Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design
Abstract
:1. Introduction
2. Ulva and Ulvan—Structural Characteristics and Extraction
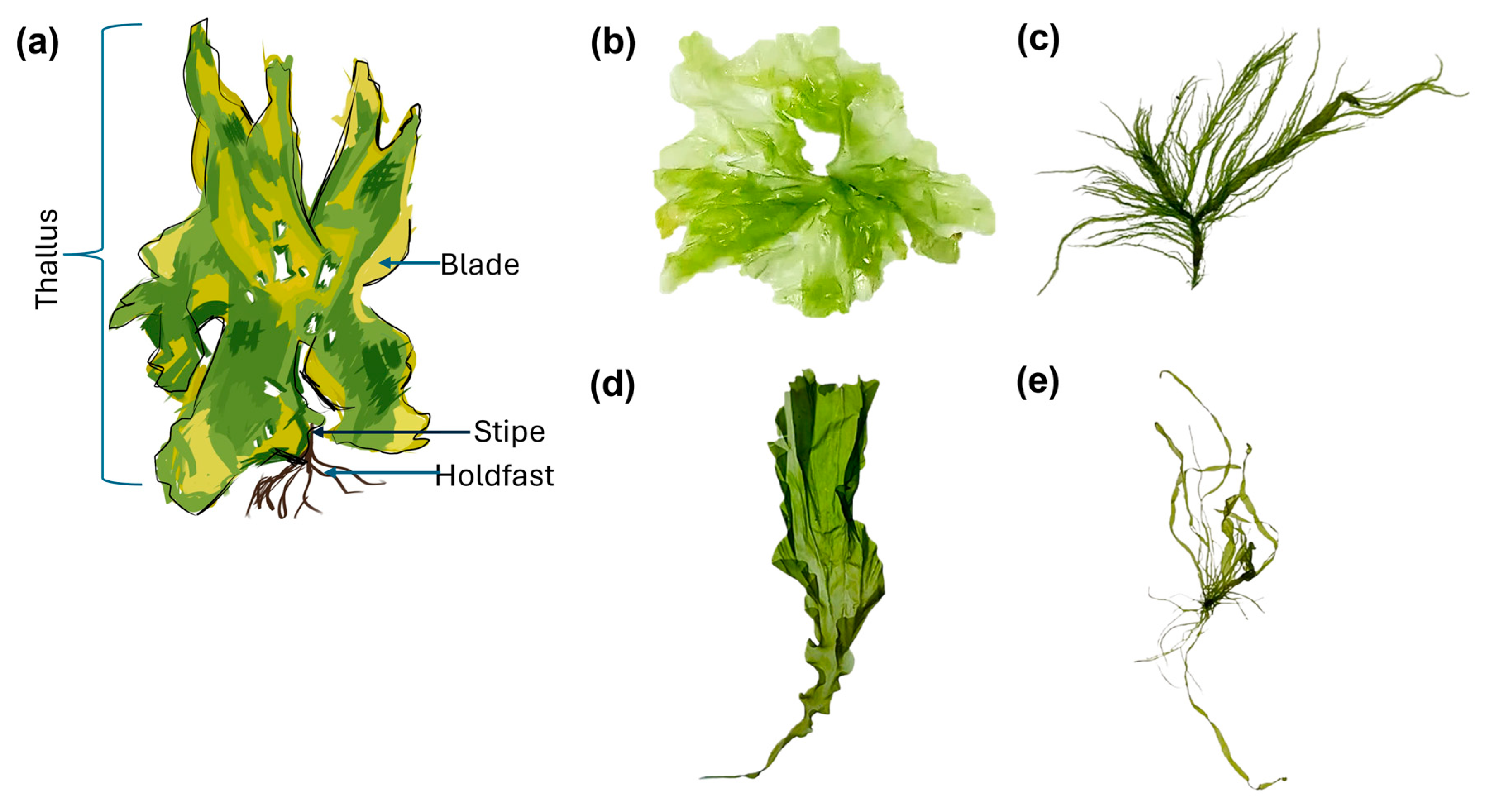
2.1. Ulva
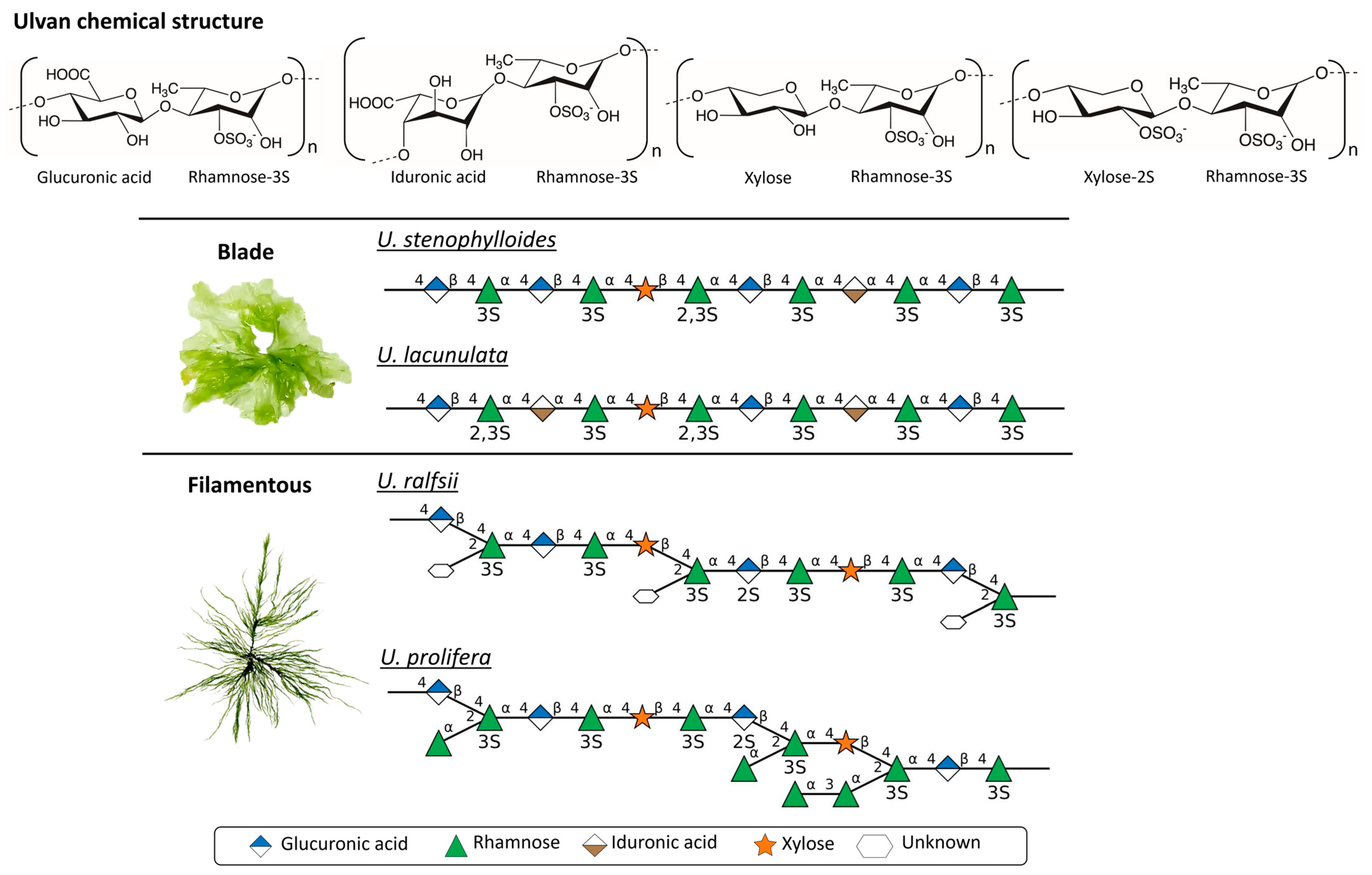
2.2. Ulvan Chemical Structure
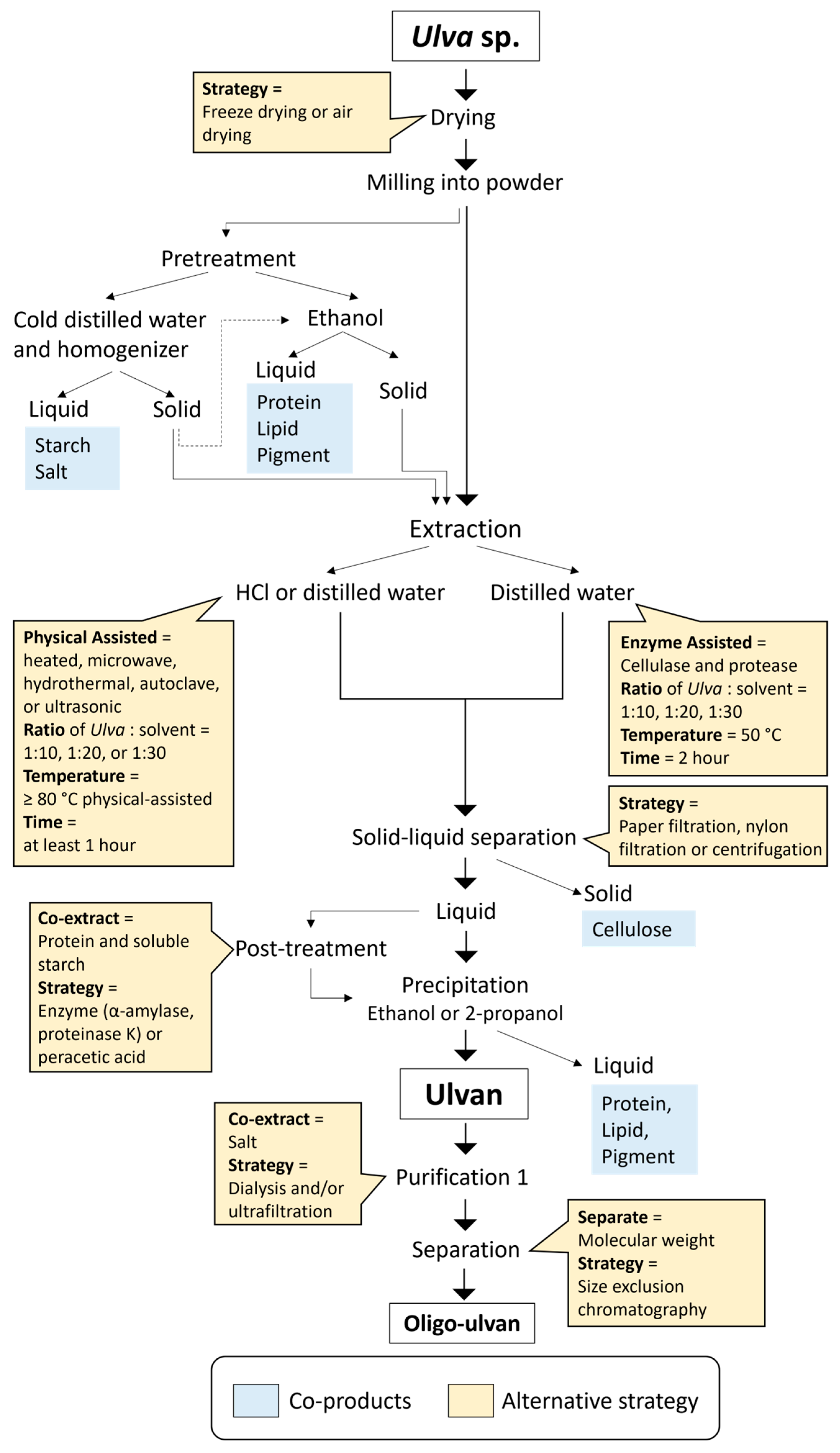
2.3. Ulvan Extraction Strategies
| Species | Yield (% dw) | MW (kDa) | Sulfate (% dw) | Extraction Solvent | T (°C) | Assisted | Solvent for Co-Product | Ref. |
|---|---|---|---|---|---|---|---|---|
| U. ohnoi | 3.50 | 105 | 17.60 | HCl (pH2) | 37 | - | - | [66] |
| U. tepida | 3.90 | 313 | 21.60 | HCl (pH2) | - | - | ||
| U. prolifera | 6.70 | 246 | 16.60 | HCl (pH2) | - | - | ||
| U. lactuca | 17.95 | - | 17.22 | Distilled water | 50 | Cellulase and protease | - | [62] |
| U. lactuca | 16.90 | 265 | 53 | NaOH | 70 | Ultrasonic | [61] | |
| 14.50 | 280 | 58 | HCl | 70 | ||||
| 12.50 | 304 | 39 | Distilled water | 70 | ||||
| U. linza | 17.00 | - | - | Citric acid | 60 | - | - | [66] |
| U. fasciata | 6.02 | - | 14.92 | Distilled water | - | Ethanol-protein and pigment | [67] | |
| 7.34 | - | 12.73 | HCl | - | ||||
| 6.74 | - | 7.760 | Na2EDTA | - | ||||
| U. lactuca | 14.22 | - | 16.82 | HCl (pH2) | 80 | - | - | [62] |
| U. linza | 29.33 | 16 | 13.78 | Oxalic acid | - | Distilled water—Starch | [66] | |
| U. intestinalis | 17.76 | 300 | - | Distilled water | - | - | [58] | |
| 23.21 | 88 | - | Acidic water (pH3) | - | Ethanol-lipid and pigment | |||
| 16.11 | 110 | - | Alkaline water (pH10) | - | ||||
| 20.41 | - | - | Distilled water | Microwave | ||||
| 17.89 | - | - | Distilled water | Autoclave 121 °C | ||||
| 23.73 | - | - | Distilled water | Ultrasonic | ||||
| U. ohnoi | 8.20 | 10.5 | 12,5 | HCl | 85 | - | Distilled water—salt | [57] |
| 7.00 | 16.3 | 12.4 | HCl | - | Ethanol—pigments | |||
| 8.10 | 10.8 | 12.5 | HCl | - | Distilled water—salt Ethanol—pigments | |||
| Ulva sp. | 0.04 | - | 18.00 | Citric acid | 90 | - | - | [68] |
| U. fenestrata, U. lactuca | 18.00 | - | 17.80 | HCl | - | - | [63,69] | |
| U. compressa | 18.00 | - | 17.80 | HCl | - | - | [69] | |
| U. lactuca, | 11.00 | - | 14.30 | Distilled water | Post-treatment α-amylase and proteinase K | Ethanol-protein and pigment | ||
| U. compressa | 11.00 | - | 9.30 | Distilled water | ||||
| U. lactuca | 41.96 | - | 23.20 | ChCl-glycerol | Peracetic acid | - | [60] | |
| U. lactuca | 3.40 | - | 15.65 | HCl (pH1.5) | - | - | [62] | |
| U. pertusa | 17.80 | 283 | 13.20 | Distilled water | - | - | [70] | |
| 20.60 | 352 | 9.20 | Distilled water | Ultrasonic | - | |||
| 25.30 | 404 | 6.80 | HCl (pH4.5) | Pretreatment cellulase at 50 °C | - | |||
| 26.70 | 300 | 3.90 | HCl (pH4.5) | - | ||||
| Ulva sp. | 30.36 | - | - | HCl (pH2) | 90 | - | - | [71] |
| 30.48 | - | 31 | Distilled water | 120 | Microwave hydrothermal | - | ||
| 30.46 | - | 40 | Distilled water | 140 | - | |||
| 30.66 | - | 50 | Distilled water | 160 | - | |||
| 30.70 | - | 20 | Distilled water | 180 | - | |||
| 30.66 | - | 21 | Distilled water | 200 | - | |||
| Ulva sp. | 11.00 | - | 11.02 | Distilled water | 120 | Hydrothermal | Pretreatment supercritical CO2 and ethanol—polyunsaturated rich lipids and phenolic content | [65] |
| 19.00 | - | 7.14 | Distilled water | 140 | ||||
| 22.00 | - | 10.09 | Distilled water | 160 | ||||
| 5.00 | - | 7.58 | Distilled water | 180 | ||||
| 5.00 | - | 7.36 | Distilled water | 200 |
3. Biological Properties of Ulvan
3.1. Biocompatibility
3.2. Immunomodulatory Effect
3.3. Anticoagulation Activity
3.4. Antimicrobial Activity
4. Ulvan in Biomaterial Design
4.1. Ulvan-Based Hydrogels
4.2. Ulvan-Based Films
4.3. Ulvan-Based Nanocomposites
4.4. Ulvan-Based Emulsions
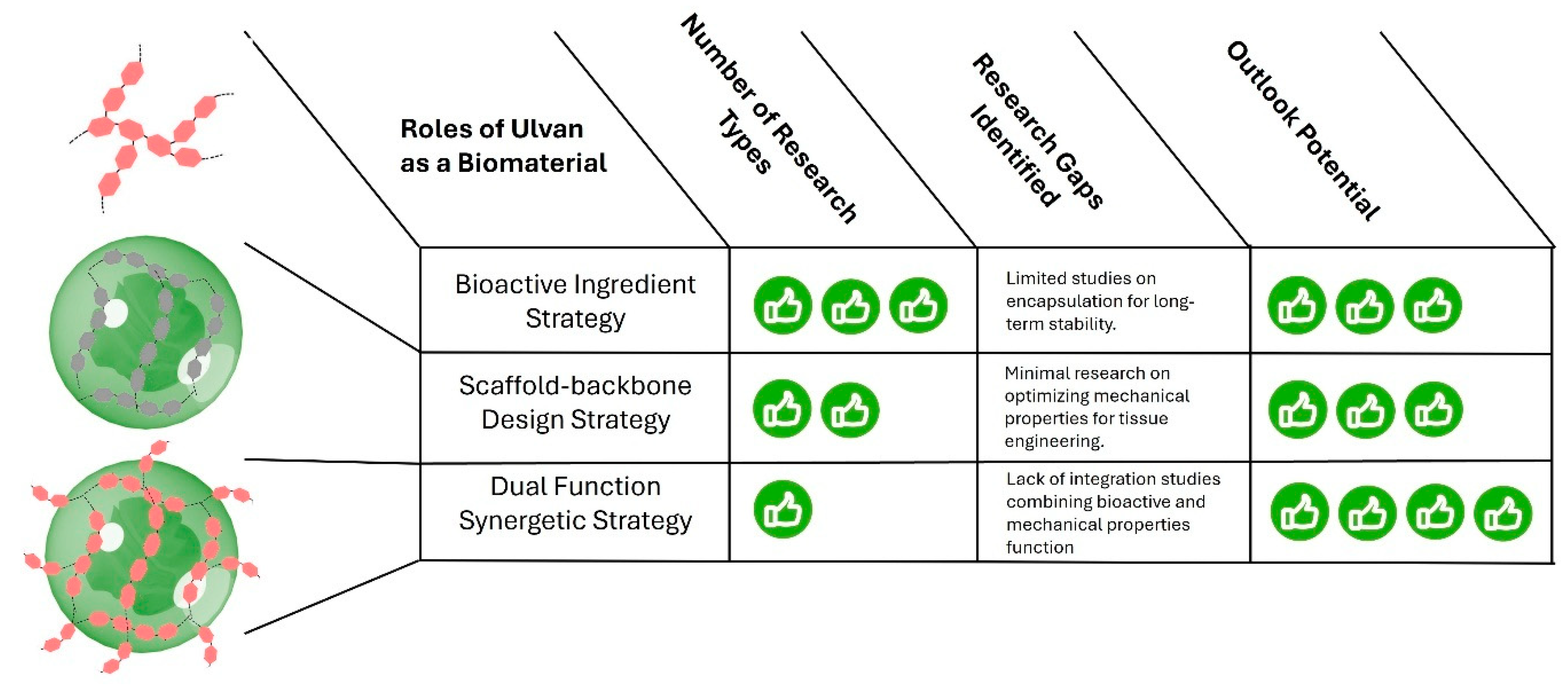
5. Comparative Advantages of Ulvan
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dw | dry weight |
| SNFG | Symbol Nomenclature for Glycans |
| ChCl | Choline Chloride |
| DES | Deep Eutectic Solvent |
| COMP | 3-hydroxy-4,7-megastigmadien-9-one |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| DGR | Daily growth rate |
| MWCO | Molecular weight cut-off |
| SEC | Size Exclusion Chromatography |
| IPEC-1 | porcine intestinal epithelial cells |
| TNF-α | tumor necrosis factor-alpha |
| IL | Interleukin |
| CXCL | C-X-C motif chemokine ligand |
| IgM | Immunoglobulin M |
| COX-2 | Cyclooxygenase-2 |
| iNOS-2 | inducible nitric oxide synthase-2 |
| NF-κB | nuclear factor kappa-B |
| MAPK | Mitogen-activated protein kinase |
| TLR | Toll-like receptors |
| HRP | Horseradish peroxidase |
| Na-CMC | Sodium carboxymethyl cellulose |
| CNC | Cellulose nanocrystals |
| PVA | Polyvinyl alcohol |
References
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-Based Cellulose: Applications, and Future Perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae Based Biorefinery—How to Make Sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Rudke, A.R.; De Andrade, C.J.; Ferreira, S.R.S. Kappaphycus alvarezii Macroalgae: An Unexplored and Valuable Biomass for Green Biorefinery Conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-104958-7. [Google Scholar]
- Cesário, M.T.; Da Fonseca, M.M.R.; Marques, M.M.; De Almeida, M.C.M.D. Marine Algal Carbohydrates as Carbon Sources for the Production of Biochemicals and Biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for Biofuels Production: Progress and Perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Sultana, F.; Wahab, M.A.; Nahiduzzaman, M.; Mohiuddin, M.; Iqbal, M.Z.; Shakil, A.; Mamun, A.-A.; Khan, M.S.R.; Wong, L.; Asaduzzaman, M. Seaweed Farming for Food and Nutritional Security, Climate Change Mitigation and Adaptation, and Women Empowerment: A Review. Aquac. Fish. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Hebbale, D. Bioethanol from Macroalgae: Prospects and Challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Ross, F.; Tarbuck, P.; Macreadie, P.I. Seaweed Afforestation at Large-Scales Exclusively for Carbon Sequestration: Critical Assessment of Risks, Viability and the State of Knowledge. Front. Mar. Sci. 2022, 9, 1015612. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of Macroalgae as Feedstocks for Biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef] [PubMed]
- Uju; Wijayanta, A.T.; Goto, M.; Kamiya, N. Great Potency of Seaweed Waste Biomass from the Carrageenan Industry for Bioethanol Production by Peracetic Acid–Ionic Liquid Pretreatment. Biomass Bioenergy 2015, 81, 63–69. [Google Scholar] [CrossRef]
- Uju; Wijayanta, A.T.; Goto, M.; Kamiya, N. High Yield Hydrolysis of Seaweed-Waste Biomass Using Peracetic Acid and Ionic Liquid Treatments. AIP Conf. Proc. 2018, 1931, 020004. [Google Scholar]
- Uju; Goto, M.; Kamiya, N. Powerful Peracetic Acid–Ionic Liquid Pretreatment Process for the Efficient Chemical Hydrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2016, 214, 487–495. [Google Scholar] [CrossRef]
- Hiraoka, M. Massive Ulva Green Tides Caused by Inhibition of Biomass Allocation to Sporulation. Plants 2021, 10, 2482. [Google Scholar] [CrossRef]
- Mantri, V.A.; Kazi, M.A.; Balar, N.B.; Gupta, V.; Gajaria, T. Concise Review of Green Algal Genus Ulva linnaeus. J. Appl. Phycol. 2020, 32, 2725–2741. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Łęska, B.; Messyasz, B.; Fabrowska, J.; Sołoducha, M.; Pikosz, M. First Isolation of Polysaccharidic Ulvans from the Cell Walls of Freshwater Algae. Algal Res. 2016, 19, 348–354. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; De Saeger, J.; Depuydt, S.; Asselman, J.; Janssen, C.; Heynderickx, P.M.; Wu, D.; Ronsse, F.; Tack, F.M.G.; et al. Harnessing Green Tide Ulva Biomass for Carbon Dioxide Sequestration. Rev. Environ. Sci. Biotechnol. 2024, 23, 1041–1061. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R.; et al. Ulva prolifera Green-Tide Outbreaks and Their Environmental Impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful Macroalgal Blooms (HMBs) in China’s Coastal Water: Green and Golden Tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic Hydrolysis and Production of Bioethanol from Common Macrophytic Green Alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.D.; Lüning, K. Growth Rates of North Sea Macroalgae in Relation to Temperature, Irradiance and Photoperiod. Helgol. Meeresunters 1980, 34, 15–29. [Google Scholar] [CrossRef]
- Chemodanov, A.; Robin, A.; Jinjikhashvily, G.; Yitzhak, D.; Liberzon, A.; Israel, A.; Golberg, A. Feasibility Study of Ulva sp. (Chlorophyta) Intensive Cultivation in a Coastal Area of the Eastern Mediterranean Sea. Biofuels Bioprod. Bioref. 2019, 13, 864–877. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar]
- Trivedi, N.; Baghel, R.S.; Bothwell, J.; Gupta, V.; Reddy, C.R.K.; Lali, A.M.; Jha, B. An Integrated Process for the Extraction of Fuel and Chemicals from Marine Macroalgal Biomass. Sci. Rep. 2016, 6, 30728. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, N.S.; Nassar, H.N.; Ismail, A.R.; Ali, H.R.; Ali, B.A.; Abdelsalam, K.M.; Mubarak, M. A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability 2023, 15, 7319. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the Sea: The Green Macroalga Ulva ohnoi as a Potential Source for Sustainable Starch Production in the Marine Biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Thygesen, A.; Fernando, D.; Ståhl, K.; Daniel, G.; Mensah, M.; Meyer, A.S. Cell Wall Configuration and Ultrastructure of Cellulose Crystals in Green Seaweeds. Cellulose 2021, 28, 2763–2778. [Google Scholar] [CrossRef]
- Pilavtepe, M.; Sargin, S.; Celiktas, M.S.; Yesil-Celiktas, O. An Integrated Process for Conversion of Zostera marina Residues to Bioethanol. J. Supercrit. Fluids 2012, 68, 117–122. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of Protein Extraction with a Stream of Byproducts from Marine Macroalgae: A Model Forms the Basis for Marine Bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Bikker, P.; Van Krimpen, M.M.; Van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; Van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the Green Seaweed Ulva lactuca to Produce Animal Feed, Chemicals and Biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a Polysaccharide from Macroalga Ulva sp.: A Review of Chemistry, Biological Activities and Potential for Food and Biomedical Applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Attasgah, R.B.; Velasco-Rodríguez, B.; Pardo, A.; Fernández-Vega, J.; Arellano-Galindo, L.; Rosales-Rivera, L.C.; Prieto, G.; Barbosa, S.; Soltero, J.F.A.; Mahmoudi, M.; et al. Development of Functional Hybrid Scaffolds for Wound Healing Applications. iScience 2022, 25, 104019. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Rêma, A.; Biscaia, S.; Cordeiro, R.; Faria, F.; et al. Hybrid Scaffolds for Bone Tissue Engineering: Integration of Composites and Bioactive Hydrogels Loaded with hDPSCs. Biomater. Adv. 2025, 166, 214042. [Google Scholar] [CrossRef] [PubMed]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Chapter 3—Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 41–106. ISBN 978-0-12-802772-1. [Google Scholar]
- Mutizabal-Aros, J.; Ramírez, M.E.; Haye, P.A.; Meynard, A.; Pinilla-Rojas, B.; Núñez, A.; Latorre-Padilla, N.; Search, F.V.; Tapia, F.J.; Saldías, G.S.; et al. Morphological and Molecular Identification of Ulva spp. (Ulvophyceae; Chlorophyta) from Algarrobo Bay, Chile: Understanding the Composition of Green Tides. Plants 2024, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Cao, X.; Li, S.; Cao, J.; Tong, Y.; Sun, Y.; Liu, J.; Zhao, S.; Cui, Q.; Zeng, Y.; et al. Distribution of Ulva prolifera, the Dominant Species in Green Tides along the Jiangsu Province Coast in the Southern Yellow Sea, China. J. Sea Res. 2023, 196, 102436. [Google Scholar] [CrossRef]
- Pari, R.F.; Uju; Wijayanta, A.T.; Ramadhan, W.; Hardiningtyas, S.D.; Kurnia, K.A.; Firmansyah, M.L.; Hana, A.; Abrar, M.N.; Wakabayashi, R.; et al. Prospecting Ulva lactuca Seaweed in Java Island, Indonesia, as a Candidate Resource for Industrial Applications. Fish Sci. 2024, 90, 795–808. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of Nutritional Composition of The Dried Seaweed Ulva lactuca from Pameungpeuk Waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119–125. [Google Scholar] [CrossRef]
- Debbarma, J.; Rao, B.M.; Murthy, L.N.; Mathew, S.; Venkateshwarlu, G.; Ravishankar, C.N. Nutritional Profiling of the Edible Seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J. Fish. 2016, 63, 81–87. [Google Scholar] [CrossRef]
- Ali, A.A.H. Overview of the Vital Roles of Macro Minerals in the Human Body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Eco-Friendly Nutrient from Ocean: Exploring Ulva Seaweed Potential as a Sustainable Food Source. J. Agric. Food Res. 2024, 17, 101239. [Google Scholar] [CrossRef]
- Luhila, Õ.; Paalme, T.; Tanilas, K.; Sarand, I. Omega-3 Fatty Acid and B12 Vitamin Content in Baltic Algae. Algal Res. 2022, 67, 102860. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, M.H.M. Biorefinery of the Macroalgae Ulva lactuca: Extraction of Proteins and Carbohydrates by Mild Disintegration. J. Appl. Phycol. 2018, 30, 1281–1293. [Google Scholar] [CrossRef]
- Huang, A.; Wu, X.; Lu, F.; Liu, F. Sustainable Production of Ulva Oligosaccharides via Enzymatic Hydrolysis: A Review on Ulvan Lyase. Foods 2024, 13, 2820. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Glasson, C.R.K.; Magnusson, M.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Carnachan, S.M. Ulvans Are Not Equal—Linkage and Substitution Patterns in Ulvan Polysaccharides Differ with Ulva Morphology. Carbohydr. Polym. 2024, 333, 121962. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, Y.; Neelamegham, S. DrawGlycan-SNFG: A Robust Tool to Render Glycans and Glycopeptides with Fragmentation Information. Glycobiology 2017, 27, 200–205. [Google Scholar] [CrossRef]
- Huang, X.; Ai, C.; Yao, H.; Zhao, C.; Xiang, C.; Hong, T.; Xiao, J. Guideline for the Extraction, Isolation, Purification, and Structural Characterization of Polysaccharides from Natural Resources. eFood 2022, 3, e37. [Google Scholar] [CrossRef]
- Ferreira, A.F. Biorefinery Concept. In Biorefineries: Targeting Energy, High Value Products and Waste Valorisation; Rabaçal, M., Ferreira, A.F., Silva, C.A.M., Costa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–20. ISBN 978-3-319-48288-0. [Google Scholar]
- Magnusson, M.; Carl, C.; Mata, L.; de Nys, R.; Paul, N.A. Seaweed Salt from Ulva: A Novel First Step in a Cascading Biorefinery Model. Algal Res. 2016, 16, 308–316. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of Chlorophyll from Wild and Farmed Ulva spp. Using Aqueous Solutions of Ionic Liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, R.; Coutinho, J.A.P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Ventura, S.P.M. Recovery of Pigments from Ulva rigida. Sep. Purif. Technol. 2021, 255, 117723. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Sims, I.M.; Carnachan, S.M.; de Nys, R.; Magnusson, M. A Cascading Biorefinery Process Targeting Sulfated Polysaccharides (Ulvan) from Ulva ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Kazemi, M.; Fathi, M.; Jahanbin, K.; Taghdir, M.; Abbaszadeh, S. Optimization of Ultrasonic-Assisted Hot Acidic Solvent Extraction of Ulvan from Ulva intestinalis of the Persian Gulf: Evaluation of Structural, Techno-Functional, and Bioactivity Properties. Food Hydrocoll. 2023, 142, 108837. [Google Scholar] [CrossRef]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Ramadhan, W.; Alamsyah, A.; Uju; Hardiningtyas, S.D.; Pari, R.F.; Wakabayashi, R.; Kamiya, N.; Goto, M. Facilitating Ulvan Extraction from Ulva lactuca via Deep Eutectic Solvent and Peracetic Acid Treatment. ASEAN J. Chem. Eng. 2024, 24, 90–101. [Google Scholar] [CrossRef]
- Ramadhan, W.; Uju; Hardiningtyas, S.D.; Pari, R.F.; Nurhayati, N.; Sevica, D. Ekstraksi Polisakarida Ulvan Dari Rumput Laut Ulva lactuca Berbantu Gelombang Ultrasonik Pada Suhu Rendah: Ultrasonic Wave Assisted Extraction of Ulvan Polysaccharide from Ulva Seaweed at Low Temperature. J. Pengolah. Has. Perikan. Indones. 2022, 25, 132–142. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of Extraction Procedures on the Chemical Structure, Antitumor and Anticoagulant Properties of Ulvan from Ulva lactuca of Tunisia Coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and Structure of Cell Wall Ulvans Recovered from Ulva spp. along the Swedish West Coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Andrade, C.; Martins, P.L.; Duarte, L.C.; Oliveira, A.C.; Carvalheiro, F. Development of an Innovative Macroalgae Biorefinery: Oligosaccharides as Pivotal Compounds. Fuel 2022, 320, 123780. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, P.; Baltrusch, K.L.; Díaz-Reinoso, B.; López-Álvarez, M.; Novoa-Carballal, R.; González, P.; González-Novoa, A.; Rodríguez-Montes, A.; Kennes, C.; Veiga, M.C.; et al. Hydrothermal Extraction of Ulvans from Ulva spp. in a Biorefinery Approach. Sci. Total Environ. 2024, 951, 175654. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Badr, H.A. Optimization of Oxalic Acid Treatment for Ulvan Extraction from Ulva linza Biomass and Its Potential Application as Fe(III) Chelator. Algal Res. 2024, 80, 103536. [Google Scholar] [CrossRef]
- Moawad, M.N.; El-Sayed, A.A.M.; Abd El Latif, H.H.; El-Naggar, N.A.; Shams El-Din, N.G.; Tadros, H.R.Z. Chemical Characterization and Biochemical Activity of Polysaccharides Isolated from Egyptian Ulva fasciata Delile. Oceanologia 2022, 64, 117–130. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Lens, P.N.L. Green Extraction and Esterification of Marine Polysaccharide (Ulvan) from Green Macroalgae Ulva sp. Using Citric Acid for Hydrogel Preparation. J. Clean. Prod. 2022, 366, 132952. [Google Scholar] [CrossRef]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan Dialdehyde-Gelatin Hydrogels for Removal of Heavy Metals and Methylene Blue from Aqueous Solution. Carbohydr. Polym. 2020, 249, 116841. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, W.; Gan, J.; Li, Y.; Pan, Y.; Li, J.; Chen, H. Physicochemical Properties and Anti-Oxidation Activities of Ulvan from Ulva pertusa Kjellm. Algal Res. 2021, 55, 102269. [Google Scholar] [CrossRef]
- André, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Microwave-Assisted Extraction of Ulva spp. Including a Stage of Selective Coagulation of Ulvan Stimulated by a Bio-Ionic Liquid. Int. J. Biol. Macromol. 2023, 225, 952–963. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva Oligosaccharides: A Systematic Review of Structure, Preparation, Biological Activities and Applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef]
- Tran, V.H.N.; Mikkelsen, M.D.; Truong, H.B.; Vo, H.N.M.; Pham, T.D.; Cao, H.T.T.; Nguyen, T.T.; Meyer, A.S.; Thanh, T.T.T.; Van, T.T.T. Structural Characterization and Cytotoxic Activity Evaluation of Ulvan Polysaccharides Extracted from the Green Algae Ulva papenfussii. Mar. Drugs 2023, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Pengzhan, Y.; Quanbin, Z.; Hong, Z.; Xizhen, N.; Zhien, L. Preparation of Polysaccharides in Different Molecular Weights from Ulva pertusa Kjellm (Chorophyta). Chin. J. Ocean. Limnol. 2004, 22, 381–385. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva armoricana Extract Protects Plants against Three Powdery Mildew Pathogens. Eur. J. Plant Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phytother. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Barros, A.A.A.; Alves, A.; Nunes, C.; Coimbra, M.A.; Pires, R.A.; Reis, R.L. Carboxymethylation of Ulvan and Chitosan and Their Use as Polymeric Components of Bone Cements. Acta Biomater. 2013, 9, 9086–9097. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Dabaghian, E.H.; Surayot, U. Water-Soluble Polysaccharides from Ulva intestinalis: Molecular Properties, Structural Elucidation and Immunomodulatory Activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular Characteristics and Immunomodulatory Activities of Water-Soluble Sulfated Polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef]
- Cho, M.; Yang, C.; Kim, S.M.; You, S. Molecular Characterization and Biological Activities of Watersoluble Sulfated Polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of Biochemical and Immunomodulatory Activity of Sulphated Polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef]
- Berri, M.; Slugocki, C.; Olivier, M.; Helloin, E.; Jacques, I.; Salmon, H.; Demais, H.; Le Goff, M.; Collen, P.N. Marine-Sulfated Polysaccharides Extract of Ulva armoricana Green Algae Exhibits an Antimicrobial Activity and Stimulates Cytokine Expression by Intestinal Epithelial Cells. J. Appl. Phycol. 2016, 28, 2999–3008. [Google Scholar] [CrossRef]
- Berri, M.; Olivier, M.; Holbert, S.; Dupont, J.; Demais, H.; Le Goff, M.; Collen, P.N. Ulvan from Ulva armoricana (Chlorophyta) Activates the PI3K/Akt Signalling Pathway via TLR4 to Induce Intestinal Cytokine Production. Algal Res. 2017, 28, 39–47. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Maray, S.O.; Abdel-Kareem, M.S.M.; Mabrouk, M.E.M.; El-Halmouch, Y.; Makhlof, M.E.M. In Vitro Assessment of Antiviral, Antimicrobial, Antioxidant and Anticancer Activities of Ulvan Extracted from the Green Seaweed Ulva lactuca. Thalassas 2023, 39, 779–790. [Google Scholar] [CrossRef]
- Vonthron-Sénécheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.-M. Antiprotozoal Activities of Organic Extracts from French Marine Seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Itoe, M.; Blunden, G.; Mota, M.; Tasdemir, D. Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds. Mar. Drugs 2013, 11, 4019–4034. [Google Scholar] [CrossRef] [PubMed]
- Botta, A.; Martínez, V.; Mitjans, M.; Balboa, E.; Conde, E.; Vinardell, M.P. Erythrocytes and Cell Line-Based Assays to Evaluate the Cytoprotective Activity of Antioxidant Components Obtained from Natural Sources. Toxicol. In Vitro 2014, 28, 120–124. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Robledo, D.; Freile-Pelegrín, Y.; Peña-Hernández, M.A.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Synergistic Effects of Sulfated Polysaccharides from Mexican Seaweeds against Measles Virus. BioMed Res. Int. 2016, 2016, 8502123. [Google Scholar] [CrossRef]
- De Araújo, I.W.F.; Rodrigues, J.A.G.; Quinderé, A.L.G.; Silva, J.D.F.T.; Maciel, G.D.F.; Ribeiro, N.A.; De Sousa Oliveira Vanderlei, E.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Analgesic and Anti-Inflammatory Actions on Bradykinin Route of a Polysulfated Fraction from Alga Ulva lactuca. Int. J. Biol. Macromol. 2016, 92, 820–830. [Google Scholar] [CrossRef]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and Anti-Tumor Activity of Alkali-Extracted Polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef]
- Das Chagas Faustino Alves, M.G.; Dore, C.M.P.G.; Castro, A.J.G.; Do Nascimento, M.S.; Cruz, A.K.M.; Soriano, E.M.; Benevides, N.M.B.; Leite, E.L. Antioxidant, Cytotoxic and Hemolytic Effects of Sulfated Galactans from Edible Red Alga Hypnea musciformis. J. Appl. Phycol. 2012, 24, 1217–1227. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.; Cruz-Suarez, L.; Sassi, J.-F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds Both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green Seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) Derived Sulphated Polysaccharides Inhibit Herpes Simplex Virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.-S.; You, S.G. In Vitro and in Vivo Immunomodulatory Activity of Sulfated Polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Sonia, K.; Meena, K.S.; Bai, S.A. In vitroCytotoxic Activity of a Sulphated Polysaccharide Ulvan against Human Breast and Glioblastoma Cell Line. Indo Glob. J. Pharm. Sci. 2022, 12, 122–127. [Google Scholar] [CrossRef]
- García-Márquez, J.; Moreira, B.R.; Valverde-Guillén, P.; Latorre-Redoli, S.; Caneda-Santiago, C.T.; Acién, G.; Martínez-Manzanares, E.; Marí-Beffa, M.; Abdala-Díaz, R.T. In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida. Pharmaceuticals 2023, 16, 660. [Google Scholar] [CrossRef]
- Yousefpour, P.; Ni, K.; Irvine, D.J. Targeted Modulation of Immune Cells and Tissues Using Engineered Biomaterials. Nat. Rev. Bioeng. 2023, 1, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Abou El Azm, N.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of Bioactive Compounds from the Sulfated Polysaccharides Extracts of Ulva lactuca: Post-Extraction Enzymatic Hydrolysis Followed by Ion-Exchange Chromatographic Fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile Green Synthesis of Zinc Oxide Nanoparticles Using Ulva lactuca Seaweed Extract and Evaluation of Their Photocatalytic, Antibiofilm and Insecticidal Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Amin, H.H. Potential Using of Ulvan Polysaccharide from Ulva lactuca as a Prebiotic in Synbiotic Yogurt Production. J. Prob. Health 2019, 7, 100208. [Google Scholar] [CrossRef]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA Enriched with Ulvan Particles as a Novel 3D Porous Scaffold Targeted for Bone Engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Vane, J.; Botting, R. Inflammation and the Mechanism of Action of Anti-Inflammatory Drugs. FASEB J. 1987, 1, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with Chitosan by Spray Drying for Industry Applications—A Review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.; Kim, S. Seaweed Polysaccharides and Their Potential Biomedical Applications. Starch 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Castro, R.; Piazzon, M.C.; Zarra, I.; Leiro, J.; Noya, M.; Lamas, J. Stimulation of Turbot Phagocytes by Ulva rigida C. Agardh Polysaccharides. Aquaculture 2006, 254, 9–20. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.; Coste, O.; Malta, E. Polymer Chitosan Nanoparticles Functionalized with Ulva ohnoi Extracts Boost in Vitro Ulvan Immunostimulant Effect in Solea senegalensis Macrophages. Algal Res. 2017, 26, 135–142. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Liu, G.; Pei, D.; Liu, Y.; Liu, Y.; Di, D. Polysaccharides from Enteromorpha prolifera Enhance the Immunity of Normal Mice. Int. J. Biol. Macromol. 2014, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium Nanoparticles Decorated with Ulva lactuca Polysaccharide Potentially Attenuate Colitis by Inhibiting NF-κB Mediated Hyper Inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-K.; Bong, M.-H.; Park, J.-C.; Moon, H.-K.; Kim, D.-W.; Lee, S.-C.; Lee, J.-H. Antioxidant and Immunomodulatory Effects of Ulva pertusa Kjellman on Broiler Chickens. J. Anim. Sci. Technol. 2011, 53, 419–428. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating Activities of Acidic Sulphated Polysaccharides Obtained from the Seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muiños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-Inflammatory Potential of Ulvan. Int. J. Biol. Macromol. 2023, 253, 126936. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy—From Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Chao, C.-C.; Wang, Y.-C.; Kenison, J.E.; Li, Z.; Rone, J.M.; Rejano-Gordillo, C.M.; Polonio, C.M.; Gutierrez-Vazquez, C.; Piester, G.; et al. Identification of Environmental Factors That Promote Intestinal Inflammation. Nature 2022, 611, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Manzoor, Z.; Koo, J.-E.; Kim, J.-E.; Byeon, S.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.-H.; Koh, Y.-S. 3-Hydroxy-4,7-Megastigmadien-9-One, Isolated from Ulva pertusa, Attenuates TLR9-Mediated Inflammatory Response by down-Regulating Mitogen-Activated Protein Kinase and NF-κB Pathways. Pharm. Biol. 2017, 55, 435–440. [Google Scholar] [CrossRef]
- Cian, R.E.; Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Drago, S.R.; Sánchez De Medina, F.; Martínez-Augustin, O. Green Alga Ulva spp. Hydrolysates and Their Peptide Fractions Regulate Cytokine Production in Splenic Macrophages and Lymphocytes Involving the TLR4-NFκB/MAPK Pathways. Mar. Drugs 2018, 16, 235. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Wassie, T.; Wang, H.; Li, T.; Xie, C.; Wu, X. Enteromorpha prolifera Polysaccharide–Zinc Complex Modulates the Immune Response and Alleviates LPS-Induced Intestinal Inflammation via Inhibiting the TLR4/NF-κB Signaling Pathway. Food Funct. 2022, 13, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K.; et al. Structural Features and Anti-Coagulant Activity of the Sulphated Polysaccharide SPS-CF from a Green Alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Adrien, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Evaluation of the Anticoagulant Potential of Polysaccharide-Rich Fractions Extracted from Macroalgae. Nat. Prod. Res. 2017, 31, 2126–2136. [Google Scholar] [CrossRef]
- Shanmugam, M.; Ramavat, B.K.; Mody, K.; Oza, R.; Tewari, A. Distribution of Heparinoid-Active Sulphated Polysaccharides in Some Indian Marine Green Algae. Indian J. Mar. Sci. 2001, 30, 222–227. [Google Scholar]
- Cui, J.; Li, Y.; Wang, S.; Chi, Y.; Hwang, H.; Wang, P. Directional Preparation of Anticoagulant-Active Sulfated Polysaccharides from Enteromorpha prolifera Using Artificial Neural Networks. Sci. Rep. 2018, 8, 3062. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of Anticoagulant Activity of Two Algal Polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, G.; Gómez-Gutiérrez, C.M.; Alarcón-Arteaga, G.; Soria-Mercado, I.E.; Ayala-Sánchez, N.E. Screening for Anticoagulant Activity in Marine Algae from the Northwest Mexican Pacific Coast. J. Appl. Phycol. 2011, 23, 495–503. [Google Scholar] [CrossRef]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated Polysaccharides from Marine Green Algae Ulva conglobata and Their Anticoagulant Activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Chen, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C. Chemical Characteristics and Anticoagulant Activities of Two Sulfated Polysaccharides from Enteromorpha linza (Chlorophyta). J. Ocean Univ. China 2013, 12, 175–182. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, Anticoagulant and Antioxidant Activities of Polysaccharide from Green Algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Setayeshmehr, M.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Poursamar, S.A.; Ismail, A.F.; Sharif, S.; Chen, X.; Berto, F. A Review on Antibacterial Biomaterials in Biomedical Applications: From Materials Perspective to Bioinks Design. Polymers 2022, 14, 2238. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, Conformation in Aqueous Solution and Antimicrobial Activity of Ulvan Extracted from Green Seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Bedoux, G.; Souak, D.; Bourgougnon, N.; Feuilloley, M.G.J.; Latire, T. Effects of Ulva sp. Extracts on the Growth, Biofilm Production, and Virulence of Skin Bacteria Microbiota: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes Strains. Molecules 2021, 26, 4763. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-Fouling Properties. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef]
- Afzal, S.; Yadav, A.K.; Poonia, A.K.; Choure, K.; Yadav, A.N.; Pandey, A. Antimicrobial Therapeutics Isolated from Algal Source: Retrospect and Prospect. Biologia 2022, 78, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Kraithong, S.; Bunyameen, N.; Theppawong, A.; Ke, X.; Lee, S.; Zhang, X.; Huang, R. Potentials of Ulva spp.—Derived Sulfated Polysaccharides as Gelling Agents with Promising Therapeutic Effects. Int. J. Biol. Macromol. 2024, 273, 132882. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.M.; de Freitas, R.A.; Ducatti, D.R.B.; Ferreira, L.G.; Gonçalves, A.G.; Colodi, F.G.; Mazepa, E.; Aranha, E.M.; Noseda, M.D.; Duarte, M.E.R. Modification of Ulvans via Periodate-Chlorite Oxidation: Chemical Characterization and Anticoagulant Activity. Carbohydr. Polym. 2018, 197, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, S.; Zhu, Y.; Ning, Y.; Chen, T.; Yang, Y.; Qi, B.; Huang, Y.; Li, Y. Crosslinking of Gelatin Schiff Base Hydrogels with Different Structural Dialdehyde Polysaccharides as Novel Crosslinkers: Characterization and Performance Comparison. Food Chem. 2024, 456, 140090. [Google Scholar] [CrossRef]
- Jaiswal, D.; James, R.; Shelke, N.B.; Harmon, M.D.; Brown, J.L.; Hussain, F.; Kumbar, S.G. Gelatin Nanofiber Matrices Derived from Schiff Base Derivative for Tissue Engineering Applications. J. Biomed. Nanotechnol. 2015, 11, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Fathi, M.; Safary, A.; Javadzadeh, Y.; Omidi, Y. In Situ Forming Alginate/Gelatin Hydrogel Scaffold through Schiff Base Reaction Embedded with Curcumin-Loaded Chitosan Microspheres for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2024, 256, 128335. [Google Scholar] [CrossRef]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M.S. Horseradish Peroxidase (HRP) as a Tool in Green Chemistry. RSC Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Parsons-Mair, H.N.; Gebicki, S.; Gebicki, J.M.; Davies, M.J. Requirements for Superoxide-Dependent Tyrosine Hydroperoxide Formation in Peptides. Biochem. J. 2004, 381, 241–248. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Bartoli, C.; Gazzarri, M.; Chiellini, F. Enzymatically Crosslinked Ulvan Hydrogels as Injectable Systems for Cell Delivery. Macromol. Chem. Phys. 2016, 217, 581–590. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Zoetebier, B.; Dijkstra, P.J.; Karperien, M. Enzymatic Co-Crosslinking of Star-Shaped Poly(Ethylene Glycol) Tyramine and Hyaluronic Acid Tyramine Conjugates Provides Elastic Biocompatible and Biodegradable Hydrogels. Bioact. Mater. 2023, 20, 53–63. [Google Scholar] [CrossRef]
- Sulastri, E.; Lesmana, R.; Zubair, M.S.; Elamin, K.M.; Wathoni, N. A Comprehensive Review on Ulvan Based Hydrogel and Its Biomedical Applications. Chemical and Pharmaceutical Bulletin 2021, 69, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yue, Z.; Winberg, P.C.; Lou, Y.-R.; Beirne, S.; Wallace, G.G. 3D Bioprinting Dermal-like Structures Using Species-Specific Ulvan. Biomater. Sci. 2021, 9, 2424–2438. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.N.; Khaled, B.M.; Liu, F.; Zhong, F. Hydrogel Beads for Designing Future Foods: Structures, Mechanisms, Applications, and Challenges. Food Hydrocoll. Health 2022, 2, 100073. [Google Scholar] [CrossRef]
- Kansandee, W.; Moonmangmee, S.; Vangpikul, S.; Kosawatpat, P.; Tamtin, M. Physicochemical Properties and in Vitro Prebiotic Activity of Ulva rigida Polysaccharides. Biocatal. Agric. Biotechnol. 2024, 59, 103252. [Google Scholar] [CrossRef]
- Ramadhan, W.; Ramadhani, F.A.; Sevica, D.; Hardiningtyas, S.D.; Desniar, D. Synthesis and Characterization of Ulvan-Alginate Hydrogel Beads as a Scaffold for Probiotic Immobilization. BIO Web Conf. 2024, 92, 02020. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and Characterization of Ulvan Polysaccharides-Based Hydrogel Films for Potential Wound Dressing Applications. Drug Des Devel Ther 2021, 15, 4213–4226. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Benelhadj, S.; Adjouman, Y.D.; Richel, A.; Blecker, C.; Sindic, M.; Boufi, S.; Attia, H.; Garna, H. Smart Ulvan Films Responsive to Stimuli of Plasticizer and Extraction Condition in Physico-Chemical, Optical, Barrier and Mechanical Properties. Int. J. Biol. Macromol. 2020, 150, 714–726. [Google Scholar] [CrossRef]
- Schmitz, L.; do Amaral, D.S.; Alarcon, O.E. Physicochemical Characterization of Ulvan Films Modified with Carnauba Wax for Enhanced Hydrophobicity. Colloid. Polym. Sci. 2024, 302, 1725–1735. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, H.; Feng, T.; Bu, X.; Cui, C.; Yang, F.; Yu, C. Advancing Soy Protein Isolate-Ulvan Film Physicochemical Properties and Antioxidant Activities through Strategic High-Pressure Homogenization Technique. Ind. Crops. Prod. 2024, 215, 118704. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and In Vivo Analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Colodi, F.G.; Ducatti, D.R.B.; Noseda, M.D.; de Carvalho, M.M.; Winnischofer, S.M.B.; Duarte, M.E.R. Semi-Synthesis of Hybrid Ulvan-Kappa-Carrabiose Polysaccharides and Evaluation of Their Cytotoxic and Anticoagulant Effects. Carbohydr. Polym. 2021, 267, 118161. [Google Scholar] [CrossRef] [PubMed]
- Madany, M.A.; Abdel-Kareem, M.S.; Al-Oufy, A.K.; Haroun, M.; Sheweita, S.A. The Biopolymer Ulvan from Ulva Fasciata: Extraction towards Nanofibers Fabrication. Int. J. Biol. Macromol. 2021, 177, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave Assisted Hydrothermal Extraction of Polysaccharides from Ulva prolifera: Functional Properties and Bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, A.; Ghariani, M.; Sellem, I.; Hamdi, M.; Feki, A.; Jaballi, I.; Nasri, M.; Amara, I.B. Extraction, Characterization and Biological Properties of Polysaccharide Derived from Green Seaweed “Chaetomorpha linum” and Its Potential Application in Tunisian Beef Sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic Extraction of Pectin from Opuntia ficus Indica Cladodes after Mucilage Removal: Optimization of Experimental Conditions and Evaluation of Chemical and Functional Properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H. Characterization, Ultrafiltration, Depolymerization and Gel Formulation of Ulvans Extracted via a Novel Ultrasound-Enzyme Assisted Method. Ultrason. Sonochem. 2024, 111, 107072. [Google Scholar] [CrossRef]
- Cheong, K.; Jesumani, V.; Khan, B.M.; Liu, Y.; Du, H. Algal Polysaccharides and Their Biological Properties. In Recent Advances in Micro and Macroalgal Processing; Rajauria, G., Yuan, Y.V., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 231–277. ISBN 978-1-119-54258-2. [Google Scholar]
- Michael, O.S.; Adetunji, C.O.; Ayeni, A.E.; Akram, M.; Inamuddin; Adetunji, J.B.; Olaniyan, M.; Muhibi, M.A. Marine Polysaccharides: Properties and Applications. In Polysaccharides; Inamuddin, Ahamed, M.I., Boddula, R., Altalhi, T., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 423–439. ISBN 978-1-119-71138-4. [Google Scholar]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]


| Polysaccharide | Source Algae | Yield Range (%) | Gelling Mechanism | Existing Function in the Current Market | Commercial Availability | Mechanical Properties | Cytocompatibility | Thermal Stability | Cross-Linking Potential | Functionalization Potential | Common Modifications | Applications of Modifications | Challenges in Modifications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulvan | Ulva spp. | 15–41 | Ionic cross-linking with divalent cations (e.g., Ca2+; CaCl2, H3BO3) | Emerging in tissue engineering, drug delivery, and bioadhesive development | Moderate, requires specialized extraction processes | Moderate elasticity, suitable for hydrogels | Excellent, supports cell adhesion and proliferation | Moderate, stable up to ~80 °C under physiological conditions | High, form strong ionic cross-links | High, easily modified with bioactive groups | Thiolated ulvan, sulfation, carboxymethylation, phosphorylation, hydrogel formation | Drug delivery, tissue engineering, bioadhesive development, antioxidant systems | Complexity in achieving uniform thiolation, scalability issues |
| Agar | Gracilaria sp., Gelidiella sp., Gelidium sp., Pterocladia, Laurencia | 10–15 | Thermal gelation via hydrogen bonding | Widely used in the food industry (gels, thickeners), limited biomedical applications | High, widely available, and established supply chain | Strong, brittle gels, limited elasticity | Good, limited applications in biomedical fields | High, retains gel properties up to ~100 °C | Moderate, limited chemical reactivity | Moderate, limited functionalization pathways | Thiolated agar, esterification, hydrogel formation, nanoparticle stabilization | Encapsulation, tissue scaffolding, bioadhesives, wound dressings | Low reactivity under mild conditions, batch variability |
| Carrageenan | Kappa-phycus spp. | 20–30 | Thermal gelation via sulfate groups. Helical structures formed via 3,6-anhydrous-galactose units and ion interactions | Predominantly in food as stabilizers and thickeners, some drug delivery systems | High, commercially available for various industries | Flexible gels, moderate strength | Moderate, may require modifications for biocompatibility | High, stable up to ~120 °C | High, versatile cross-linking potential | High, supports diverse chemical modifications | Sulfation, hydrogel formation, derivatization for drug delivery | Drug release matrices, bioadhesive, biocompatible scaffolds | Control over sulfation levels, stability in physiological conditions |
| Alginate | Macrocystis spp., Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Sargassum sp., Ascophyllum nodosum | 15–35 | Ionic cross-linking with Ca2+ or other divalent ions | Extensively in food, pharmaceuticals, and wound care products | High, extensively used, and widely produced | High elasticity, robust structural integrity | Excellent, widely used in tissue engineering | High, stable across wide temperature ranges (~150 °C) | High, readily cross-links with divalent ions | High, extensively modified for various uses | Calcium cross-linking, thiolation, carboxylation, hydrogel formation, esterification | Controlled release systems, wound care, tissue scaffolding | High dependency on cross-linking agents, cost of modification processes |
| Fucoidan | Fucus vesiculosus, Cladosiphon okamuranus, Laminaria japonica, Undaria pinnatifida | 5–10 | Not a primary gelling agent, it interacts through sulfated domains | Limited use in niche biomedical applications (anticoagulants, drug carriers) | Low, niche market with limited availability | Weak mechanical properties, limited application | Variable, dependent on sulfation level | Moderate, sensitive to heat above ~70 °C | Low, limited cross-linking capability | Moderate functionalization depends on sulfate groups | Sulfation, desulfation, acetylation, hydrogel formation, anti-coagulant enhancement | Anti-inflammatory agents, drug carriers, heparin substitutes | Variability in biological activity, cost-intensive extraction and modification |
| Laminaran | Laminaria spp. | 10–20 | Weak hydrogen bonding and limited gel formation | Occasionally, in nutraceuticals and research-grade biomaterials | Low, specialized production with limited supply | Low mechanical strength, not a primary gelling agent | Moderate, limited data on cytocompatibility | Low, weak stability under heat, <60 °C | Low, rarely used for cross-linking | Low, not typically functionalized extensively | Oxidation, acetylation, hydrogel formation, nanoparticle delivery systems | Nanoparticle stabilizers, immune enhancement, tissue scaffolds | Low stability under physiological conditions, complex modification processes |
| Porphyran | Porphyra spp. | 10–15 | Thermal gelation and hydrogen bonding | Emerging in antioxidant-rich supplements and basic drug delivery systems | Moderate, emerging commercial interest | Moderate strength, suitable for soft applications | Good, supports basic biomedical applications | Moderate, stable under mild thermal conditions (~80 °C) | Moderate potential for chemical derivatization | Moderate, supports basic functionalization | Sulfation, esterification, hydrogel formation, antioxidant enhancement | Antioxidant applications, drug delivery, immune modulation | Limited structural studies, stability in industrial applications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Mar. Drugs 2025, 23, 56. https://doi.org/10.3390/md23020056
Pari RF, Uju U, Hardiningtyas SD, Ramadhan W, Wakabayashi R, Goto M, Kamiya N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Marine Drugs. 2025; 23(2):56. https://doi.org/10.3390/md23020056
Chicago/Turabian StylePari, Rizfi Fariz, Uju Uju, Safrina Dyah Hardiningtyas, Wahyu Ramadhan, Rie Wakabayashi, Masahiro Goto, and Noriho Kamiya. 2025. "Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design" Marine Drugs 23, no. 2: 56. https://doi.org/10.3390/md23020056
APA StylePari, R. F., Uju, U., Hardiningtyas, S. D., Ramadhan, W., Wakabayashi, R., Goto, M., & Kamiya, N. (2025). Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Marine Drugs, 23(2), 56. https://doi.org/10.3390/md23020056









