Advanced Technologies for Large Scale Supply of Marine Drugs
Abstract
1. Introduction
2. Aquaculture
2.1. Aquaculture Methods: Mariculture vs. Captive Breeding
2.2. Aquaculture-Derived Compounds: Clinical Development
2.3. Innovative Technologies for Sustainable Marine Bioprospecting
3. Chemical Total Synthesis and Semi-Synthesis
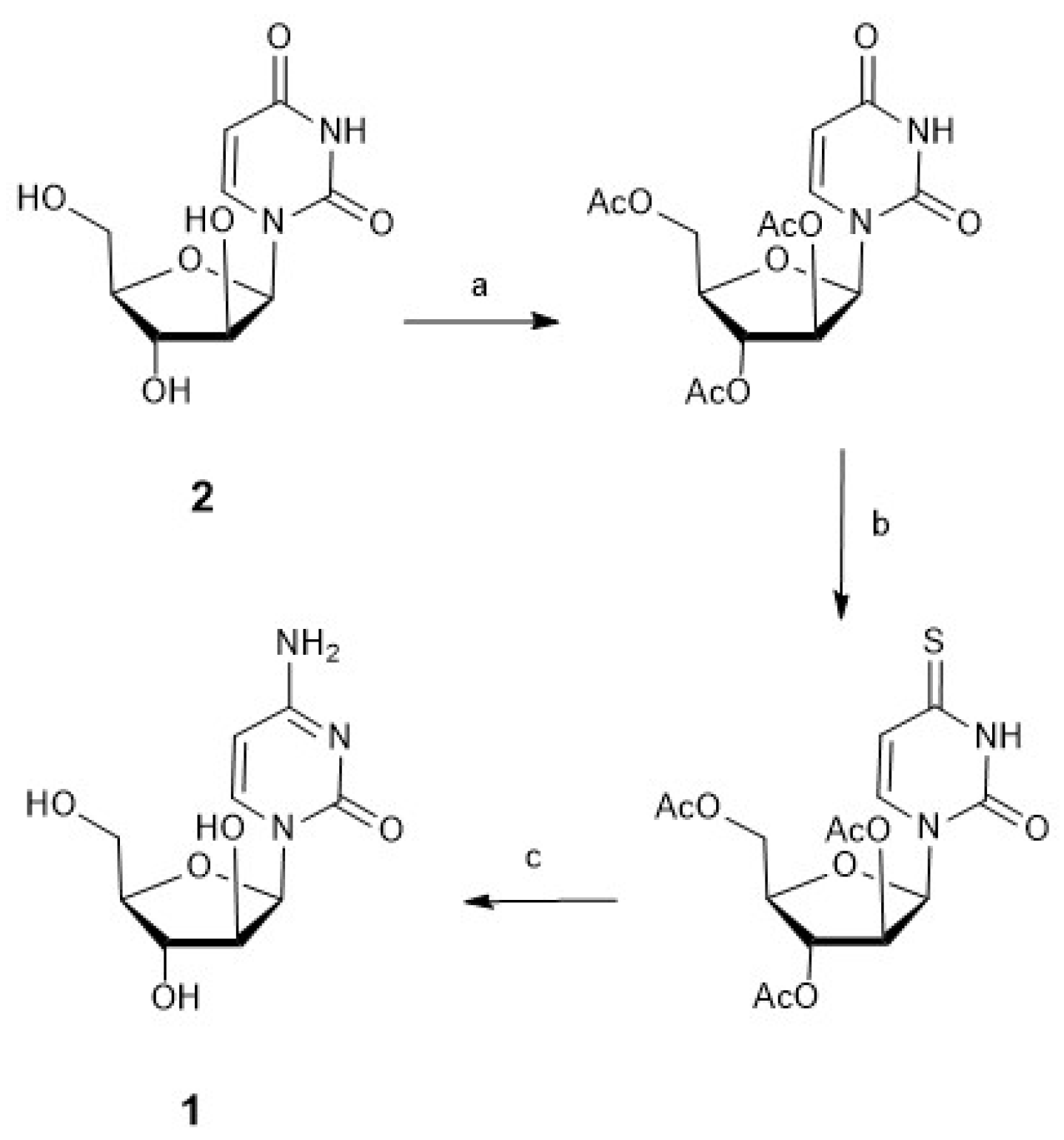
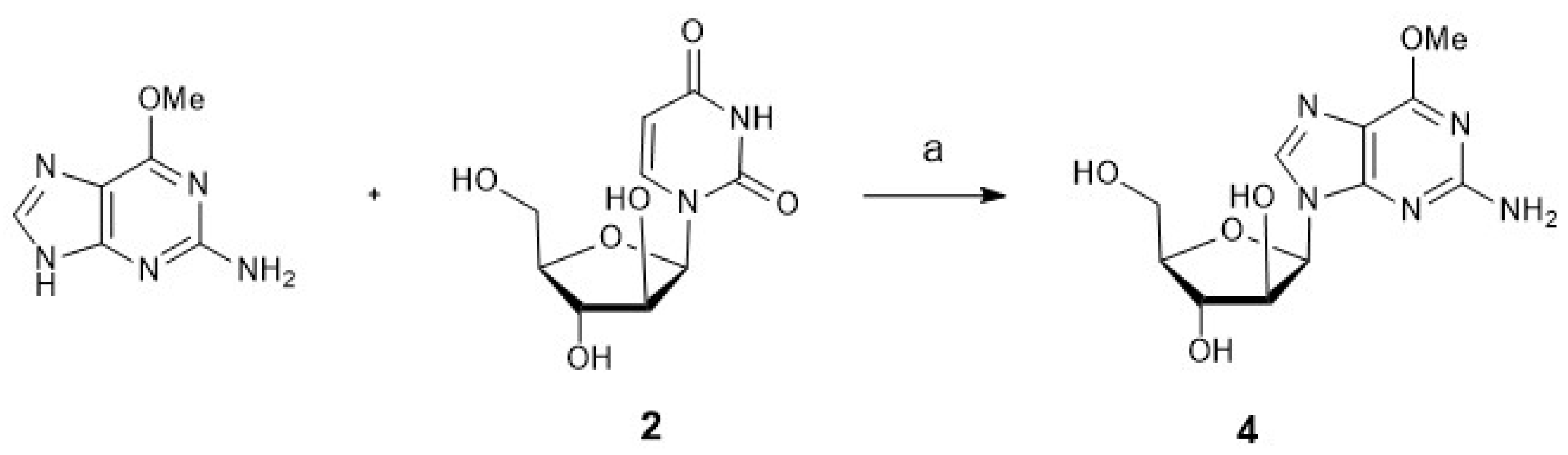
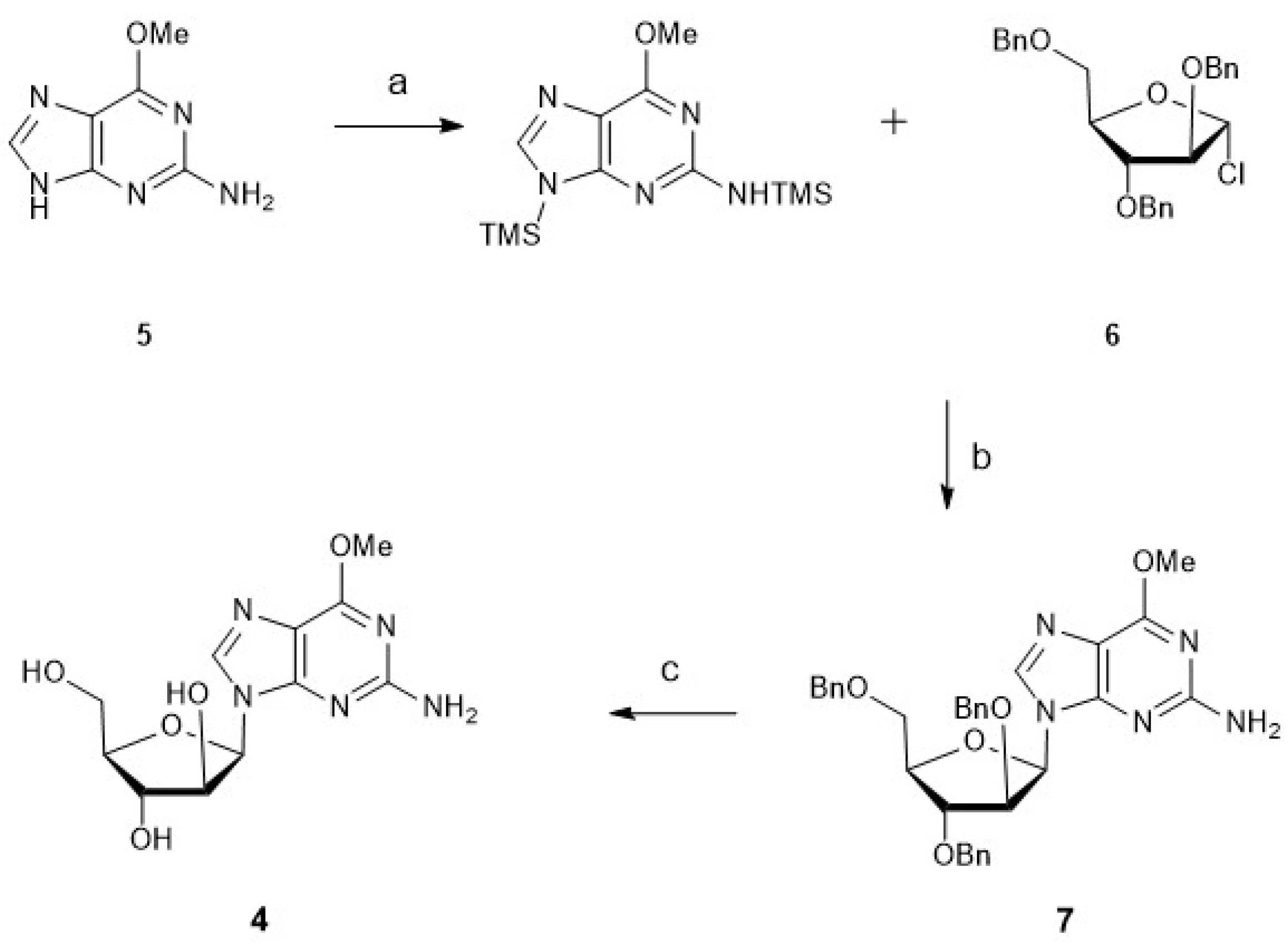
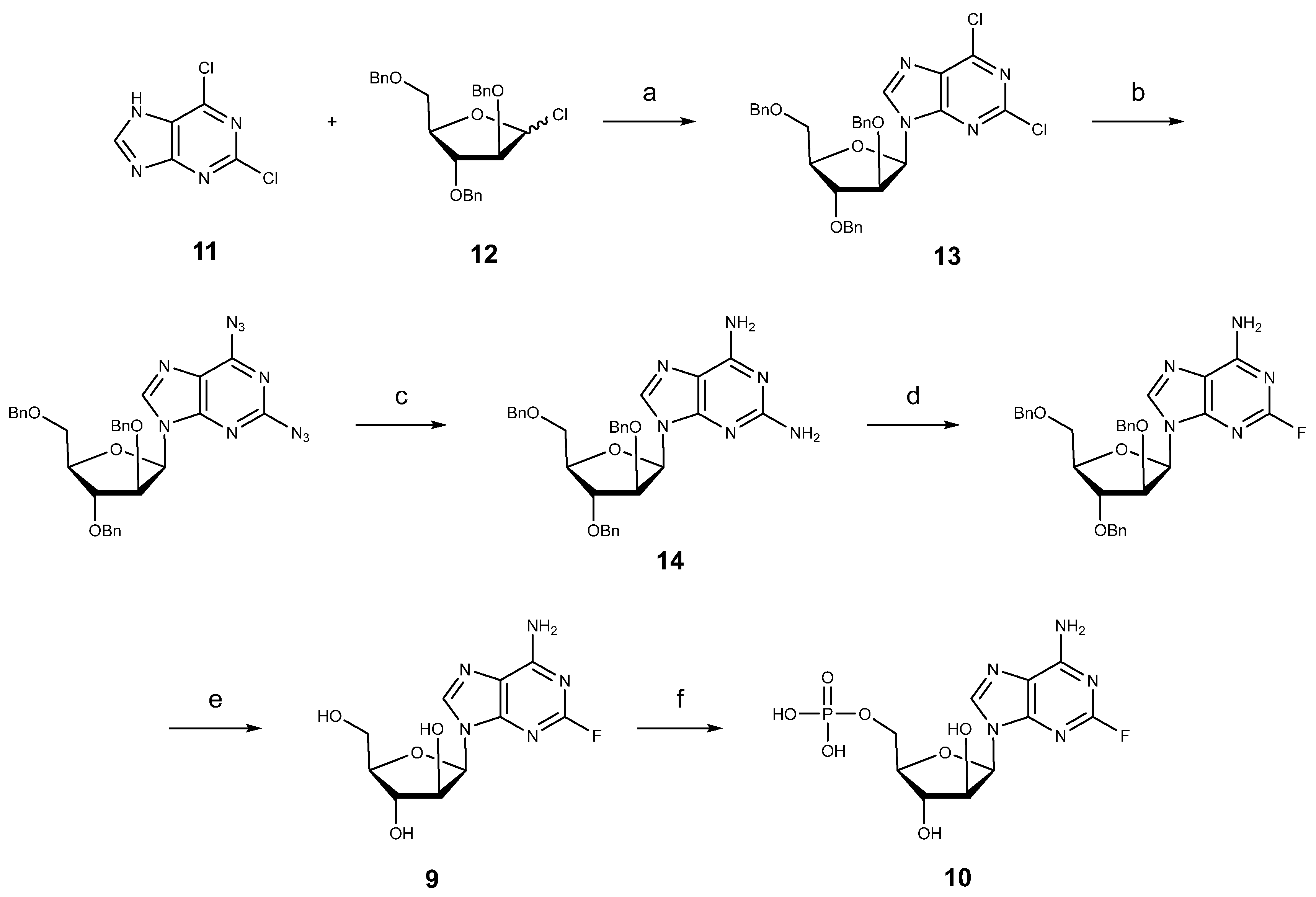
3.1. Synthesis of Nucleosides: Cytarabine, Nelarabine, and Vidarabine
3.1.1. Cytarabine
3.1.2. Nelarabine
3.1.3. Vidarabine
3.1.4. Fludarabine Phosphate
3.2. Synthesis of Depsipeptide: Plitidepsin
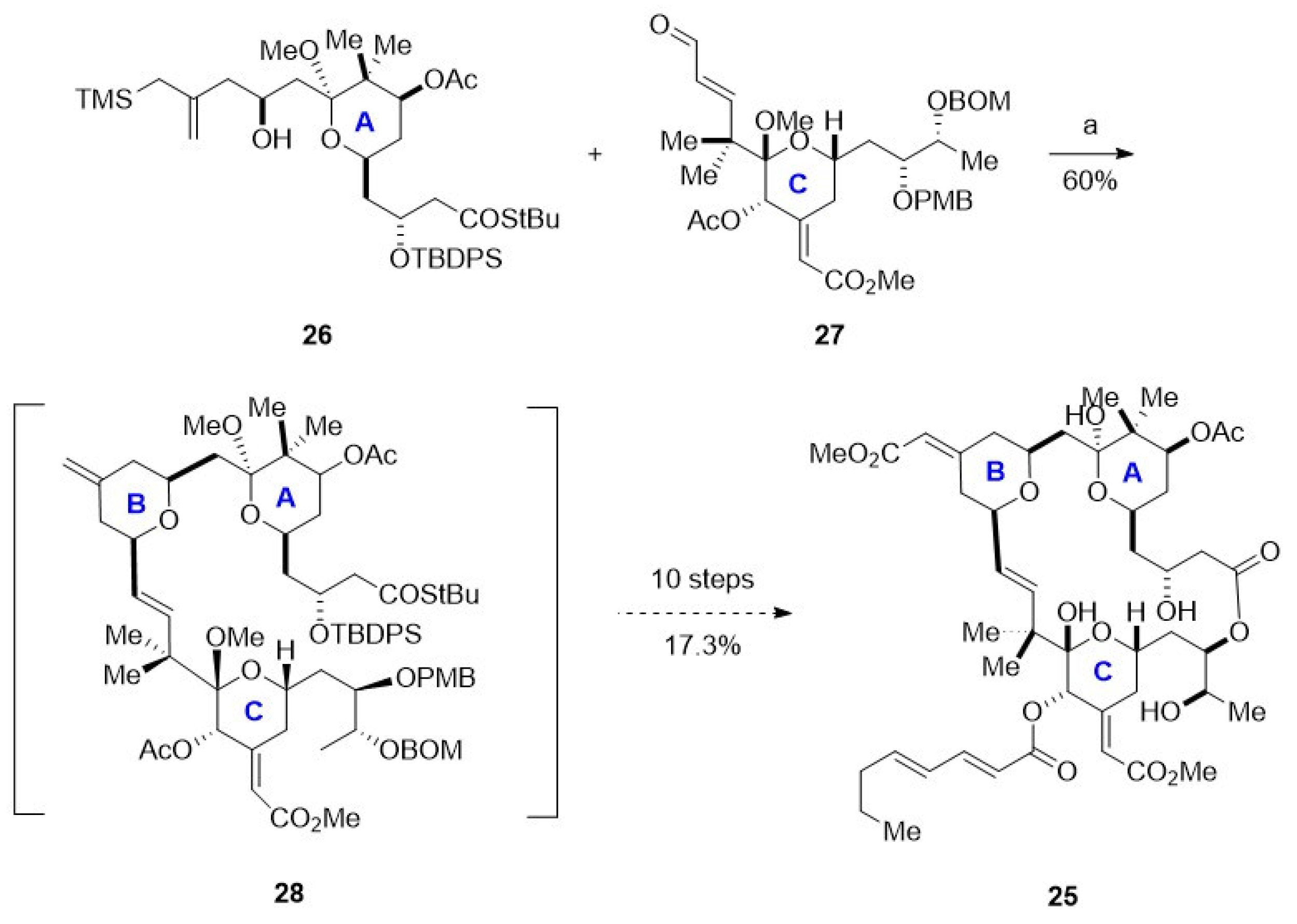
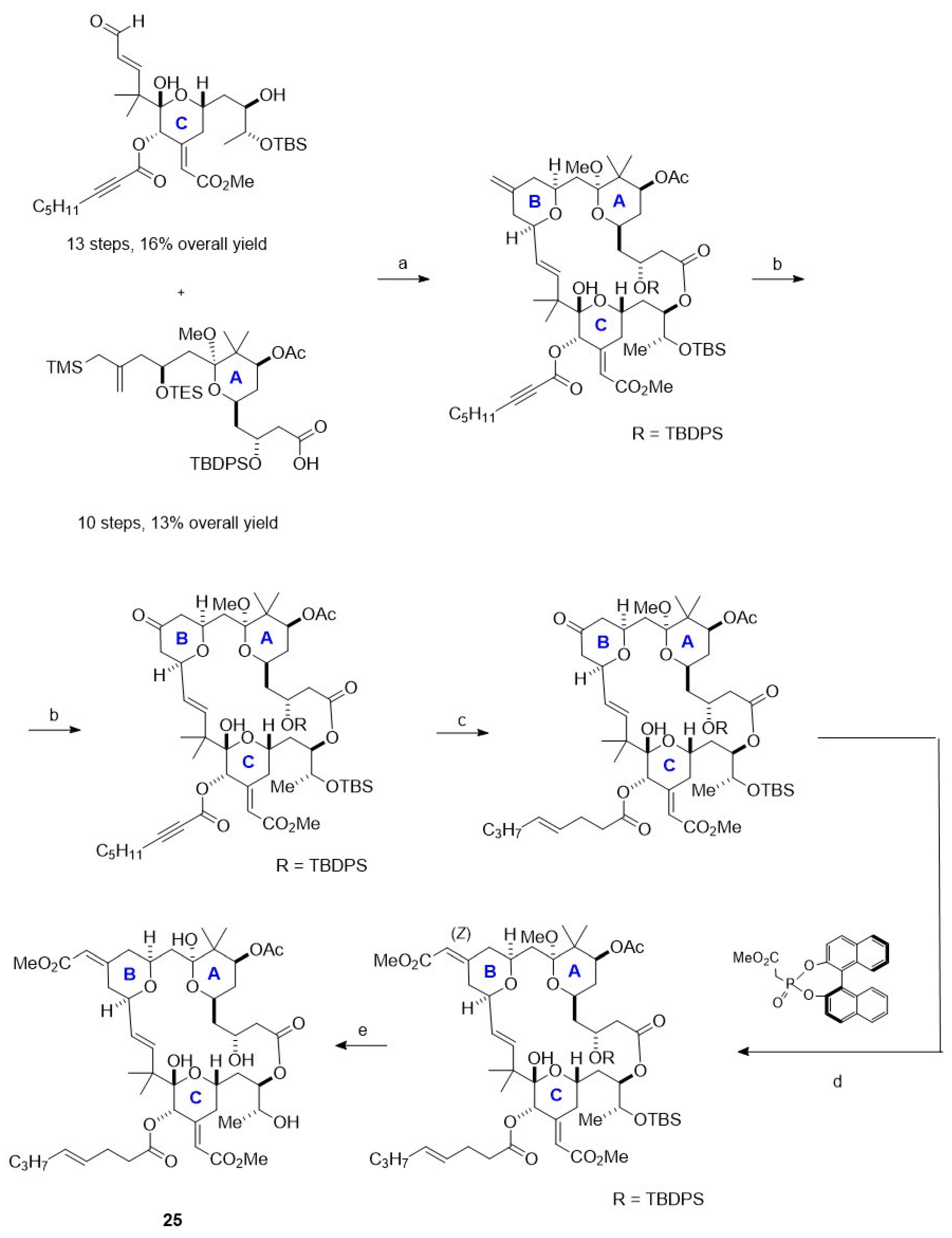
3.3. Synthesis of Macrolide: Bryostatin-1
3.4. Synthesis of Alkaloid: Trabectedine
4. Chemoenzymatic Synthesis of Marine Natural Products
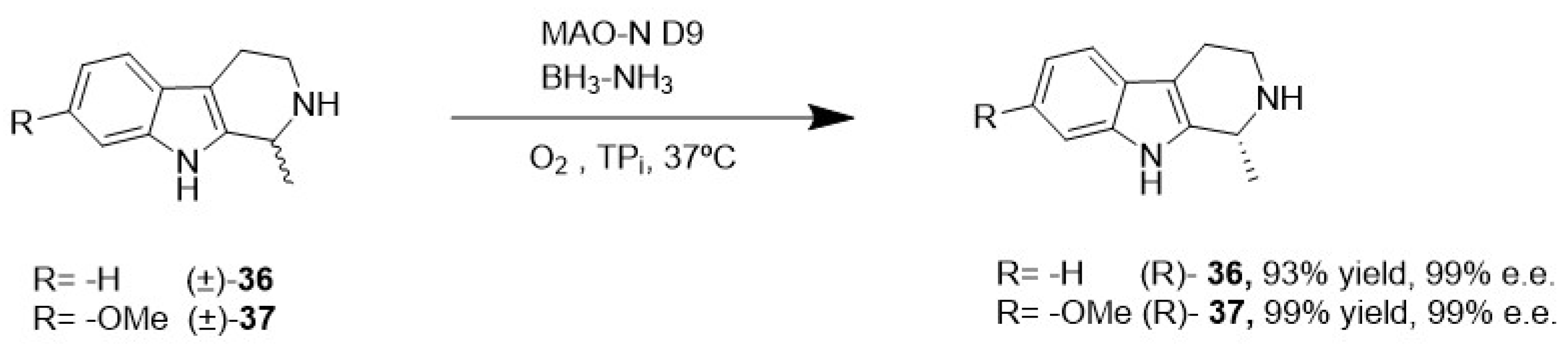
4.1. Enzymatic Desymmetrization of Meso-Compounds, Kinetic Resolution, and Deracemization
4.2. Enzymatic Carbon–Carbon Bond Formation
4.3. Biocatalytic Oxidation
5. Production of MNPs Using Biotechnological Approaches
5.1. Fermentation: Axenic Macroscale Culture and Mixed Fermentation
5.1.1. Axenic Macroscale Cultures
5.1.2. Mixed Fermentation or Co-Culture
5.2. Ex Vivo Biosynthesis or In Vitro Multienzyme Synthesis
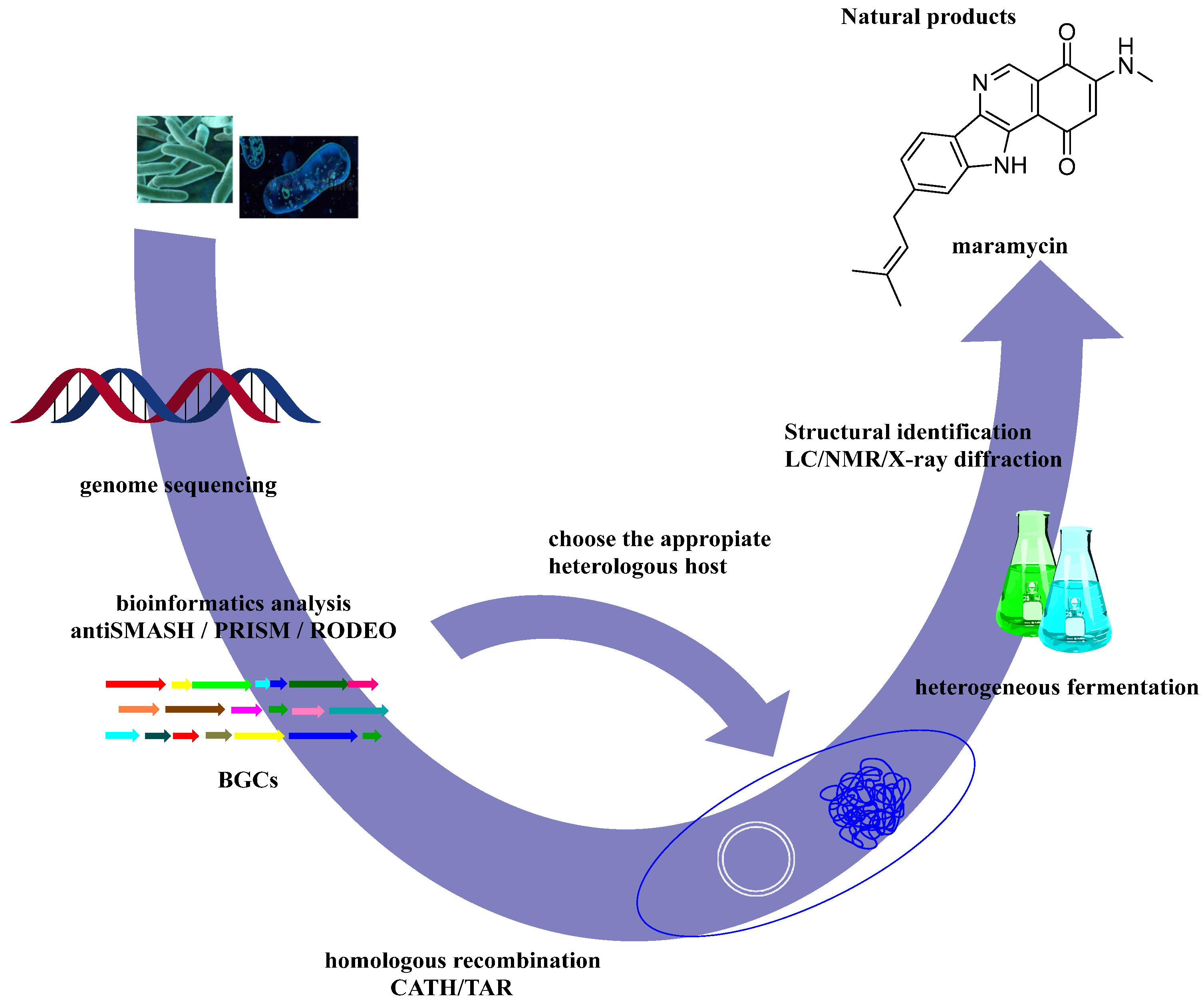
5.3. Heterologous Expression of Biosynthetic Gene Clusters (BGCs)
5.4. Combinatorial Biosynthesis and Metabolic Engineering
6. Computer-Aided Approaches to Development of Marine Natural Products
7. Artificial Intelligence in Marine Novel Drug Discovery
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ALL | Acute lymphoblastic leukemia |
| ADC | Antibody drug conjugate |
| Ara-A | Arabinosyl adenine |
| Ara-C | Arabinosyl cytosine |
| Ara-G | Arabinosyl guanine |
| Ara-U | Arabinosyl uracil |
| BGC | Biosynthetic gene cluster |
| CAL B | Candida antarctica lipase B |
| Cbz | Carboxybenzyl |
| CDFT | Conceptual Density Functional Theory |
| CDFT-CP | Conceptual Density Functional Theory based Computational Peptidology |
| DCW | Dry cell weight |
| DFT | Density functional theory |
| EBA | Enterobacter aerogenes |
| Et-743 | Trabectedin |
| F-ara-A | F-Arabinosyl adenosine |
| FDA | Food and Drug Administration |
| FDMO | FAD dependent monooxygenase |
| GMPs | Good Manufacturing Practices |
| HDMS | Hexamethyldisilazane |
| HPLC | High performance liquid chromatography |
| I-SMEL | In situ Marine moleculE Logger |
| MNPs | Marine Natural Products |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADP+ | Nicotinamide adenine dinucleotide phosphate |
| NCS | (S)-Norcoclaurine Synthase |
| PCR | Protein C Reactive |
| PCT | Patent Cooperation Treaty |
| PSTs | Paralytic shellfish toxins |
| PKC | Protein Kinase C |
| RCM | Ring closing metathesis |
| SA | Salicylic acid |
| STS | Soft tissue sarcoma |
| TEMPO | 2,2,6,6-tetramethylpiperidinyloxy |
References
- Gomes, N.G.M.; Madureira-Carvalho, Á.; Dias-da-Silva, D.; Valentão, P.; Andrade, P.B. Biosynthetic Versatility of Marine-Derived Fungi on the Delivery of Novel Antibacterial Agents against Priority Pathogens. Biomed. Pharmacother. 2021, 140, 111756–111776. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Azha, N.U.; Farooq, R.; Sheikh, S.A.; Ganie, M.A.; Parray, M.N.; Mushtaq, H.; Hameed, I.; Lone, M.A. Exploring the Potential of Marine Natural Products in Drug Development: A Comprehensive Review. Phytochem. Lett. 2024, 59, 124–135. [Google Scholar] [CrossRef]
- Bocharova, E.A.; Kopytina, N.I.; Slynko, E.E. Anti-Tumour Drugs of Marine Origin Currently at Various Stages of Clinical Trials (Review). Regul. Mech. Biosyst. 2021, 12, 365–380. [Google Scholar] [CrossRef]
- Trincone, A. Enzymatic Processes in Marine Biotechnology. Mar. Drugs 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.-H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia 2017, 71, 646–652. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Bilela, L.L.; Moulin, C.; Taffin-De-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Kong, D.-X.; Jiang, Y.-Y.; Zhang, H.-Y. Marine Natural Products as Sources of Novel Scaffolds: Achievement and Concern. Drug Discov. Today 2010, 15, 884–886. [Google Scholar] [CrossRef]
- Wright, G.D. Unlocking the Potential of Natural Products in Drug Discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching Cancer Pharmacology with Drugs of Marine Origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef]
- Mahajan, P.; Sufiyan, M.; Anwer, Z. Marine drugs. Int. J. Creat. Res. Thoughts 2022, 10, 18–40. [Google Scholar]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of Marine Natural Products in Drug Research. Bioorg. Med. Chem. 2021, 35, 116058–116088. [Google Scholar] [CrossRef]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentaõ, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-Derived Anticancer Agents: Clinical Benefits, Innovative Mechanisms, and New Targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine Cyclic Peptides: Antimicrobial Activity and Synthetic Strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jin, Y.; Hou, X.; Zhu, Y.; Chen, D.; Tai, J.; Chen, Q.; Shi, C.; Ye, J.; Wu, M.; et al. Application of Marine Natural Products against Alzheimer’s Disease: Past, Present and Future. Mar. Drugs 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2020. Mar. Drugs 2020, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bio-active Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef]
- Papon, N.; Copp, B.R.; Courdavault, V. Marine Drugs: Biology, Pipelines, Current and Future Prospects for Production. Biotechnol. Adv. 2022, 54, 107871–107881. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Developing Natural Product Drugs: Supply Problems and How They Have Been Overcome. Pharmacol. Ther. 2016, 162, 1–9. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine Invertebrate Metabolites with Anticancer Activities: Solutions to the “Supply Problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Drug Development: The Journey of a Medicine from Lab to Shelf. Pharm. J. 2015, 12, 926–932. [Google Scholar]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial Production of the Tunicate-Derived Antitumor Cyclic Depsipeptide Didemnin B. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, O. Why a Landmark Treaty to Stop Ocean Biopiracy Could Stymie Research. Nature 2020, 580, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug Development from Marine Natural Products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M.; et al. The Essentials of Marine Biotechnology. Front. Mar. Sci. 2021, 8, 629629–629681. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Liu, W. Cell-Free Synthetic Biology for in vitro Biosynthesis of Pharmaceutical Natural Products. Synth. Syst. Biotechnol. 2018, 3, 83–89. [Google Scholar] [CrossRef]
- Kanase, H.R.; Singh, K.N.M. Marine Pharmacology: Potential, Challenges, and Future in India. J. Med. Sci. 2018, 38, 49–53. [Google Scholar]
- Khalil, A.S.; Collins, J.J. Synthetic Biology: Applications Come of Age. Nat. Rev. Genet. 2010, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, Z.; Sun, J.; Lee, S.Y. Metabolic Engineering for the Microbial Production of Marine Bioactive Compounds. Biotechnol. Adv. 2017, 35, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; McCarthy, C.; Eustace, S.; FitzPatrick, R.E.; Delahunt, E.; De Vito, G. Mineral Rich Algae with Pine Bark Improved Pain, Physical Function and Analgesic Use in Mild-Knee Joint Osteoarthritis, Compared to Glucosamine: A Randomized Controlled Pilot Trial. Complement. Ther. Med. 2020, 50, 102349–102356. [Google Scholar] [CrossRef]
- Benkendorff, K. Aquaculture and the Production of Pharmaceuticals and Nutraceuticals. In New Technologies in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2009; pp. 866–891. [Google Scholar]
- Leal, M.C.; Ferrier-Pagès, C.; Calado, R.; Thompson, M.E.; Frischer, M.E.; Nejstgaard, J.C. Coral Feeding on Microalgae Assessed with Molecular Trophic Markers. Mol. Ecol. 2014, 23, 3870–3876. [Google Scholar] [CrossRef] [PubMed]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Vlachou, P.; Le Goff, G.; Alonso, C.; Álvarez, P.A.; Gallard, J.-F.; Fokialakis, N.; Ouazzani, J. Innovative Approach to Sustainable Marine Invertebrate Chemistry and a Scale-Up Technology for Open Marine Ecosystems. Mar. Drugs 2018, 16, 152. [Google Scholar] [CrossRef]
- Page, M.J.; Handley, S.J.; Northcote, P.T.; Cairney, D.; Willan, R.C. Successes and Pitfalls of the Aquaculture of the Sponge Mycale Hentscheli. Aquaculture 2011, 312, 52–61. [Google Scholar] [CrossRef]
- Potts, M.B.; McMillan, E.A.; Rosales, T.I.; Kim, H.S.; Ou, Y.-H.; Toombs, J.E.; Brekken, R.A.; Minden, M.D.; MacMillan, J.B.; White, M.A. Mode of Action and Pharmacogenomic Biomarkers for Exceptional Responders to Didemnin B. Nat. Chem. Biol. 2015, 11, 401–408. [Google Scholar] [CrossRef]
- Chun, H.G.; Davies, B.; Hoth, D.; Suffness, M.; Plowman, J.; Flora, K.; Grieshaber, C.; Leyland-Jones, B. Didemnin B. The First Marine Compound Entering Clinical Trials as an Antineoplastic Agent. Invest. New Drugs 1986, 4, 279–284. [Google Scholar] [CrossRef]
- Ankisetty, S.; Khan, S.I.; Avula, B.; Gochfeld, D.; Khan, I.A.; Slattery, M. Chlorinated Didemnins from the Tunicate Trididemnum solidum. Mar. Drugs 2013, 11, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, C.; Francesch, A.; Galmarini, C.M.; Aviles, P.; Munt, S. Ecteinascidin-743 (Yondelis®). Aplidin® and Irvalec®. In Anticancer Agents from Natural Products; CRC Press: Boca Raton, FL, USA, 2012; Volume 2, pp. 291–316. [Google Scholar]
- Delgado-Calle, J.; Kurihara, N.; Atkinson, E.G.; Nelson, J.; Miyagawa, K.; Galmarini, C.M.; Roodman, G.D.; Bellido, T. Aplidin (Plitidepsin) Is a Novel Anti-Myeloma Agent with Potent Anti-Resorptive Activity Mediated by Direct Effects on Osteoclasts. Oncotarget 2019, 10, 2709–2721. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Valentão, P.; Andrade, P.B.; Pereira, R.B. Plitidepsin to Treat Multiple Myeloma. Drugs Today 2020, 56, 337–347. [Google Scholar] [CrossRef]
- Schaufelberger, D.E.; Koleck, M.P.; Beutler, J.A.; Vatakis, A.M.; Alvarado, A.B.; Andrews, P.; Marzo, L.V.; Muschik, G.M.; Roach, J.; Ross, J.T.; et al. The Large-Scale Isolation of Bryostatin 1 from Bugula neritina Following Current Good Manufacturing Practices. J. Nat. Prod. 1991, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Kallifidas, D.; Dhakal, D.; Chen, M.; Chen, Q.-Y.; Kokkaliari, S.; Colon Rosa, N.A.; Ratnayake, R.; Bruner, S.D.; Paul, V.J.; Ding, Y.; et al. Biosynthesis of Dolastatin 10 in Marine Cyanobacteria, a Prototype for Multiple Approved Cancer Drugs. Org. Lett. 2024, 26, 1321–1325. [Google Scholar] [CrossRef]
- Matsumoto-Elliott, O.; Sanchez, L.M. I-SMEL a Big Catch! ACS Cent. Sci. 2023, 9, 2006–2008. [Google Scholar] [CrossRef]
- Mauduit, M.; Derrien, M.; Grenier, M.; Greff, S.; Molinari, S.; Chevaldonné, P.; Simmler, C.; Pérez, T. In situ Capture and Real-Time Enrichment of Marine Chemical Diversity. ACS Cent. Sci. 2023, 9, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.; Salib, M.N.; Chase, A.B.; Hammerlindl, H.; Muskat, M.N.; Luedtke, S.; da Silva, E.B.; O’Donoghue, A.J.; Wu, L.F.; Altschuler, S.J.; et al. Small Molecule in situ Resin Capture Provides a Compound First Approach to Natural Product Discovery. Nat. Commun. 2024, 15, 5230. [Google Scholar] [CrossRef]
- Bauer, A. Story of Eribulin Mesylate: Development of the Longest Drug Synthesis. In Synthesis of Heterocycles in Contemporary Medicinal Chemistry. Topics in Heterocyclic Chemistry; Casar, Z., Ed.; Springer: Cham, Switzerland, 2016; Volume 44, pp. 209–270. [Google Scholar]
- Nicolaou, K.C.; Pan, S.; Shelke, Y.; Rigol, S.; Bao, R.; Das, D.; Ye, Q. A Unified Strategy for the Total Syntheses of Eribulin and a Macrolactam Analogue of Halichondrin B. Proc. Natl. Acad. Sci. USA 2022, 119, e2208938119. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.; Lu, X.; Hollenbaugh, J.A.; Cho, J.H.; Amblard, F.; Schinazi, R.F. Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs. Chem. Rev. 2016, 116, 14379–14455. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S. Sponges, Cancer Chemotherapy, and Cellular Aging. Perspect. Biol. Med. 1963, 6, 215–227. [Google Scholar] [CrossRef]
- Wang, J.J.; Selawry, O.S.; Vietti, T.J.; Bodey, G.P. Prolonged Infusion of Arabinosyl Cytosine in Childhood Leukemia. Cancer 1970, 25, 1–6. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Z.; Jia, G.; Li, H.; Wu, X. Preparing Method for Cytarabine. Patent 03153692.1, CN1302004C, 28 February 2005. [Google Scholar]
- Robak, T.; Lech-Maranda, E.; Korycka, A.; Robak, E. Purine Nucleoside Analogs as Immunosuppressive and Antineoplastic Agents: Mechanism of Action and Clinical Activity. Curr. Med. Chem. 2006, 13, 3165–3189. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.; Eaddy, J.; Dawson, M. Enzymic Acylation of 506U78 (2-Amino-9-Beta-D-Arabinofuranosyl-6-Methoxy-9H-Purine), a Powerful New Anti-Leukaemic Agent. Biotechnol. Appl. Biochem. 1999, 29, 229–233. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Nimmanapalli, R.; Ravandi, F.; Keating, M.J.; Gandhi, V. Forodesine, an Inhibitor of Purine Nucleoside Phosphorylase, Induces Apoptosis in Chronic Lymphocytic Leukemia Cells. Blood 2006, 108, 2392–2398. [Google Scholar] [CrossRef]
- Osborne, W.R.A.; Chen, S.-H.; Giblett, E.R.; Biggar, W.D.; Ammann, A.A.; Scott, C.R. Purine Nucleoside Phosphorylase Deficiency. J. Clin. Investig. 1977, 60, 741–746. [Google Scholar] [CrossRef]
- Rodriguez, C.O.; Gandhi, V. Arabinosylguanine-Induced Apoptosis of T-Lymphoblastic Cells: Incorporation into DNA Is a Necessary Step. Cancer Res. 1999, 59, 4937–4943. [Google Scholar]
- Gravatt, L.C.; Chaffee, S.; Hebert, M.E.; Halperin, E.C.; Friedman, H.S.; Kurtzberg, J. Efficacy and Toxicity of 9-Beta-D-Arabinofuranosylguanine (AraG) as an Agent to Purge Malignant T Cells from Murine Bone Marrow: Application to an in vivo T-Leukemia Model. Leukemia 1993, 7, 1261–1267. [Google Scholar]
- Cohen, M.H.; Johnson, J.R.; Massie, T.; Sridhara, R.; McGuinn, W.D.; Abraham, S.; Booth, B.P.; Goheer, M.A.; Morse, D.; Chen, X.H.; et al. Approval Summary: Nelarabine for the Treatment of T-Cell Lymphoblastic Leukemia/Lymphoma. Clin. Cancer Res. 2006, 12, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Ford Hutman, R. Shorla Pharma Announces FDA Filing Acceptance and Priority Review for T-Cell Leukemia Treatment on April 23, 2021. Available online: https://www.businesswire.com/news/home/20210423005122/en/Shorla-Pharma-Announces-FDA-Filing-Acceptance-and-Priority-Review-for-T-cell-Leukemia-Treatment (accessed on 20 December 2024).
- Krenitsky, T.A.; Koszalka, G.W.; Jones, L.A.; Averett, D.R.; Moorman, A.R. Antiviral Compounds. EP Patent 0294114, 7 December 1988. [Google Scholar]
- Zhai, Y.; Wang, J. Synthetic Method for Preparing Nelarabine. Patent 201310460155.9, CN103483409A, 24 September 2013. [Google Scholar]
- Buchanan, R.A.; Hess, F. Vidarabine (Vira-A®): Pharmacology and Clinical Experience. Pharmacol. Ther. 1980, 8, 143–171. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral Lead Compounds from Marine Sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Gandhi, V. Evolution of the Arabinosides and the Pharmacology of Fludarabine. Drugs 1994, 47, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Chubb, S.; Alexander, L.; Montgomery, J.A. Comparison of the Toxicity and Metabolism of 9-Beta-D-Arabinofuranosyl-2-Fluoroadenine and 9-Beta-D-Arabinofuranosyladenine in Human Lymphoblastoid Cells. Cancer Res. 1980, 40, 2349–2355. [Google Scholar]
- Gandhi, V.; Chen, W.; Ayres, M.; Rhie, J.; Madden, T.; Newman, R. Plasma and Cellular Pharmacology of 8-Chloro-Adenosine in Mice and Rats. Cancer Chemother. Pharmacol. 2002, 50, 85–94. [Google Scholar] [CrossRef]
- Gerrie, A.S.; Zypchen, L.N.; Connors, J.M. Fludarabine and Rituximab for Relapsed or Refractory Hairy Cell Leukemia. Blood 2012, 119, 1988–1991. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chubb, S.; Plunkett, W. Termination of DNA Synthesis by 9-Beta-D-Arabinofuranosyl-2-Fluoroadenine. A Mechanism for Cytotoxicity. J. Biol. Chem. 1990, 265, 16617–16625. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Saunders, P.P. Metabolism and Action of Purine Nucleoside Analogs. Pharmacol. Ther. 1991, 49, 239–268. [Google Scholar] [CrossRef]
- Montgomery, J.; Hewson, K. Nucleosides of 2-Fluoroadenine. J. Med. Chem. 1969, 12, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A. Anticancer and Antiviral Activity of 9-β-d-Arabinofuranosyl-2-Fluoroadenine. Patent 4188378, US4188378A, 12 February 1980. [Google Scholar]
- Montgomery, J.A.; Shortnacy, A.T. Prodrug Derivatives of 9-β-d-Arabinofuranosyl-2-Fluoroadenine. US 4357324, 2 November 1982. [Google Scholar]
- Kshirsagar, S.W.; Deshpande, M.S.; Sonawane, S.P.; Maikap, G.C.; Gurjar, M.K. Simple Modification To Obtain High Quality Fludarabine. Org. Process. Res. Dev. 2012, 16, 840–842. [Google Scholar] [CrossRef]
- Farina, P.; Petrucciani, L.; Colombo, P.; Caprioli, G. A Process for the Preparation of Fludarabine Phosphate from 2-Fluoroadenine. EP1464708A1, 3 April 2003. [Google Scholar]
- Shen, C.; Liu, J.; Ouyang, W.; Ding, H.; Bai, J.; Xiao, Q. Practical Synthesis of Fludarabine and Nelarabine. Synthesis 2020, 52, 417–423. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martin, A. Plitidepsin: Design, Development, and Potential Place in Therapy. Drug Des. Devel. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Venkatesan, J. Introduction to Marine Biomaterials. In Marine Biomaterials; CRC Press: Boca Raton, FL, USA, 2013; pp. 3–16. [Google Scholar]
- Pelay-Gimeno, M.; Albericio, F.; Tulla-Puche, J. Synthesis of Complex Head-to-Side-Chain Cyclodepsipeptides. Nat. Protoc. 2016, 11, 1924–1947. [Google Scholar] [CrossRef]
- Zhang, H.; Hui, Z.; Cai, M.; Huang, S.; Shi, W.; Liang, M.; Lin, Y.; Shen, J.; Sui, M.; Li, X.; et al. Integration of Microbial and Chemical Synthesis for the Efficient Production of Plitidepsin, a Promising Anticancer and Antiviral Agent. BioRxiv 2023, 1–7. [Google Scholar] [CrossRef]
- Rodríguez, I.; Polanco, C.; Cuevas, F.; Mandez, P.; Cuevas, C.; Gallego, P.; Munt, S.; Manzanares, I. Synthetic Methods for Aplidin, and New Antitumoral Derivatives, Methods of Making and Using Them. WO2002002596A2, 7 February 2001. [Google Scholar]
- Kortmansky, J.; Schwartz, G.K. Bryostatin-1: A Novel PKC Inhibitor in Clinical Development. Cancer Investig. 2003, 21, 924–936. [Google Scholar] [CrossRef]
- Etcheberrigaray, R.; Tan, M.; Dewachter, I.; Kuipéri, C.; Van der Auwera, I.; Wera, S.; Qiao, L.; Bank, B.; Nelson, T.J.; Kozikowski, A.P.; et al. Therapeutic Effects of PKC Activators in Alzheimer’s Disease Transgenic Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 11141–11146. [Google Scholar] [CrossRef]
- Kornberg, M.D.; Smith, M.D.; Shirazi, H.A.; Calabresi, P.A.; Snyder, S.H.; Kim, P.M. Bryostatin-1 Alleviates Experimental Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, 2186–2191. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, X.-T.; Jiang, X.; Tian, J. Bryostatin-1: A Promising Compound for Neurological Disorders. Front. Pharmacol. 2023, 14, 1187411. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The Isolation and Structure of a Remarkable Marine Animal Antineoplastic Constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Manaviazar, S.; Hale, K.J. Total Synthesis of Bryostatin 1: A Short Route. Angew. Chem. Int. Ed. 2011, 50, 8786–8789. [Google Scholar] [CrossRef]
- Manaviazar, S.; Frigerio, M.; Bhatia, G.S.; Hummersone, M.G.; Aliev, A.E.; Hale, K.J. Enantioselective Formal Total Synthesis of the Antitumor Macrolide Bryostatin 7. Org. Lett. 2006, 8, 4477–4480. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Hardman, C.T.; Ho, S.; Jeffreys, M.S.; Maclaren, J.K.; Quiroz, R.V.; Ryckbosch, S.M.; Shimizu, A.J.; Sloane, J.L.; Stevens, M.C. Scalable Synthesis of Bryostatin 1 and Analogs, Adjuvant Leads against Latent HIV. Science 2017, 358, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Rychnovsky, S.D.; Kim, J. Triphenylphosphine-Catalyzed Isomerizations of Enynes to (E,E,E)-Trienes: Phenol as a Cocatalyst. J. Org. Chem. 1994, 59, 2659–2660. [Google Scholar] [CrossRef]
- Moore, B.M.; Seaman, F.C.; Hurley, L.H. NMR-Based Model of an Ecteinascidin 743−DNA Adduct. J. Am. Chem. Soc. 1997, 119, 5475–5476. [Google Scholar] [CrossRef]
- Corey, E.J.; Gin, D.Y.; Kania, R.S. Enantioselective Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 1996, 118, 9202–9203. [Google Scholar] [CrossRef]
- Cuevas, C.; Francesch, A. Development of Yondelis® (Trabectedin, ET-743). A Semisynthetic Process Solves the Supply Problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- Martinez, E.J.; Corey, E.J. A New, More Efficient, and Effective Process for the Synthesis of a Key Pentacyclic Intermediate for Production of Ecteinascidin and Phthalascidin Antitumor Agents. Org. Lett. 2000, 2, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 2002, 124, 6552–6554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Bois-Choussy, M.; Zhu, J. Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 2006, 128, 87–89. [Google Scholar] [CrossRef]
- Zheng, S.; Chan, C.; Furuuchi, T.; Wright, B.J.D.; Zhou, B.; Guo, J.; Danishefsky, S.J. Stereospecific Formal Total Synthesis of Ecteinascidin 743. Angew. Chem. Int. Ed. 2006, 45, 1754–1759. [Google Scholar] [CrossRef]
- Fishlock, D.; Williams, R.M. Synthetic Studies on Et-743. Assembly of the Pentacyclic Core and a Formal Total Synthesis. J. Org. Chem. 2008, 73, 9594–9600. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Ma, D. A Scalable Total Synthesis of the Antitumor Agents Et-743 and Lurbinectedin. Angew. Chem. Int. Ed. 2019, 58, 3972–3975. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, D.; Hu, Z.; Zheng, Z.; Chen, X. A New Approach to the Synthesis of L-3-Hydroxy-4-Methoxy-5-Methyl-Phenylalanine Derivatives from l-Tyrosine. Tetrahedron Asymmetry 2010, 21, 39–42. [Google Scholar] [CrossRef]
- Cuevas, C.; Pérez, M.; Martín, M.J.; Chicharro, J.L.; Fernández-Rivas, C.; Flores, M.; Francesch, A.; Gallego, P.; Zarzuelo, M.; de la Calle, F.; et al. Synthesis of Ecteinascidin ET-743 and Phthalascidin Pt-650 from Cyanosafracin B. Org. Lett. 2000, 2, 2545–2548. [Google Scholar] [CrossRef]
- Tirkkonen, H.; Brown, K.V.; Niemczura, M.; Faudemer, Z.; Brown, C.; Ponomareva, L.V.; Helmy, Y.A.; Thorson, J.S.; Nybo, S.E.; Metsä-Ketelä, M.; et al. Engineering BioBricks for Deoxysugar Biosynthesis and Generation of New Tetracenomycins. ACS Omega 2023, 8, 21237–21253. [Google Scholar] [CrossRef]
- Keasling, J.D.; Mendoza, A.; Baran, P.S. A Constructive Debate. Nature 2012, 492, 188–189. [Google Scholar] [CrossRef]
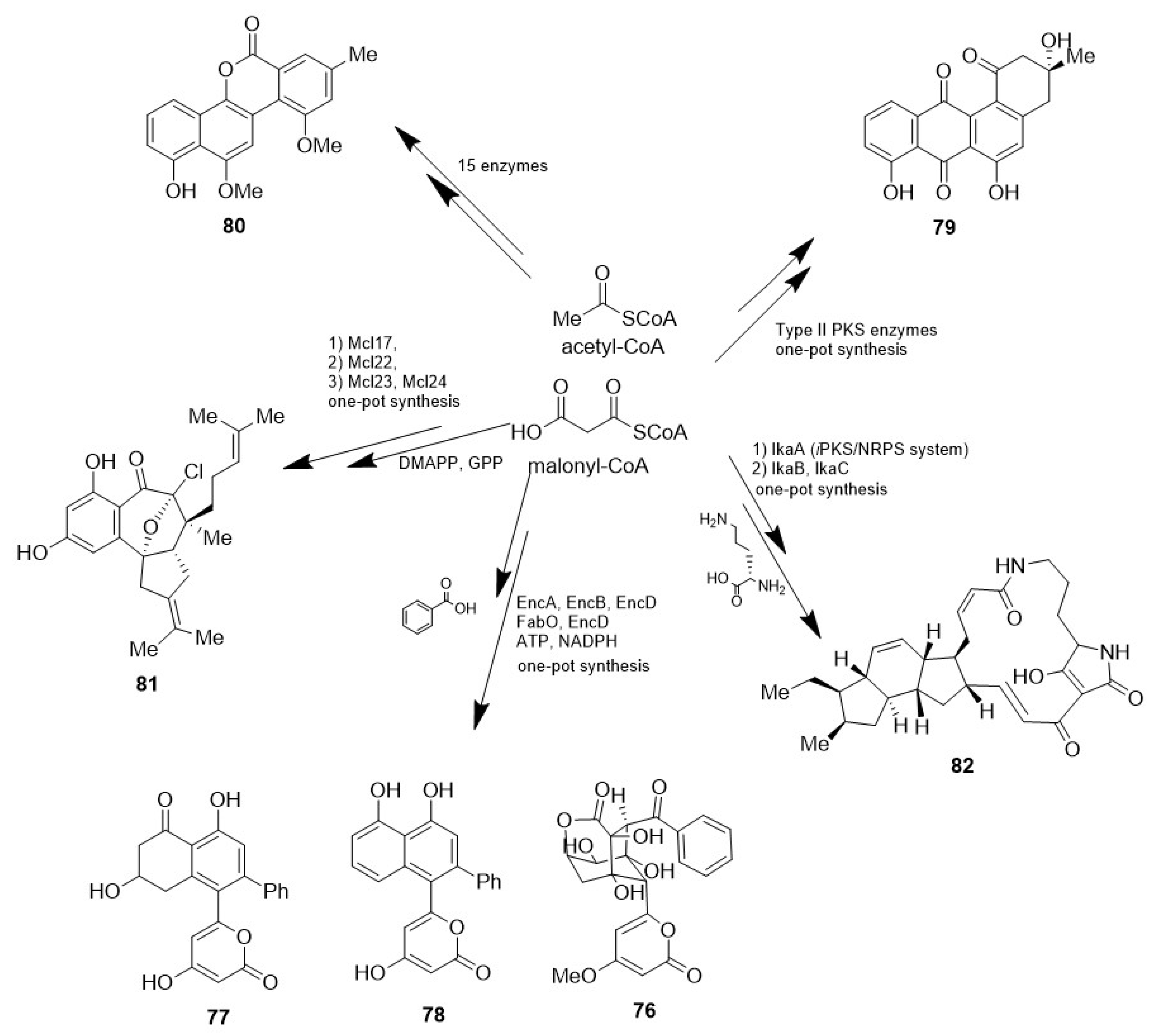
- Teufel, R.; Kaysser, L.; Villaume, M.T.; Diethelm, S.; Carbullido, M.K.; Baran, P.S.; Moore, B.S. One-Pot Enzymatic Synthesis of Merochlorin A and B. Angew. Chem. Int. Ed. 2014, 53, 11019–11022. [Google Scholar] [CrossRef] [PubMed]
- Tanifuji, R.; Minami, A.; Oguri, H.; Oikawa, H. Total Synthesis of Alkaloids Using Both Chemical and Biochemical Methods. Nat. Prod. Rep. 2020, 37, 1098–1121. [Google Scholar] [CrossRef]
- Yu, S.; Park, S.Y.; Kim, D.H.; Yun, E.J.; Kim, K.H. Multi-Step Enzymatic Production and Purification of 2-Keto-3-Deoxy-Galactonate from Red-Macroalgae-Derived Agarose. Mar. Drugs 2022, 20, 288. [Google Scholar] [CrossRef]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Paquette, L.A.; Romine, J.L.; Lin, H.S.; Wright, J. Total Synthesis of (+)-Ikarugamycin. 1. Stereocontrolled Construction of the Decahydro-as-Indacene Subunit. J. Am. Chem. Soc. 1990, 112, 9284–9292. [Google Scholar] [CrossRef]
- Devine, P.N.; Howard, R.M.; Kumar, R.; Thompson, M.P.; Truppo, M.D.; Turner, N.J. Extending the Application of Biocatalysis to Meet the Challenges of Drug Development. Nat. Rev. Chem. 2018, 2, 409–421. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the Third Wave of Biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tanifuji, R.; Oguri, H. Chemo-Enzymatic Total Synthesis: Current Approaches toward the Integration of Chemical and Enzymatic Transformations. Beilstein J. Org. Chem. 2024, 20, 1693–1712. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Resch, V. The Role of Biocatalysis in the Asymmetric Synthesis of Alkaloids. RSC Adv. 2013, 3, 17602–17632. [Google Scholar] [CrossRef]
- Cigan, E.; Eggbauer, B.; Schrittwieser, J.H.; Kroutil, W. The Role of Biocatalysis in the Asymmetric Synthesis of Alkaloids—An Update. RSC Adv. 2021, 11, 28223–28270. [Google Scholar] [CrossRef]
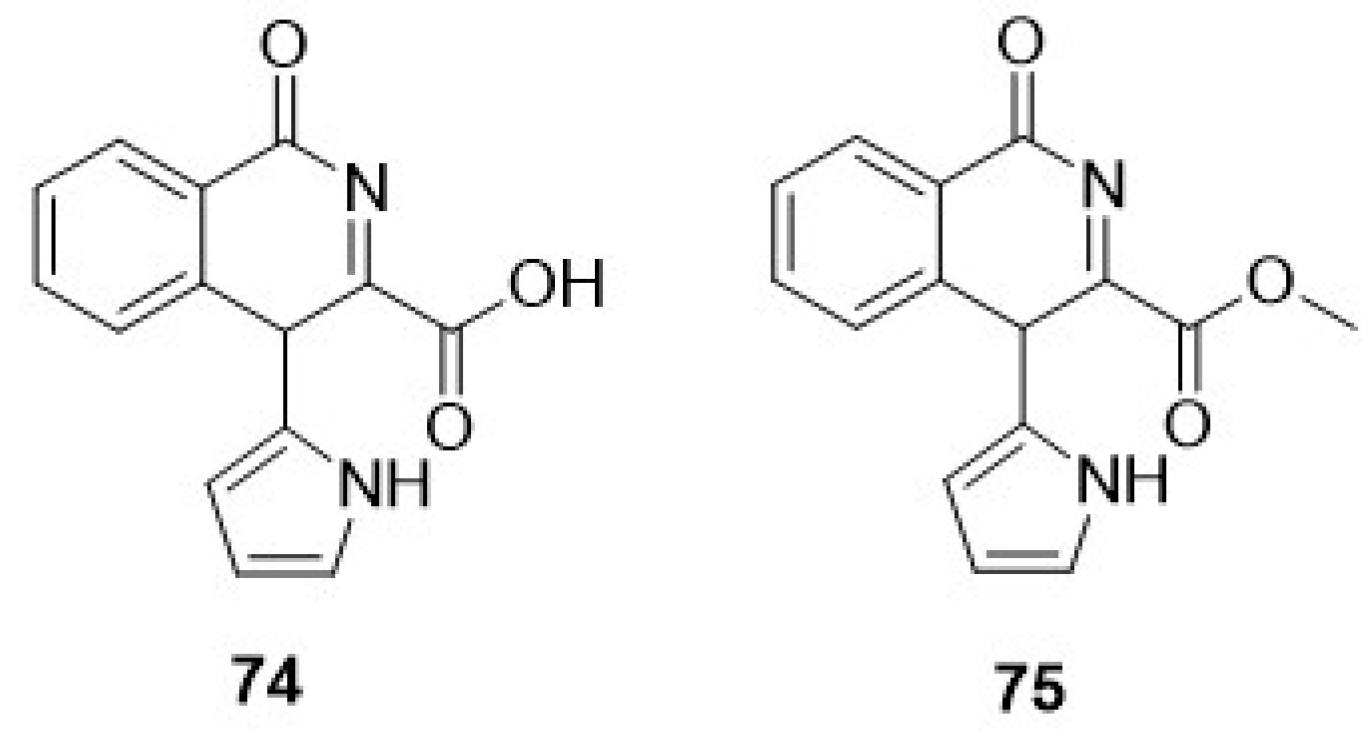
- Chen, B.S.; Zhang, D.; de Souza, F.Z.R.; Liu, L. Recent Advances in the Synthesis of Marine-Derived Alkaloids via Enzymatic Reactions. Mar. Drugs 2022, 20, 368. [Google Scholar] [CrossRef]
- Li, J.; Amatuni, A.; Renata, H. Recent Advances in the Chemoenzymatic Synthesis of Bioactive Natural Products. Curr. Opin. Chem. Biol. 2020, 55, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Edmunds, G.; Gibbons, C.; Zhang, J.; Gadi, M.R.; Zhu, H.; Fang, J.; Liu, X.; Kong, Y.; Wang, P.G. Toward Automated Enzymatic Synthesis of Oligosaccharides. Chem. Rev. 2018, 118, 8151–8187. [Google Scholar] [CrossRef]
- Cho, Y.T.; Adak, A.K.; Su, Y.Y.; Chang, T.W.; Lin, C.C. Chemoenzymatic Total Synthesis of the Neuritogenic Echinoderm Ganglioside LLG-5 and Related Analogues. Adv. Synth. Catal. 2022, 364, 3573–3588. [Google Scholar] [CrossRef]
- Kaneko, M.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Neuritogenic Activity of Gangliosides from Echinoderms and Their Structure-Activity Relationship. Chem. Pharm. Bull. 2007, 55, 462–463. [Google Scholar] [CrossRef]
- Rich, J.R.; Withers, S.G. A Chemoenzymatic Total Synthesis of the Neurogenic Starfish Ganglioside LLG-3 Using an Engineered and Evolved Synthase. Angew. Chem. Int. Ed. 2012, 51, 8640–8643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lian, Z.; Peng, X.; Li, Z.; Zhu, H. Applications of Higenamine in Pharmacology and Medicine. J. Ethnopharmacol. 2017, 196, 242–252. [Google Scholar] [CrossRef]
- Trisrivirat, D.; Sutthaphirom, C.; Pimviriyakul, P.; Chaiyen, P. Dual Activities of Oxidation and Oxidative Decarboxylation by Flavoenzymes. ChemBioChem 2022, 23, 666–679. [Google Scholar] [CrossRef]
- Ibraheem, W.; Wils, Q.; Camiade, E.; Ahmed, E.; Thibonnet, J.; Thiery, E.; Petrignet, J. Synthesis and Antibacterial Activity of Racemic Paecilocin A and Its Derivatives against Methicillin-Sensitive and -Resistant Staphylococcus aureus. Tetrahedron Lett. 2021, 67, 152888–152893. [Google Scholar] [CrossRef]
- Sreelakshmi, C.; Bhaskar Rao, A.; Lakshmi Narasu, M.; Janardhan Reddy, P.; Reddy, B.V.S. Chemoenzymatic Total Synthesis of Paecilocin A and 3-Butyl-7-Hydroxyphthalide. Tetrahedron Lett. 2014, 55, 1303–1305. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Badiei, A.; Ziarani, M.; Reza, A. Chemoenzymatic Enantioselective Formal Synthesis of (−)-Gephyrotoxin-223. Iran. J. Chem. Chem. Eng. 2006, 25, 31–38. [Google Scholar]
- Kanakkanthara, A.; Northcote, P.T.; Miller, J.H. Peloruside A: A Lead Non-Taxoid-Site Microtubule-Stabilizing Agent with Potential Activity against Cancer, Neurodegeneration, and Autoimmune Disease. Nat. Prod. Rep. 2016, 33, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, H.; Mollitor, J.; Schneider, C. A Chemoenzymatic Approach to the Stereocontrolled Synthesis of the C1-C11 Fragment of (+)-Peloruside A. Eur. J. Org. Chem. 2010, 2010, 3908–3918. [Google Scholar] [CrossRef]
- Eggen, M.; Georg, G.I. The Cryptophycins: Their Synthesis and Anticancer Activity. Med. Res. Rev. 2002, 22, 85–101. [Google Scholar] [CrossRef]
- Beck, Z.Q.; Aldrich, C.C.; Magarvey, N.A.; Georg, G.I.; Sherman, D.H. Chemoenzymatic Synthesis of Cryptophycin/Arenastatin Natural Products. Biochemistry 2005, 44, 13457–13466. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Khatri, Y.; Brody, S.I.; Zhu, C.; Pietraszkiewicz, H.; Valeriote, F.A.; Sherman, D.H. A Versatile Chemoenzymatic Synthesis for the Discovery of Potent Cryptophycin Analogs. ACS Chem. Biol. 2020, 15, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Idowu, T.O.; Iwalewa, E.O.; Aderogba, M.A.; Akinpelu, B.A.; Ogundaini, A.O. Anticonceptive, Anti-Inflammatory and Antioxidant Activities of Eleagnine: An Alkaloid Isolated from Chrysophyllum albidum Seed Cotyledons. J. Biol. Sci. 2006, 6, 1029–1034. [Google Scholar] [CrossRef]
- Callaway, J.C. Various Alkaloid Profiles in Decoctions of Banisteriopsis Caapi. J. Psychoact. Drugs 2005, 37, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, D.; Green, A.P.; Pontini, M.; Willies, S.C.; Rowles, I.; Frank, A.; Grogan, G.; Turner, N.J. Engineering an Enantioselective Amine Oxidase for the Synthesis of Pharmaceutical Building Blocks and Alkaloid Natural Products. J. Am. Chem. Soc. 2013, 135, 10863–10869. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Gupta, R. Role of Psilocybin in the Treatment of Depression. Ther. Adv. Psychopharmacol. 2017, 7, 54–56. [Google Scholar] [CrossRef]
- Fricke, J.; Sherwood, A.; Kargbo, R.; Orry, A.; Blei, F.; Naschberger, A.; Rupp, B.; Hoffmeister, D. Enzymatic Route toward 6-Methylated Baeocystin and Psilocybin. ChemBioChem 2019, 20, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Kevvai, K.; Grewal, P.S.; Narcross, L.; Choi, B.; Bourgeois, L.; Dueber, J.E.; Martin, V.J.J. A Yeast Platform for High-Level Synthesis of Tetrahydroisoquinoline Alkaloids. Nat. Commun. 2020, 11, 3337–3346. [Google Scholar] [CrossRef]
- Tanifuji, R.; Oguri, H. A Chemo-Enzymatic Approach for the Rapid Assembly of Tetrahydroisoquinoline Alkaloids and Their Analogs. In Modern Natural Product Synthesis; Springer Nature: Singapore, 2024; pp. 145–161. [Google Scholar]
- Faheem; Karan Kumar, B.; Venkata Gowri Chandra Sekhar, K.; Chander, S.; Kunjiappan, S.; Murugesan, S. 1,2,3,4-Tetrahydroisoquinoline (THIQ) as Privileged Scaffold for Anticancer de Novo Drug Design. Expert Opin. Drug Discov. 2021, 16, 1119–1147. [Google Scholar] [CrossRef]
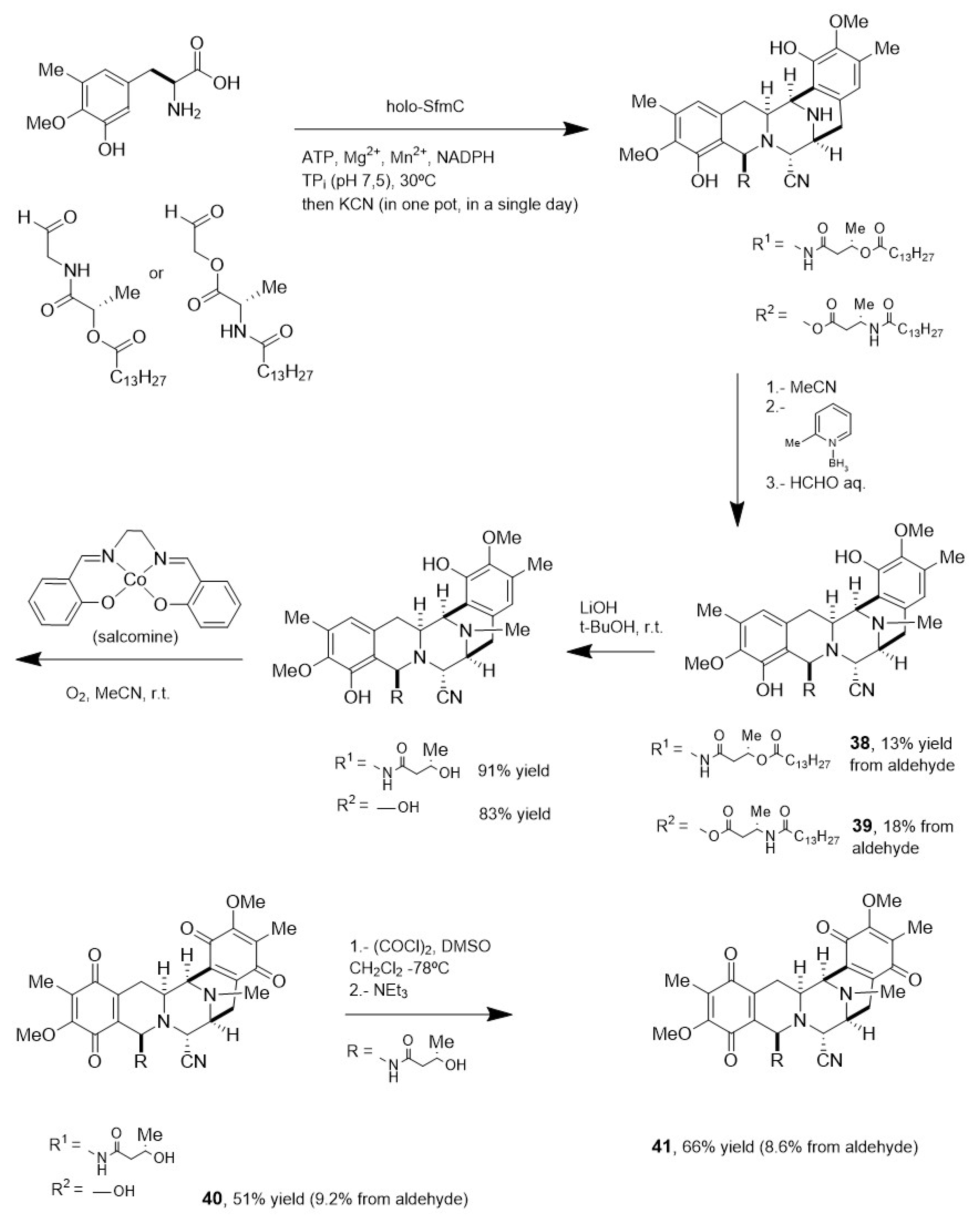
- Tanifuji, R.; Koketsu, K.; Takakura, M.; Asano, R.; Minami, A.; Oikawa, H.; Oguri, H. Chemo-Enzymatic Total Syntheses of Jorunnamycin A, Saframycin A, and N-Fmoc Saframycin Y3. J. Am. Chem. Soc. 2018, 140, 10705–10709. [Google Scholar] [CrossRef] [PubMed]
- Tanifuji, R.; Haraguchi, N.; Oguri, H. Chemo-Enzymatic Total Syntheses of Bis-Tetrahydroisoquinoline Alkaloids and Systematic Exploration of the Substrate Scope of SfmC. Tetrahedron Chem. 2022, 1, 100010–100020. [Google Scholar] [CrossRef]
- Hampson, D.R.; Manalo, J.L. The Activation of Glutamate Receptors by Kainic Acid and Domoic Acid. Nat. Toxins 1998, 6, 153–158. [Google Scholar] [CrossRef]
- Stathakis, C.I.; Yioti, E.G.; Gallos, J.K. Total Syntheses of (–)-α-Kainic Acid. Eur. J. Org. Chem. 2012, 2012, 4661–4673. [Google Scholar] [CrossRef]
- Chekan, J.R.; McKinnie, S.M.K.; Moore, M.L.; Poplawski, S.G.; Michael, T.P.; Moore, B.S. Scalable Biosynthesis of the Seaweed Neurochemical, Kainic Acid. Angew. Chem. Int. Ed. 2019, 58, 8454–8457. [Google Scholar] [CrossRef]
- Newmister, S.A.; Gober, C.M.; Romminger, S.; Yu, F.; Tripathi, A.; Parra, L.L.L.; Williams, R.M.; Berlinck, R.G.S.; Joullié, M.M.; Sherman, D.H. OxaD: A Versatile Indolic Nitrone Synthase from the Marine-Derived Fungus Penicillium oxalicum F30. J. Am. Chem. Soc. 2016, 138, 11176–11184. [Google Scholar] [CrossRef]
- Mikkola, R.; Andersson, M.A.; Hautaniemi, M.; Salkinoja-Salonen, M.S. Toxic Indole Alkaloids Avrainvillamide and Stephacidin B Produced by a Biocide Tolerant Indoor Mold Aspergillus westerdijkiae. Toxicon 2015, 99, 58–67. [Google Scholar] [CrossRef]
- Sharma, G.; Magdoff-Fairchild, B.; Sharma, G.M.; Buyer, J.S.; Pomerantz, M.W.; Hall, L.D.; Sanders, J.K.M.; Bax, A.; Freeman, R.; Morris, G.A.; et al. Manzacidins A-C, Novel Tetrahydropyrimidine Alkaloids from the Okinawan Marine Sponge Hymeniacidon sp. J. Org. Chem. 1991, 56, 4574–4576. [Google Scholar]
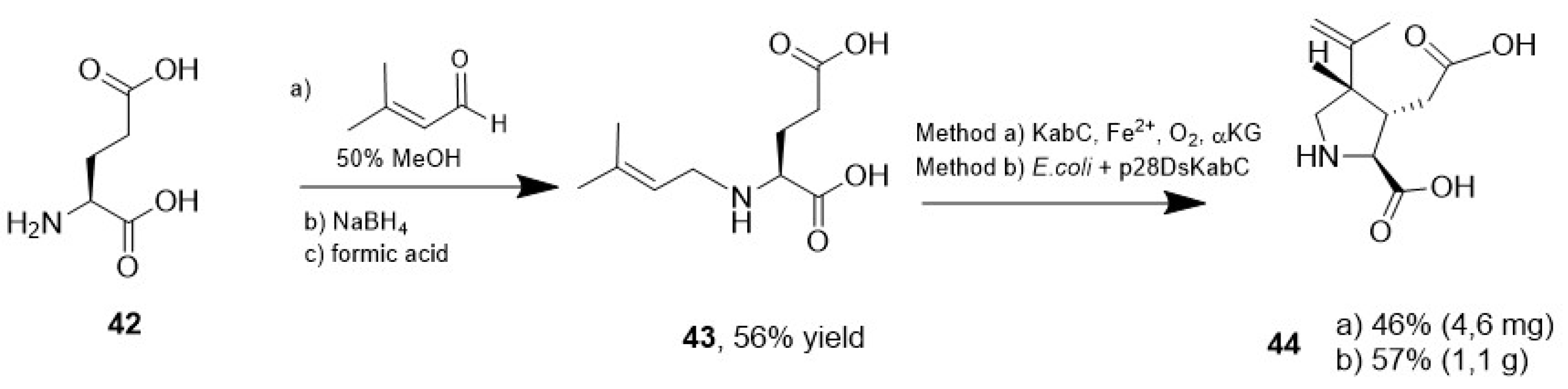
- Zwick, C.R.; Renata, H. Remote C-H Hydroxylation by an α-Ketoglutarate-Dependent Dioxygenase Enables Efficient Chemoenzymatic Synthesis of Manzacidin C and Proline Analogs. J. Am. Chem. Soc. 2018, 140, 1165–1169. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Romero, E.O.; Pyser, J.B.; Yazarians, J.A.; Narayan, A.R.H. Chemoenzymatic Total Synthesis of Natural Products. Acc. Chem. Res. 2021, 54, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
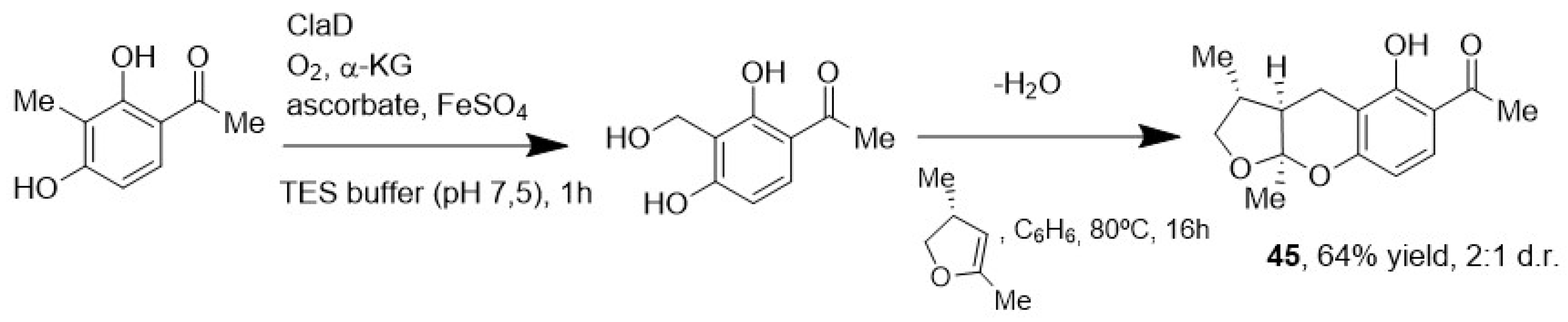
- Baker Dockrey, S.A.; Doyon, T.J.; Perkins, J.C.; Narayan, A.R.H. Whole-cell Biocatalysis Platform for Gram-scale Oxidative Dearomatization of Phenols. Chem. Biol. Drug Des. 2019, 93, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, A.L.; Ellinwood, D.C.; Hinze, M.E.; DeLuca, R.J.; Du Bois, J.; Hall, S.; Narayan, A.R.H. C–H Hydroxylation in Paralytic Shellfish Toxin Biosynthesis. J. Am. Chem. Soc. 2018, 140, 11863–11869. [Google Scholar] [CrossRef]
- Lukowski, A.L.; Denomme, N.; Hinze, M.E.; Hall, S.; Isom, L.L.; Narayan, A.R.H. Biocatalytic Detoxification of Paralytic Shellfish Toxins. ACS Chem. Biol. 2019, 14, 941–948. [Google Scholar] [CrossRef]
- Vila, M.A.; Brovetto, M.; Gamenara, D.; Bracco, P.; Zinola, G.; Seoane, G.; Rodríguez, S.; Carrera, I. Production of Cis-1,2-Dihydrocatechols of High Synthetic Value by Whole-Cell Fermentation Using Escherichia coli JM109 (PDTG601): A Detailed Study. J. Mol. Catal. B Enzym. 2013, 96, 14–20. [Google Scholar] [CrossRef]
- Banwell, M.G.; Loong, D.T.J. A Chemoenzymatic Total Synthesis of the Phytotoxic Undecenolide (-)-Cladospolide A. Org. Biomol. Chem. 2004, 2, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Thale, Z.; Kinder, F.R.; Bair, K.W.; Bontempo, J.; Czuchta, A.M.; Versace, R.W.; Phillips, P.E.; Sanders, M.L.; Wattanasin, S.; Crews, P. Bengamides Revisited: New Structures and Antitumor Studies. J. Org. Chem. 2001, 66, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Banwell, M.G.; McRae, K.J. A Chemoenzymatic Total Synthesis of Ent -Bengamide E. J. Org. Chem. 2001, 66, 6768–6774. [Google Scholar] [CrossRef]
- Baidilov, D.; Rycek, L.; Trant, J.F.; Froese, J.; Murphy, B.; Hudlicky, T. Chemoenzymatic Synthesis of Advanced Intermediates for Formal Total Syntheses of Tetrodotoxin. Angew. Chem. Int. Ed. 2018, 57, 10994–10998. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.; Rycek, L.; Hajicek, J.; Baidilov, D.; Hudlicky, T. Tetrodotoxin: History, Biology, and Synthesis. Angew. Chem. Int. Ed. 2019, 58, 18338–18387. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H. An Open-Label, Multi-Dose Efficacy and Safety Study of Intramuscular Tetrodotoxin in Patients with Severe Cancer-Related Pain. J. Pain Symptom. Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef]
- Zabala, A.O.; Xu, W.; Chooi, Y.H.; Tang, Y. Characterization of a Silent Azaphilone Gene Cluster from Aspergillus Niger ATCC 1015 Reveals a Hydroxylation-Mediated Pyran-Ring Formation. Chem. Biol. 2012, 19, 1049–1059. [Google Scholar] [CrossRef]
- Sato, M.; Winter, J.M.; Kishimoto, S.; Noguchi, H.; Tang, Y.; Watanabe, K. Combinatorial Generation of Chemical Diversity by Redox Enzymes in Chaetoviridin Biosynthesis. Org. Lett. 2016, 18, 1446–1449. [Google Scholar] [CrossRef]
- Kaur, K.; Wu, X.; Fields, J.K.; Johnson, D.K.; Lan, L.; Pratt, M.; Somoza, A.D.; Wang, C.C.C.; Karanicolas, J.; Oakley, B.R.; et al. The Fungal Natural Product Azaphilone-9 Binds to HuR and Inhibits HuR-RNA Interaction in vitro. PLoS ONE 2017, 12, e0175471. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Tahara, H.; Inokoshi, J.; Tanaka, H.; Masuma, R.; Omura, S. New Brominated and Halogen-Less Derivatives and Structure-Activity Relationship of Azaphilones Inhibiting Gp120-CD4 Binding. J. Antibiot. 1998, 51, 1004–1011. [Google Scholar] [CrossRef]
- Tang, J.-L.; Zhou, Z.-Y.; Yang, T.; Yao, C.; Wu, L.-W.; Li, G.-Y. Azaphilone Alkaloids with Anti-Inflammatory Activity from Fungus Penicillium sclerotiorum Cib-411. J. Agric. Food Chem. 2019, 67, 2175–2182. [Google Scholar] [CrossRef]
- Stark, L.M.; Pekari, K.; Sorensen, E.J. A Nucleophile-Catalyzed Cycloisomerization Permits a Concise Synthesis of (+)-Harziphilone. Proc. Natl. Acad. Sci. USA 2004, 101, 12064–12066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Germain, A.R.; Porco, J.A. Synthesis of Azaphilones and Related Molecules by Employing Cycloisomerization of o-Alkynylbenzaldehydes. Angew. Chem. Int. Ed. 2004, 43, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Pyser, J.B.; Baker Dockrey, S.A.; Benítez, A.R.; Joyce, L.A.; Wiscons, R.A.; Smith, J.L.; Narayan, A.R.H. Stereodivergent, Chemoenzymatic Synthesis of Azaphilone Natural Products. J. Am. Chem. Soc. 2019, 141, 18551–18559. [Google Scholar] [CrossRef] [PubMed]
- Baker Dockrey, S.A.; Lukowski, A.L.; Becker, M.R.; Narayan, A.R.H. Biocatalytic Site- and Enantioselective Oxidative Dearomatization of Phenols. Nat. Chem. 2018, 10, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, X.; Xu, D.; Fu, X.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from Fungi and Their Bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- Sib, A.; Gulder, T.A.M. Stereoselective Total Synthesis of Bisorbicillinoid Natural Products by Enzymatic Oxidative Dearomatization/Dimerization. Angew. Chem. 2017, 56, 12888–12891. [Google Scholar] [CrossRef]
- Sib, A.; Gulder, T.A.M. Chemo-enzymatic Total Synthesis of Oxosorbicillinol, Sorrentanone, Rezishanones B and C, Sorbicatechol A, Bisvertinolone, and (+)-Epoxysorbicillinol. Angew. Chem. Int. Ed. 2018, 57, 14650–14653. [Google Scholar] [CrossRef]
- Müller, J.I.; Gulder, T.A.M. Chemoenzymatic Total Synthesis of Sorbicillactone A. Commun. Chem. 2024, 7, 39–44. [Google Scholar] [CrossRef]
- Milzarek, T.M.; Gulder, T.A.M. Chemo-Enzymatic Total Synthesis of the Spirosorbicillinols. Commun. Chem. 2023, 6, 187–191. [Google Scholar] [CrossRef]
- Milzarek, T.M.; Schuler, S.; Matura, A.; Gulder, T.A.M. Evaluation of the Substrate Promiscuity of SorbC for the Chemo-Enzymatic Total Synthesis of Structurally Diverse Sorbicillinoids. ACS Catal. 2022, 12, 1898–1904. [Google Scholar] [CrossRef]
- Sib, A.; Milzarek, T.M.; Herrmann, A.; Oubraham, L.; Müller, J.I.; Pichlmair, A.; Brack-Werner, R.; Gulder, T.A.M. Chemoenzymatic Total Synthesis of Sorbicatechol Structural Analogues and Evaluation of Their Antiviral Potential. ChemBioChem 2020, 21, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, B. Cell Cultures from Marine Invertebrates: Obstacles, New Approaches and Recent Improvements. J. Biotechnol. 1999, 70, 133–153. [Google Scholar] [CrossRef]
- Rinkevich, B. Marine Invertebrate Cell Cultures: New Millennium Trends. Mar. Biotechnol. 2005, 7, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Barnay-Verdier, S.; Dall’Osso, D.; Joli, N.; Olivré, J.; Priouzeau, F.; Zamoum, T.; Merle, P.L.; Furla, P. Establishment of Primary Cell Culture from the Temperate Symbiotic Cnidarian, Anemonia viridis. Cytotechnology 2013, 65, 697–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rinkevich, B. Cell Cultures from Marine Invertebrates: New Insights for Capturing Endless Stemness. Mar. Biotechnol. 2011, 13, 345–354. [Google Scholar] [CrossRef]
- Ventura, P.; Toullec, G.; Fricano, C.; Chapron, L.; Meunier, V.; Röttinger, E.; Furla, P.; Barnay-Verdier, S. Cnidarian Primary Cell Culture as a Tool to Investigate the Effect of Thermal Stress at Cellular Level. Mar. Biotechnol. 2018, 20, 144–154. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the Chemical Diversity Through Microorganisms Co-Culture: Current Status and Outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite Induction via Microorganism Co-Culture: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Panter, F.; Bader, C.D.; Müller, R. Synergizing the Potential of Bacterial Genomics and Metabolomics to Find Novel Antibiotics. Chem. Sci. 2021, 12, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
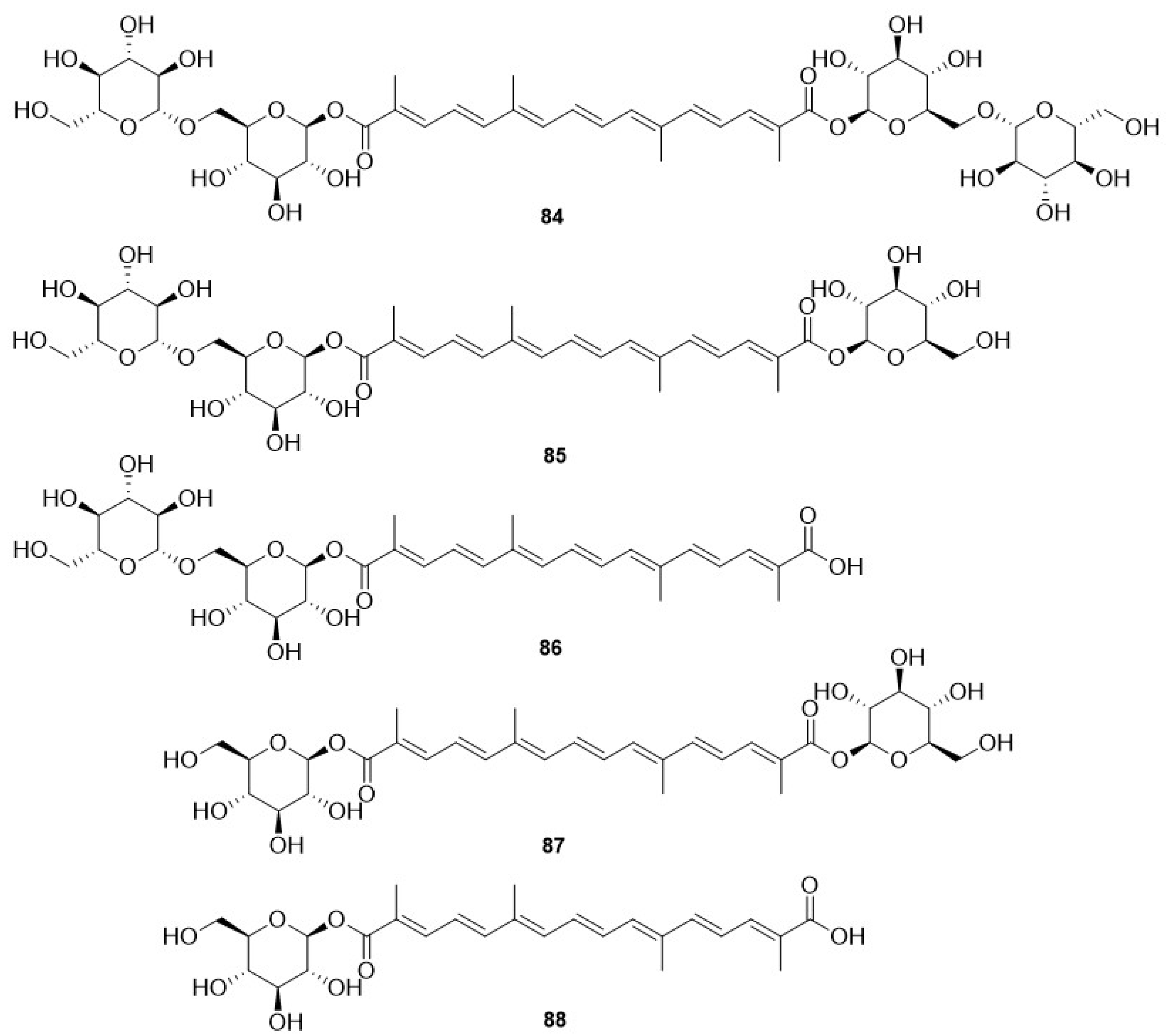
- Zhou, Q.; Hotta, K.; Deng, Y.; Yuan, R.; Quan, S.; Chen, X. Advances in Biosynthesis of Natural Products from Marine Microorganisms. Microorganisms 2021, 9, 2551. [Google Scholar] [CrossRef]
- Zhang, J.; Petersen, S.D.; Radivojevic, T.; Ramirez, A.; Pérez-Manríquez, A.; Abeliuk, E.; Sánchez, B.J.; Costello, Z.; Chen, Y.; Fero, M.J.; et al. Combining Mechanistic and Machine Learning Models for Predictive Engineering and Optimization of Tryptophan Metabolism. Nat. Commun. 2020, 11, 4880–4893. [Google Scholar] [CrossRef]
- Zhang, J.J.; Tang, X.; Moore, B.S. Genetic Platforms for Heterologous Expression of Microbial Natural Products. Nat. Prod. Rep. 2019, 36, 1313–1332. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible Cyanobacterial Genus Arthrospira: Actual State of the Art in Cultivation Methods, Genetics, and Application in Medicine. Front. Microbiol. 2017, 8, 2541. [Google Scholar] [CrossRef] [PubMed]
- Searle, P.A.; Molinski, T.F. Phorboxazoles A and B: Potent Cytostatic Macrolides from Marine Sponge Phorbas species. J. Am. Chem. Soc. 1995, 117, 8126–8131. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Guang, X.Z.; Skepper, C.K.; Molinski, T.F. Phorbasides A-E, Cytotoxic Chlorocyclopropane Macrolide Glycosides from the Marine Sponge Phorbas sp. CD Determination of C-Methyl Sugar Configurations. J. Org. Chem. 2008, 73, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Zheng, Y.Y.; Jia, Y.; Feng, J.; Huang, J.; Huang, J.; Wang, C.Y.; Yao, G. Screening and Genetic Engineering of Marine-Derived Aspergillus terreus for High-Efficient Production of Lovastatin. Microb. Cell Fact. 2024, 23, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.S.; de Carvalho, C.C.C.R. Mimicking Marine Conditions to Improve Prodigiosin Yields in Bioreactor. Processes 2024, 12, 1794. [Google Scholar] [CrossRef]
- Islan, G.A.; Rodenak-Kladniew, B.; Noacco, N.; Duran, N.; Castro, G.R. Prodigiosin: A Promising Biomolecule with Many Potential Biomedical Applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef] [PubMed]
- Srilekha, V.; Krishna, G.; Sreelatha, B.; Jagadeesh Kumar, E.; Rajeshwari, K.V.N. Prodigiosin: A Fascinating and the Most Versatile Bioactive Pigment with Diverse Applications. Syst. Microbiol. Biomanufacturing 2024, 4, 66–76. [Google Scholar] [CrossRef]
- Anwar, M.M.; Albanese, C.; Hamdy, N.M.; Sultan, A.S. Rise of the Natural Red Pigment ‘Prodigiosin’ as an Immunomodulator in Cancer. Cancer Cell Int. 2022, 22, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Goodbey, W.T. Biotechnology and Its Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 118–149. ISBN 9780128177266. [Google Scholar]
- Farag, M.A.; Deavours, B.E.; de Fáltima, Â.; Naoumkina, M.; Dixon, R.A.; Sumner, L.W. Integrated Metabolite and Transcript Profiling Identify a Biosynthetic Mechanism for Hispidol in Medicago truncatula Cell Cultures. Plant Physiol. 2009, 151, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, L. Elicitor-like Effects of Low-Energy Ultrasound on Plant (Panax ginseng) Cells: Induction of Plant Defense Responses and Secondary Metabolite Production. Appl. Microbiol. Biotechnol. 2002, 59, 51–57. [Google Scholar] [CrossRef]
- Farag, M.A.; Al-Mahdy, D.A.; Meyer, A.; Westphal, H.; Wessjohann, L.A. Metabolomics Reveals Biotic and Abiotic Elicitor Effects on the Soft Coral Sarcophyton ehrenbergi Terpenoid Content. Sci. Rep. 2017, 7, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Maamoun, A.A.; Meyer, A.; Wessjohann, L.A. Salicylic Acid and Its Derivatives Elicit the Production of Diterpenes and Sterols in Corals and Their Algal Symbionts: A Metabolomics Approach to Elicitor SAR. Metabolomics 2018, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nishi, A. Effect of Elicitors on the Production of Secondary Metabolites; Elsevier Science: New York, NY, USA, 1994; Volume 14, pp. 135–151. [Google Scholar]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tansel Tanrıkul, T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of New Metabolites from Sponge-Associated Fungus Aspergillus carneus by OSMAC Approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Strongman, D.B.; Miller, J.D.; Calhoun, L.; Findlay, J.A.; Whitney, N.J. The Biochemical Basis for Interference Competition among Some Lignicolous Marine Fungi. Bot. Mar. 1987, 30, 21–26. [Google Scholar] [CrossRef]
- Grant Burgess, J.; Jordan, E.M.; Bregu, M.; Mearns-Spragg, A.; Boyd, K.G. Microbial Antagonism: A Neglected Avenue of Natural Products Research. In Progress in Industrial Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 70, pp. 27–32. [Google Scholar]
- Sonnenbichler, J.; Dietrich, J.; Peipp, H. Investigations Concerning the Induction of the Biosynthesis of Toxic Secondary Metabolites in Basidiomycetes. Biol. Chem. Hoppe-Seyler 1994, 375, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Correa, M.; Tengerdy, R.P. Xylanase Production by Fungal Mixed Culture Solid Substrate Fermentation on Sugar Cane Bagasse. Biotechnol. Lett. 1998, 20, 45–47. [Google Scholar] [CrossRef]
- Wiesel, I.; Rehm, H.J.; Bisping, B. Improvement of Tempe Fermentations by Application of Mixed Cultures Consisting of Rhizopus sp. and Bacterial Strains. Appl. Microbiol. Biotechnol. 1997, 47, 218–225. [Google Scholar] [CrossRef]
- Selegato, D.M.; Castro-Gamboa, I. Enhancing Chemical and Biological Diversity by Co-Cultivation. Front. Microbiol. 2023, 14, 1117559–1117583. [Google Scholar] [CrossRef] [PubMed]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of Co-Culture on the Metabolism of Marine Microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, W.; Ma, Z. Induced Production of Cytochalasans in Co-Culture of Marine Fungus Aspergillus flavipes and Actinomycete streptomyces sp. Nat. Prod. Res. 2016, 30, 1718–1723. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual Induction of New Microbial Secondary Metabolites by Fungal Bacterial Co-Cultivation. Front. Microbiol. 2017, 8, 1284–1294. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A-D: Induction of Cytotoxic Diterpenoid Biosynthesis by Marine Microbial Competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, 17–30. [Google Scholar] [CrossRef]
- Shin, D.; Byun, W.S.; Moon, K.; Kwon, Y.; Bae, M.; Um, S.; Lee, S.K.; Oh, D.C. Coculture of Marine Streptomyces sp. with Bacillus sp. Produces a New Piperazic Acid-Bearing Cyclic Peptide. Front. Chem. 2018, 6, 498–510. [Google Scholar] [CrossRef]
- Mandelare, P.E.; Adpressa, D.A.; Kaweesa, E.N.; Zakharov, L.N.; Loesgen, S. Coculture of Two Developmental Stages of a Marine-Derived Aspergillus alliaceus Results in the Production of the Cytotoxic Bianthrone Allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, Y. Marinamide, a Novel Alkaloid and Its Methyl Ester Produced by the Application of Mixed Fermentation Technique to Two Mangrove Endophytic Fungi from the South China Sea. Chin. Sci. Bull. 2006, 51, 1426–1430. [Google Scholar] [CrossRef]
- Lodhi, A.F.; Zhang, Y.; Adil, M.; Deng, Y. Antibiotic Discovery: Combining Isolation Chip (IChip) Technology and Co-Culture Technique. Appl. Microbiol. Biotechnol. 2018, 102, 7333–7341. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, L.W.; Charles, M.J.; Haltli, B.A.; Marchbank, D.H.; Kerr, R.G. An Ichip-Domesticated Sponge Bacterium Produces an N-Acyltyrosine Bearing an α-Methyl Substituent. Org. Lett. 2019, 21, 7768–7771. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Mateos, A.I.; Roura Padrosa, D.; Paradisi, F. Multistep Enzyme Cascades as a Route towards Green and Sustainable Pharmaceutical Syntheses. Nat. Chem. 2022, 14, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ardao, I.; Hwang, E.T.; Zeng, A.-P. In vitro Multienzymatic Reaction Systems for Biosynthesis. In Fundamentals and Application of New Bioproduction Systems. Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 137, pp. 153–184. [Google Scholar]
- Roessner, C.A.; Scott, I. Achieving Natural Product Synthesis and Diversity via Catalytic Networking Ex vivo. Chem. Biol. 1996, 3, 325–330. [Google Scholar] [CrossRef]
- Fecik, R.A. Natural Product Biosynthesis Moves in vitro. Nat. Chem. Biol. 2007, 3, 531–532. [Google Scholar] [CrossRef]
- Sattely, E.S.; Fischbach, M.A.; Walsh, C.T. Total Biosynthesis: In vitro Reconstitution of Polyketide and Nonribosomal Peptide Pathways. Nat. Prod. Rep. 2008, 25, 757–793. [Google Scholar] [CrossRef]
- Pfleger, B.F.; Prather, K.L.J. Biological Synthesis Unbounded? Nat. Biotechnol. 2015, 33, 1148–1149. [Google Scholar] [CrossRef] [PubMed]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Betancor, L.; Luckarift, H.R. Co-Immobilized Coupled Enzyme Systems in Biotechnology. Biotechnol. Genet. Eng. Rev. 2010, 27, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, M.; Wibowo, M.; Williams, S.E.; Gotfredsen, C.H.; Sigrist, R.; Souza, L.D.O.; Cowled, M.S.; Charusanti, P.; Gren, T.; Saha, S.; et al. Maramycin, a Cytotoxic Isoquinolinequinone Terpenoid Produced through Heterologous Expression of a Bifunctional Indole Prenyltransferase/Tryptophan Indole-Lyase in S. Albidoflavus. ACS Chem. Biol. 2024, 19, 1303–1310. [Google Scholar] [CrossRef]
- Kopp, F.; Marahiel, M.A. Where Chemistry Meets Biology: The Chemoenzymatic Synthesis of Nonribosomal Peptides and Polyketides. Curr. Opin. Biotechnol. 2007, 18, 513–520. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Chen, X.; Sun, X.; Yan, Y.; Shen, X.; Yuan, Q. Biosynthesis of Aromatic Polyketides in Microorganisms Using Type II Polyketide Synthases. Microb. Cell Fact. 2020, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. Biosynthesis of Aromatic Polyketides. In Biosynthesis. Topics in Current Chemistry; Leeper, F.J., Vederas, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 209, pp. 1–51. [Google Scholar]
- McDaniel, R.; Ebert-Khosla, S.; Hopwood, D.A.; Khosla, C. Rational Design of Aromatic Polyketide Natural Products by Recombinant Assembly of Enzymatic Subunits. Nature 1995, 375, 549–554. [Google Scholar] [CrossRef]
- Cheng, Q.; Xiang, L.; Izumikawa, M.; Meluzzi, D.; Moore, B.S. Enzymatic Total Synthesis of Enterocin Polyketides. Nat. Chem. Biol. 2007, 3, 557–558. [Google Scholar] [CrossRef]
- Kalaitzis, J.A.; Cheng, Q.; Thomas, P.M.; Kelleher, N.L.; Moore, B.S. In vitro Biosynthesis of Unnatural Enterocin and Wailupemycin Polyketides. J. Nat. Prod. 2009, 72, 469–472. [Google Scholar] [CrossRef]
- Kharel, M.K.; Pahari, P.; Lian, H.; Rohr, J. Enzymatic Total Synthesis of Rabelomycin, an Angucycline Group Antibiotic. Org. Lett. 2010, 12, 2814–2817. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, Mode-of-Action, New Natural Products, and Synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef]
- Pahari, P.; Kharel, M.K.; Shepherd, M.D.; Van Lanen, S.G.; Rohr, J. Enzymatic Total Synthesis of Defucogilvocarcin M and Its Implications for Gilvocarcin Biosynthesis. Angew. Chem. Int. Ed. 2012, 51, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Kaysser, L.; Bernhardt, P.; Nam, S.-J.; Loesgen, S.; Ruby, J.G.; Skewes-Cox, P.; Jensen, P.R.; Fenical, W.; Moore, B.S. Merochlorins A–D, Cyclic Meroterpenoid Antibiotics Biosynthesized in Divergent Pathways with Vanadium-Dependent Chloroperoxidases. J. Am. Chem. Soc. 2012, 134, 11988–11991. [Google Scholar] [CrossRef]
- Popescu, R.; Heiss, E.H.; Ferk, F.; Peschel, A.; Knasmueller, S.; Dirsch, V.M.; Krupitza, G.; Kopp, B. Ikarugamycin Induces DNA Damage, Intracellular Calcium Increase, P38 MAP Kinase Activation and Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 709–710, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dong, F.; Da, L.; Yang, X.; Wang, X.; Weng, J.; Feng, L.; Zhu, L.; Zhang, Y.; Zhang, Z.; et al. Ikarugamycin Inhibits Pancreatic Cancer Cell Glycolysis by Targeting Hexokinase 2. FASEB J. 2020, 34, 3943–3955. [Google Scholar] [CrossRef]
- Greunke, C.; Glöckle, A.; Antosch, J.; Gulder, T.A.M. Biocatalytic Total Synthesis of Ikarugamycin. Angew. Chem. Int. Ed. 2017, 56, 4351–4355. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Weidner, C.H.; Perni, R.B.; Napier, J.J. An Enantioselective and Highly Convergent Synthesis of (+)-Ikarugamycin. J. Am. Chem. Soc. 1989, 111, 8036–8037. [Google Scholar] [CrossRef]
- Paquette, L.A.; Macdonald, D.; Anderson, L.G. Total Synthesis of (+)-Ikarugamycin. 2. Elaboration of the Macrocyclic Lactam and Tetramic Acid Substructures and Complete Assembly of the Antibiotic. J. Am. Chem. Soc. 1990, 112, 9292–9299. [Google Scholar] [CrossRef]
- Roush, W.R.; Wada, C.K. Application of.Eta.4-Diene Iron Tricarbonyl Complexes in Acyclic Stereocontrol: Asymmetric Synthesis of the as-Indacene Unit of Ikarugamycin (A Formal Total Synthesis). J. Am. Chem. Soc. 1994, 116, 2151–2152. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive Prediction of Secondary Metabolite Structure and Biological Activity from Microbial Genome Sequences. Nat. Commun. 2020, 11, 6058–6067. [Google Scholar] [CrossRef]
- Tietz, J.I.; Schwalen, C.J.; Patel, P.S.; Maxson, T.; Blair, P.M.; Tai, H.C.; Zakai, U.I.; Mitchell, D.A. A New Genome-Mining Tool Redefines the Lasso Peptide Biosynthetic Landscape. Nat. Chem. Biol. 2017, 13, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kouprina, N.; Larionov, V. Selective Isolation of Genomic Loci from Complex Genomes by Transformation-Associated Recombination Cloning in the Yeast Saccharomyces cerevisiae. Nat. Protoc. 2008, 3, 371–377. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, X.; Gabrieli, T.; Lou, C.; Ebenstein, Y.; Zhu, T.F. Cas9-Assisted Targeting of CHromosome Segments CATCH Enables One-Step Targeted Cloning of Large Gene Clusters. Nat. Commun. 2015, 6, 8101. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic Assembly of DNA Molecules up to Several Hundred Kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Baral, B.; Akhgari, A.; Metsä-Ketelä, M. Activation of Microbial Secondary Metabolic Pathways: Avenues and Challenges. Synth. Syst. Biotechnol. 2018, 3, 163–178. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.; Yu, X.; Jiang, Q.; Zheng, K.; Xu, J.; Wang, P. Recent Advances in the Heterologous Expression of Biosynthetic Gene Clusters for Marine Natural Products. Mar. Drugs 2022, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, J.H.; Choi, H.; Pereira, A.R.; Ban, Y.H.; Yoo, Y.J.; Kim, E.; Park, J.W.; Sherman, D.H.; Gerwick, W.H.; et al. Heterologous Production of 4- O -Demethylbarbamide, a Marine Cyanobacterial Natural Product. Org. Lett. 2012, 14, 5824–5827. [Google Scholar] [CrossRef] [PubMed]
- Sun, A. Heterologous Expression of Beauvericin in Aspergillus Nidulans; Anhui University: Hefei, China, 2016. [Google Scholar]
- Jin, S.; Chen, H.; Zhang, J.; Lin, Z.; Qu, X.; Jia, X.; Lei, C. Analyzing and Engineering of the Biosynthetic Pathway of Mollemycin A for Enhancing Its Production. Synth. Syst. Biotechnol. 2024, 9, 445–452. [Google Scholar] [CrossRef]
- Burkhardt, I.; de Rond, T.; Chen, P.Y.T.; Moore, B.S. Ancient Plant-like Terpene Biosynthesis in Corals. Nat. Chem. Biol. 2022, 18, 664–669. [Google Scholar] [CrossRef]
- Fox Ramos, A.E.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural Products Targeting Strategies Involving Molecular Networking: Different Manners, One Goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef]
- Malek Zerikly, G.L.C. Strategies for the Discovery of New Natural Products by Genome Mining. ChemBioChem 2009, 10, 625–633. [Google Scholar] [CrossRef]
- Chandra Mohana, N.; Yashavantha Rao, H.C.; Rakshith, D.; Mithun, P.R.; Nuthan, B.R.; Satish, S. Omics Based Approach for Biodiscovery of Microbial Natural Products in Antibiotic Resistance Era. J. Genet. Eng. Biotechnol. 2018, 16, 1–8. [Google Scholar] [CrossRef]
- Milanowski, D.J.; Gustafson, K.R.; Kelley, J.A.; McMahon, J.B. Caulibugulones A−F, Novel Cytotoxic Isoquinoline Quinones and Iminoquinones from the Marine Bryozoan Caulibugula Intermis. J. Nat. Prod. 2004, 67, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Park, A.; Schmitz, F.J.; van Altena, I. Perfragilins A and B, Cytotoxic Isoquinolinequinones from the Bryozoan Membranipora Perfragilis. J. Nat. Prod. 1993, 56, 1431–1433. [Google Scholar] [CrossRef]
- Pettit, G.R.; Knight, J.C.; Collins, J.C.; Herald, D.L.; Pettit, R.K.; Boyd, M.R.; Young, V.G. Antineoplastic Agents 430. Isolation and Structure of Cribrostatins 3, 4, and 5 from the Republic of Maldives Cribrochalina Species. J. Nat. Prod. 2000, 63, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Frincke, J.M.; Faulkner, D.J. Antimicrobial Metabolites of the Sponge Reniera sp. J. Am. Chem. Soc. 1982, 104, 265–269. [Google Scholar] [CrossRef]
- Kim, Y.; Ji, Y.; Kim, N.-H.; Van Tu, N.; Rho, J.-R.; Jeong, E. Isoquinolinequinone Derivatives from a Marine Sponge (Haliclona sp.) Regulate Inflammation in In vitro System of Intestine. Mar. Drugs 2021, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Inturrisi, C.E. Clinical Pharmacology of Opioids for Pain. Clin. J. Pain 2002, 18, 53–513. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Stueckle, T.A.; Tse, W.; Rojanasakul, Y.; Dinu, C.Z. Digitoxin and Its Analogs as Novel Cancer Therapeutics. Exp. Hematol. Oncol. 2012, 1, 4. [Google Scholar] [CrossRef]
- Altamirano, J.; Li, Y.; Desantiago, J.; Piacentino, V.; Houser, S.R.; Bers, D.M. The Inotropic Effect of Cardioactive Glycosides in Ventricular Myocytes Requires Na+-Ca2+ Exchanger Function. J. Physiol. 2006, 575, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.R.; Shaw, W.M.; Ellis, T. Biosynthesis of Therapeutic Natural Products Using Synthetic Biology. Adv. Drug Deliv. Rev. 2016, 105, 96–106. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-Lactam Antibiotics: An Overview from a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 208, 112829–112854. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, M.; Wibowo, M.; Gren, T.; Jarmusch, S.A.; Sterndorff, E.B.; Booth, T.; Henriksen, N.N.S.E.; Whitford, C.M.; Jiang, X.; Jørgensen, T.S.; et al. Biosynthesis of the Azoxy Compound Azodyrecin from Streptomyces mirabilis P8-A2. ACS Chem. Biol. 2024, 19, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, Z.; Zhao, H.; Ang, E.L. Recent Advances in Combinatorial Biosynthesis for Drug Discovery. Drug Des. Devel. Ther. 2015, 9, 823–833. [Google Scholar]
- Khosla, C.; Keasling, J.D. Keasling Metabolic Engineering for Drug and Development. Nat. Rev. Drug Discov. 2003, 2, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Yim, G.; Waglechner, N.; Wang, W.; Pawlowski, A.C.; Wright, G.D. Hidden Antibiotics in Actinomycetes Can Be Identified by Inactivation of Gene Clusters for Common Antibiotics. Nat. Biotechnol. 2019, 37, 1149–1154. [Google Scholar] [CrossRef]
- Moraga, A.R.; Nohales, P.F.; Pérez, J.A.F.; Gómez-Gómez, L. Glucosylation of the Saffron Apocarotenoid Crocetin by a Glucosyltransferase Isolated from Crocus sativus Stigmas. Planta 2004, 219, 955–966. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, D.; Liu, C.; Hu, Z.; Li, H.; Lou, S. Research Progress in Heterologous Crocin Production. Mar. Drugs 2024, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Wang, Y.; Mei, X.; Yao, M.; Chen, Y.; Liu, H.; Xiao, W.; Yuan, Y. Heterologous Biosynthesis and Manipulation of Crocetin in Saccharomyces cerevisiae. Microb. Cell Fact. 2017, 16, 54–70. [Google Scholar] [CrossRef]
- Wang, W.; He, P.; Zhao, D.; Ye, L.; Dai, L.; Zhang, X.; Sun, Y.; Zheng, J.; Bi, C. Construction of Escherichia coli Cell Factories for Crocin Biosynthesis. Microb. Cell Fact. 2019, 18, 120–131. [Google Scholar] [CrossRef]
- Xie, L.; Luo, Z.; Jia, X.; Mo, C.; Huang, X.; Suo, Y.; Cui, S.; Zang, Y.; Liao, J.; Ma, X. Synthesis of Crocin I and Crocin II by Multigene Stacking in Nicotiana benthamiana. Int. J. Mol. Sci. 2023, 24, 14139. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pourkarimi, S.; Hallajisani, A.; Nouralishahi, A.; Alizadehdakhel, A.; Golzary, A. Factors Affecting Production of Beta-Carotene from Dunaliella salina Microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771–101785. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative Assessment on the Extraction of Carotenoids from Microalgal Sources: Astaxanthin from H. pluvialis and β-Carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Murphy, C.D.; Clark, B.R.; Amadio, J. Metabolism of Fluoroorganic Compounds in Microorganisms: Impacts for the Environment and the Production of Fine Chemicals. Appl. Microbiol. Biotechnol. 2009, 84, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; El Omari, M.; König, G.M. Biohalogenation: Nature’s Way to Synthesize Halogenated Metabolites. J. Nat. Prod. 2009, 72, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Huang, F.; Deng, H.; Schaffrath, C.; Spencer, J.B.; O’Hagan, D.; Naismith, J.H. Crystal Structure and Mechanism of a Bacterial Fluorinating Enzyme. Nature 2004, 427, 561–565. [Google Scholar] [CrossRef]
- Eustáquio, A.S.; Pojer, F.; Noel, J.P.; Moore, B.S. Discovery and Characterization of a Marine Bacterial SAM-Dependent Chlorinase. Nat. Chem. Biol. 2007, 4, 69–74. [Google Scholar] [CrossRef]
- Eustáquio, A.S.; Mcglinchey, R.P.; Liu, Y.; Hazzard, C.; Beer, L.L.; Florova, G.; Alhamadsheh, M.M.; Lechner, A.; Kale, A.J.; Kobayashi, Y.; et al. Biosynthesis of the Salinosporamide A Polyketide Synthase Substrate Chloroethylmalonyl-Coenzyme A from S-Adenosyl-L-Methionine. Proc. Natl. Acad. Sci. USA 2009, 106, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Eustáquio, A.S.; O’Hagan, D.; Moore, B.S. Engineering Fluorometabolite Production: Fluorinase Expression in Salinispora tropica Yields Fluorosalinosporamide. J. Nat. Prod. 2010, 73, 378–382. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Pereira, F. Marine Drug Discovery through Computer-Aided Approaches. Mar. Drugs 2023, 21, 452. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Aires-de-Sousa, J. Computational Methodologies in the Exploration of Marine Natural Product Leads. Mar. Drugs 2018, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Understanding the Chemical Reactivity and Biological Properties of Patellamides Using Theoretical and Computational Methods. Comput. Theor. Chem. 2023, 1229, 114329–114339. [Google Scholar] [CrossRef]
- Baur, P.; Kühl, M.; Comba, P.; Behrendt, L. Possible Functional Roles of Patellamides in the Ascidian-Prochloron Symbiosis. Mar. Drugs 2022, 20, 272. [Google Scholar] [CrossRef]
- Parr, W.Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989; ISBN 0-19-504279-4. [Google Scholar]
- Geerlings, P.; Chamorro, E.; Chattaraj, P.K.; De Proft, F.; Gázquez, J.L.; Liu, S.; Morell, C.; Toro-Labbé, A.; Vela, A.; Ayers, P. Conceptual Density Functional Theory: Status, Prospects, Issues. Theor. Chem. Acc. 2020, 139, 36–54. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual Density Functional Theory Based Electronic Structure Principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.; Nurkolis, F.; Park, M.N.; Heriyanto, D.S.; Taslim, N.A.; Tallei, T.E.; Permatasari, H.K.; Tjandrawinata, R.R.; Moon, S.; Kim, B. Green Seaweed Caulerpa racemosa as a Novel Non-Small Cell Lung Cancer Inhibitor in Overcoming Tyrosine Kinase Inhibitor Resistance: An Analysis Employing Network Pharmacology, Molecular Docking, and In vitro Research. Mar. Drugs 2024, 22, 272. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial Intelligence to Deep Learning: Machine Intelligence Approach for Drug Discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Farghali, H.; Canová, N.K.; Arora, M. The Potential Applications of Artificial Intelligence in Drug Discovery and Development. Physiol. Res. 2021, 70, 715–722. [Google Scholar] [CrossRef]
- De Farias Silva, C.E.; Costa, G.Y.S.C.M.; Ferro, J.V.; de Oliveira Carvalho, F.; da Gama, B.M.V.; Meili, L.; dos Santos Silva, M.C.; Almeida, R.M.R.G.; Tonholo, J. Application of Machine Learning to Predict the Yield of Alginate Lyase Solid-State Fermentation by Cunninghamella echinulata: Artificial Neural Networks and Support Vector Machine. React. Kinet. Mech. Catal. 2022, 135, 3155–3171. [Google Scholar] [CrossRef]
- Barchi, A.C.; Ito, S.; Escaramboni, B.; Neto, P.d.O.; Herculano, R.D.; Miranda, M.C.R.; Passalia, F.J.; Rocha, J.C.; Núñez, E.G.F. Artificial Intelligence Approach Based on Near-Infrared Spectral Data for Monitoring of Solid-State Fermentation. Process Biochem. 2016, 51, 1338–1347. [Google Scholar] [CrossRef]
- Larrañaga, P.; Calvo, B.; Santana, R.; Bielza, C.; Galdiano, J.; Inza, I.; Lozano, J.A.; Armañanzas, R.; Santafé, G.; Pérez, A.; et al. Machine Learning in Bioinformatics. Brief Bioinform. 2006, 7, 86–112. [Google Scholar] [CrossRef]
- Azuaje, F. Computational Models for Predicting Drug Responses in Cancer Research. Brief Bioinform. 2017, 18, 820–829. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Guo, X.N.; Zhu, K.X.; Peng, W.; Zhou, H.M. Artificial Neural Network—Genetic Algorithm to Optimize Wheat Germ Fermentation Condition: Application to the Production of Two Anti-Tumor Benzoquinones. Food Chem. 2017, 227, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, X.; Zheng, J.H.; Ren, D.F.; Lu, J. Mixed Fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by Random-Centroid Optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, H.; Santos, M.; Pedraza, L.; Testera, A.M. Advanced Technologies for Large Scale Supply of Marine Drugs. Mar. Drugs 2025, 23, 69. https://doi.org/10.3390/md23020069
Martínez H, Santos M, Pedraza L, Testera AM. Advanced Technologies for Large Scale Supply of Marine Drugs. Marine Drugs. 2025; 23(2):69. https://doi.org/10.3390/md23020069
Chicago/Turabian StyleMartínez, Henar, Mercedes Santos, Lucía Pedraza, and Ana M. Testera. 2025. "Advanced Technologies for Large Scale Supply of Marine Drugs" Marine Drugs 23, no. 2: 69. https://doi.org/10.3390/md23020069
APA StyleMartínez, H., Santos, M., Pedraza, L., & Testera, A. M. (2025). Advanced Technologies for Large Scale Supply of Marine Drugs. Marine Drugs, 23(2), 69. https://doi.org/10.3390/md23020069






