.3390/md23100402
Xiao J, Wu P, Zhang Y, Lv Q, Chi Y, Xu W, Lin W, Cheng Z. New Polyketides and a Ferroptosis Inhibitor from the Marine-Derived Fungus Diaporthe searlei CS-HF-1. Marine Drugs. 2025; 23(10):402. https://doi.org/10.3390/md23100402
Chicago/Turabian StyleXiao, Jicheng, Peng Wu, Yan Zhang, Qi Lv, Yulang Chi, Wei Xu, Wenzhen Lin, and Zhongbin Cheng. 2025. "New Polyketides and a Ferroptosis Inhibitor from the Marine-Derived Fungus Diaporthe searlei CS-HF-1" Marine Drugs 23, no. 10: 402. https://doi.org/10.3390/md23100402
APA StyleXiao, J., Wu, P., Zhang, Y., Lv, Q., Chi, Y., Xu, W., Lin, W., & Cheng, Z. (2025). New Polyketides and a Ferroptosis Inhibitor from the Marine-Derived Fungus Diaporthe searlei CS-HF-1. Marine Drugs, 23(10), 402. https://doi.org/10.3390/md23100402





