Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review
Abstract
:1. Introduction
2. Methodology
3. Chemical Bioactive Compounds from Macroalgae
3.1. Biodiversity of Algae
3.2. Seaweed Cultivation and Harvesting
3.3. Biochemical Composition of Algae
3.3.1. The Extraction Methods of Biocompounds from Seaweeds
3.3.2. Proximate Nutritional Composition
3.4. Active Metabolites from Seaweeds
3.4.1. Polysaccharides (MAPs)
3.4.2. Terpenoids Content
3.4.3. Seaweeds Lipids, Fatty Acids (AFs), and Sterols
| Green Algae | Red Algae | Brown Algae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acids | Cladophora albida | Ulva intestinalis | Caulerpa lentillifera | Ulva lactuca | Ceramium deslongchampsii (as Ceramium strictum) | Gracilaria gracilaris | Porphyra dioica | Palmaria palmata | Stephanocystis hakodatensis (as Cystoseira hakodatensis) | Dictyota dichotoma | Undaria pinnatifida | Sargassum horneri | Padina boergesenii |
| C16:0 | 33.04 ± 0.52 | 31.05 ± 11.10 | 33.69 ± 0.64 | 33.39 ± 12.87 | 24.00 ± 0.60 | 27.1 ± 1.2 | 25.71 ± 3.40 | 24.32 ± 1.11 | 18.49 ± 0.30 | 24.75 ± 0.32 | 11.51 ± 0.01 | 25.24 ± 1.91 | 49.20 ± 0.30 |
| C18:0 | 1.28 ± 0.19 | n.d.a | 13.57 ± 0.91 | 2.21 ± 1.32 | 3.30 ± 0.20 | 4.6 ± 0.8 | 3.53 ± 1.93 | 12.45 ± 6.74 | n.d.a | 2.85 ± 0.08 | 0.64 ± 0.02 | 28.90 ± 2.33 | 2.30 ± 0.10 |
| ∑SFA | 50.03 ± 0.56 | 42.80 ± 25.17 | 29.80 ± 1.65 | 54.95 ± 26.77 | 34.80 | 34.9 ± 0.9 | 34.32 ± 4.49 | 36.77 ± 6.95 | n.d.a | 35.98 ± 0.47 | 12.15 | 26.98 ± 0.00 | 58.00 ± 0.40 |
| C16:1 | 13.90 ± 0.09 | 4.85 ± 4.31 | n.d.a | 3.31 ± 3.64 | 7.30 ± 0.30 | 2.8 ± 0.8 | 10.78 ± 11.90 | 2.03 ± 0.43 | 0.63 ± 0.08 | 15.49 ± 0.09 | 1.66 ± 0.06 | n.d.a | 3.20 ± 0.30 |
| C18:1 | 12.51 ± 0.02 | 12.35 ± 4.03 | n.d.a | 8.88 ± 4.49 | 18.30 ± 0.20 | 9.7 ± 0.4 | 2.85 ± 0.63 | 2.82 ± 0.54 | 11.08 ± 0.24 | 8.49 ± 0.13 | 6.21 ± 0.11 | n.d.a | 16.80 ± 0.80 |
| ∑MUFA | 27.73 ± 0.11 | 23.60 ± 1.69 | 9.08 ± 2.75 | 15.45 ± 7.71 | 30.30 | 12.5 ± 0.7 | 13.63 ± 9.94 | 4.85 ± 0.56 | n.d.a | 24.28 ± 0.13 | 10.35 | 14.24 ± 0.00 | 20.50 ± 0.80 |
| C18:2(LA) | 15.54 ± 0.22 | 7.10 ± 1.83 | n.d.a | 5.54 ± 3.57 | 2.00 ± 0.10 | 2 ± 0.4 | 1.67 ± 0.04 | 0.45 ± 0.19 | 6.95 ± 0.15 | 5.55 ± 0.02 | 3.87 ± 0.08 | n.d.a | 3.30 ± 0.10 |
| C18:3 (ALA) | n.d.a | 7.85 ± 9.26 | n.d.a | 5.64 ± 6.03 | 15.10 ± 0.30 | 2.7 ± 0.2 | 1.95 ± 0.07 | n.d.a | 6.87 ± 0.18 | 2.63 ± 0.20 | 19.84 ± 0.42 | n.d.a | 2.20 ± 0.1 |
| ∑PUFA | 16.24 ± 0.24 | 25.95 ± 15.76 | 13.06 ± 0.32 | 17.62 ± 19.64 | 24.90 | 5.26 ± 1.4 | 13.78 ± 12.42 | 0.45 ± 0.19 | n.d.a | 19.74 ± 0.67 | 23.71 | 29.00 ± 0.00 | 6.40 ± 0.50 |
| C20:4(ARA) | 1.37 ± 0.07 | n.d.a. | 2.84 ± 0.53 | 2.50 ± 4.03 | 3.90 ± 0.10 | 35.4 ± 1.5 | 3.16 ± 0.19 | 0.92 ± 0.17 | 16.59 ± 0.11 | 11.46 ± 0.59 | 0.00 | 23.04 ± 0.52 | 2.0 ± 0.1 |
| C20:5(EPA) | 2.02 ± 0.05 | 0.55 ± 0.35 | n.d.a | 1.69 ± 1.12 | n.d.a | 5.5 ± 0.2 | 33.42 ± 18.27 | 51.68 ± 6.47 | 12.96 ± 0.18 | 6.57 ± 0.22 | 13.15 ± 0.02 | n.d.a | 0.3 ± 0.02 |
| C22:6 (DHA) | 0.86 ± 0.03 | n.d.a | n.d.a | 0.65 ± 0.63 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | 8.55 ± 0.37 | n.d.a | n.d.a |
| ∑HUFA | 4.25 | 0.55 | 2.84 | 4.84 | 3.90 | 40.9 | 36.58 | 52.6 ± 6.40 | 29.55 | 18.03 | 21.70 | 23.04 | 2.3 |
| ∑FA | 98.25 | 92.9 | 54.78 | 92.86 | 93.90 | 93.56 | 98.31 | 3578 | n.d.a | 98.03 | 68.11 | 93.26 | 87.2 |
| ω-6/ω-3 | 6.73 | 0.35 ± 0.21 | 0.79 ± 0.05 | 0.77 ± 0.48 | n.d.a | 2.47 | 1.22 ± 1.49 | - | 1.32 | 3.52 | 67.86 | 0.99 ± 0.30 | 1.4 ± 0.03 |
| References | [120] | [120] | [123] | [120,124] | [120] | [125] | [125] | [126] | [120] | [120] | [118] | [123] | [127] |
3.4.4. Proteins and Amino Acids
3.4.5. Pigments Content from Seaweeds
3.4.6. Polyphenols from Seaweeds
3.4.7. Vitamins from Marine Macroalgae
| Green algae | ||||||||||
| Seaweed species | Cladophora vagabunda | Cladophora vagabunda | Ulva lactuca | Ulva lactuca | Ulva rigida | Ulva fasciata | Ulva intestinalis | Ulva flexuosa | Ulva intestinalis | Udotea argentea |
| Region | Black Sea | Black Sea | Black Sea | Ireland coast | Atlantic waters | Indian waters | Black Sea | Indian waters | Gulf of Gökova of Aegean Sea | Mauritius coast |
| Vitamin Content | ||||||||||
| Vitamin A; mg/100 g d.w. | 0.151 ± 1.83 | 0.58 ± 0.03 | 0.57 ± 0.06 | 0.017 | 9581 | - | 0.49 ± 0.05 | - | 0.081 ± 1.54 | - |
| Vitamin C; mg/100 g d.w. | 89.665 ± 2.58 | 149.66 ± 0.58 | 146.63 ± 0.95 | 0.242 | 9.42 | 0.38 ± 0.04 | 136.16 ± 0.85 | 0.36 ± 0.02 | 147 ± 2.00 | 435 |
| Vitamin E; mg/100 g d.w. | 8.132 ± 1.03 | 8.54 ± 0.63 | 8.22 ± 0.11 | 0.024 | 19.70 | - | 9.93 ± 0.83 | - | 5.13 ± 1.03 | - |
| Vitamin B1; mg/100 g d.w. | 0.153 ± 0.02 | 4.16 ± 0.25 | 3.72 ± 0.25 | - | 0.47 | - | 3.95 ± 0.52 | - | 0.17 ± 0.1 | 48.3 |
| Vitamin B2; mg/100 g d.w. | 0.893 ± 0.16 | 0.89 ± 0.06 | 0.99 ± 0.07 | 0.533 | 0.199 | 0.32 ± 0.29 | 0.97 ± 0.02 | 0.26 ± 0.32 | 0.89 ± 0.02 | - |
| Vitamin B3; mg/100 g d.w.; ppm * | 2.495 ± 0.19 | 2.59 ± 0.32 | 2.97 ± 0.28 | 98 * | 0.5 | 1.02 ± 0.41 | 1.84 ± 0.45 | 0.92 ± 0.48 | 2.42 ± 0.09 | 32.8 |
| References | [30] | [49] | [49] | [204] | [54] | [55] | [49] | [55] | [56] | [199] |
| Red algae | ||||||||||
| Seaweed species | Palmaria palmata | Porphyra umbilicalis | Gracilaria edulis | Ceramium virgatum | Gracilaria cortica | Gracilaria edulis | Gracilaria corticata | Chondruscrispus | Porphyra umbilicalis | Palmaria palmata |
| Region | Atlantic waters | Atlantic waters | Indian waters | Black sea | Southeast coast of India | Southeast coast of India | Mauritius coast | Spanish coast | Ireland coast | Barents Sea |
| Vitamin Content | ||||||||||
| Vitamin A; mg/100 g d.w. *; mg/g ** | 1.59 * | 3.65 * | - | - | 2.67 ± 0.30 ** | 2.07 ± 0.06 ** | - | 0.1 * | 3.65 * | - |
| Vitamin C; mg/100 g d.w. *; mg/g **; ppm *** | 6.34–34.5 * | 4.214 * | 0.25 ± 0.06 * | 50.0 ± 0.5 * | 14.66 ± 0.23 ** | 13.41 ± 0.57 ** | - | 10 *** | 12.885 * | - |
| Vitamin E; mg/100 g d.w.; mg/g ** | 2.2–13.9 | - | - | 250 ± 1.1 | 1.40 ± 0.10 ** | 1.49 ± 0.10 ** | - | - | 0.114 | - |
| Vitamin B1; mg/100 g d.w.; mg/g ** | 0.073–1.56 | 0.144 | - | 4.2 ± 0.3 | 0.38 ± 0.02 ** | 0.36 ± 0.02 ** | 23.3 | 0.1 | 0.077 | - |
| Vitamin B2; mg/100 g d.w.; mg/g **; µg/100 g *** | 0.51–1.91 | 0.36 | 0.12 ± 0.15 | 6.6 ± 0.4 | 0.05 ± 0.01 ** | 1.54 ± 0.07 ** | - | 2.5 | 0.274 | 35.38 ± 2.22 *** |
| Vitamin B3; mg/100 g d.w.; mg/g **; µg/100 g *** | 1.89 | - | 0.52 ± 0.28 | 15.0 ± 0.6 | 1.54 ± 0.39 ** | 1.10 ± 0.29 ** | - | 3.2 | 0.761 | 18.6 ± 1.10 *** |
| Vitamin B12; mg/100 g d.w.; ppm **; µg/100 g *** | - | - | - | - | - | - | 26.9 | 0.6 ** | 0.769 *** | - |
| References | [54] | [54] | [55] | [57] | [59] | [59] | [199] | [204] | [205] | [206] |
| Brown algae | ||||||||||
| Seaweed species | Fucus vesiculosus | Laminaria digitata | Undariapinnatifida | Padina gymnospora | Gongolaria barbata | Himanthalia elongata | Sargassum obovatum | Padina boryana | Himanthalia elongata | Ascophyllum nodosum |
| Region | Atlantic waters | Atlantic waters | Atlantic waters | Indian waters | Black sea | North-Eastern Atlantic Ocean | Mauritius coast | Mauritius coast | Spanish coast | Ireland coast |
| Vitamin Content | ||||||||||
| Vitamin A; mg/100 g d.w. | 0.30–7 | - | 0.04–0.22 | - | - | - | - | - | 0.079 | - |
| Vitamin C; mg/100 g d.w. | 14.124 | 35.5 | 5.29 | 0.29 ± 0.02 | 22.0 ± 1.2 | - | - | - | 28.56 | 0.654 |
| Vitamin E; mg/100 g d.w.; µg/g d.w. * | - | 3.43 | 1.4–2.5 | - | 120 ± 1.9 | 33.3 ± 4.2 * | - | - | 5.8 | 0.029 |
| Vitamin B1; mg/100 g d.w.; g/g d.w. ** | 0.02 | 1.250 | 0.17–0.30 | - | 2.3 ± 0.5 | 0.14 ± 0.02 ** | 56.8 | 8.87 | 0.020 | 0.216 |
| Vitamin B2; mg/100 g d.w.; g/g d.w. ** | 0.035 | 0.138 | 0.23–1.4 | 0.08 ± 0.18 | 5.5 ± 0.8 | 1.14 ± 0.14 ** | 3.27 | 1.67 | 0.020 | 0.058- |
| Vitamin B3; mg/100 g d.w. | - | 61.2 | 2.56 | 0.34 ± 0.16 | 22.0 ± 0.9 | - | 17.3 | - | - | - |
| Vitamin B12; mg/100 g d.w.; µg/100 g d.w. *** | - | - | - | - | - | - | - | 24.5 | - | 1.840 *** |
| References | [54] | [54] | [54] | [55] | [57] | [62] | [199] | [199] | [204] | [205] |
3.4.8. Mineral Content of Seaweeds
4. The Relationship Between the Biological Activities of Biocompounds—Potential Health and Nutraceutical Application
4.1. Antitumoral Activity
4.2. Antioxidant Activity
| Type of Seaweed | Bioactive Metabolites/ Compounds | Mechanism of Action | Biological Activity | References |
|---|---|---|---|---|
| Antitumoral activity | ||||
| Polysaccharides | ||||
| Fucus vesiculosus—brown algae | Fucoidan | Decreases colony formation, cancer cell formation, and cell adhesion. | Antitumoral activity | [215] |
| Ecklonia radiata—brown algae | Fucoidan | Decreases cancer cell formation and cell adhesion. | Antitumoral activity | [215] |
| Sargassum elegans—brown algae | Fucoidan | Decreases colony formation, cancer cell formation, and cell adhesion. | Antitumoral activity | [215] |
| Fucus vesiculosu—brown algae | Fucoidans | Decreases cancer cell sphere formation and cell adhesion. | Antitumoral activity | [216] |
| Ulva conglobata—green algae | Fucoidans | Induced apoptosis and decreased the cell cycle in HT-29 cells in S and G2/M phases and accumulation in cells in G1 phase. It has been proven that H2O2 has an antioxidant effect on HT-29 cells. | Antitumoral activity | [217] |
| Sargassum pallidum—brown algae | Fucoidans | Decreases colony formation, cancer cell sphere formation, and cell adhesion. | Antitumoral activity | [218] |
| Codium isthmocladum—green algae | Sulphated galactan | Reducing cell invasion, colony-forming capacity, and membrane glycoconjugates and reducing solid tumour growth and metastasis. | Antitumor activity | [219] |
| Ulva Lactuca—green algae | Polysaccharides | Decreased the level of methane dicarboxylic aldehyde and inhibited the activation of signaling pathways in human uveal melanoma cells | Antitumor activity | [220] |
| Pyopia haitanensis—red algae | Porphyrans | Direct cytotoxic effects, inducing the oxidative stress and apoptosis in cells, and causing the cell G0–G1 phase arrest. | Antitumoral activity | [73] |
| Chondrus armatus—red algae | Carrageenans | Decreases cell viability of cancerous cells. | Antitumoral activity | [221] |
| Capsosiphon fulvescens—green algae | Sulfated glucuronorhamnoxylan | Inhibits the growth of HT-29 human colon cancer cells. | Antitumor activity | [222] |
| Udotea flabellum—green algae | Sulfated galactans | The anti-proliferative activity was dependent on their degree of sulfation. | Antitumoral activity | [223] |
| Sargassum cinereum—brown algae | Polysaccharides | Decreases cell viability of cancerous cells. | Antitumoral activity | [224] |
| Terpenoides | ||||
| Dictyota bartayresiana—brown algae | SiO2–ZnO nanocomposites with diterpenes from algae | Antitumor effect on HT29. Antimicrobian effect. Excellent antioxidant activity. | Antitumor potential | [225] |
| Pigments | ||||
| Undaria pinnatifida—brown algae | Fucoxanthin | Decreases levels of vascular endothelial growth factor (VEGF)-C, VEGF receptor-3, nuclear factor kappa β, phospho-Akt, and phospho-PI3K in HLEC. Decreases micro-lymphatic vascular density in an MDA-MB-231 nude mouse model of breast cancer. | Antitumoral activity on breast cancer. | [226] |
| Polyphenols | ||||
| Sargassum tenerrimum—brown algae | Polyphenol compounds | Polyphenols have anti-cancer activity against HeLa cells. | Antitumoral activity | [227] |
| Antioxidant activity | ||||
| Polysaccharides | ||||
| Nizamuddinia zanardinii—brown algae | Fucoidan | It decreases the intracellular production of ROS, having a protective effect | Antioxidant activity | [229] |
| Sargassum fusiforme (as Hizikia fusiforme)—brown algae | Fucoidan | It reduced apoptosis by eliminating intracellular ROS, by increasing intracellular SOD-1 and CAT expressed by up-regulation of Nrf2. Prevented cell death. | Antioxidant activity | [230] |
| Undaria pinnatifida—brown algae | Fucoidan | Decreases cell death, intracellular ROS, and lipid peroxidation | Antioxidant activity | [71] |
| Ecklonia maxima—brown algae | Sulfated polysaccharides | It decreases oxidative stress and cell death, improves inhibition of MMPs. | Antioxidant, activity | [231] |
| Padina boryana—brown algae | Sulfated polysaccharides | Decreases cell death, intracellular ROS, and lipid peroxidation | Antioxidant activity | [232] |
| Ulva australis (as Ulva pertusa)—green algae | Ulvan | Showed antioxidant activity by increasing antioxidant enzymes CAT, SOD, GPx | Antioxidant activity | [233] |
| Sargassum fulvellum—brown algae | Polysaccharides | Decreases cell death, decreases intracellular ROS and lipid peroxidation | Antioxidant activity | [234] |
| Sargassum wightii—brown algae | Sulfated polygalacto-pyranosyl-fucopyranan | The presence of sulphate groups in the composition of the isolated polysaccharide seems to play a major role in the scavenging potential of free radicals. | Antioxidant activity | [235] |
| Ulva lactuca—green algae | Polysaccharides | protective effect on renal lesions by decreasing atrophy and serum levels of creatinine and cystatin C. Decreases oxidative stress in the kidneys. | Kidney injury caused by oxidative stress | [236] |
| Terpenoides | ||||
| Laurencia tristicha—red algae | Laurane-type sesquiterpene | Different methods of evaluating antioxidant activity | Antioxidant activity | [237] |
| Fatty acids | ||||
| Grateloupia turuturu—red algae | EPA and PUFA | Free radical scavenging activity: DPPH and ABTS | Antioxidant activity | [238] |
| Palmaria palmata—red algae | EPA | Antioxidant assay by DPPH and ABTS | Antioxidant activity | [126] |
| Proteins | ||||
| Red, brown, and green seaweeds | Proteins, peptides, lectins | For antioxidant activity of amino acids: Test DPPH and ABTS assay | Antioxidant activity | [136] |
| Gracilariopsis lemaneiformis—red algae | Peptide sequence ELWKTF | Scavenging DPPH free radicals assay | Antioxidant activity | [239] |
| Pyropia yezoensis—red algae | Amino acid: Taurine | Different methods of evaluating antioxidant activity | Antioxidant activty | [240] |
| Rodophyta–Porfira spp. | Amino acids inred algae | Evaluate antioxidant capacity—DPPH, ferrous ion-chelating, ABTS, FRAP, β-carotene/linoleic acid, and ORAC | Antioxidant activity | [241] |
| Pigments | ||||
| Ulva lactuca—green algae | Chlorophyll a and b; total carotenoids | Antioxidants activity by DPPH, FRAP, TEAC assay | Antioxidant activity | [49] |
| Ulva intestinalis—green algae | Chlorophyll a and b; total carotenoids | Antioxidants activity by DPPH, FRAP, TEAC assay | Antioxidant activity | [49] |
| Cladophora vagabunda—green algae | Chlorophyll a and b; total carotenoids | Antioxidants activity by DPPH, FRAP, TEAC assay | Antioxidant activity | [49] |
| Gracilaria corticata—red algae | R-phycoerythrin | Resulted activity by MTT assay, and the colon cancer cell lines SW620 and HCT-116 were inhibited by the compound in a concentration-dependent manner. | Antioxidant activity | [241] |
| Caulerma racemosa—green algae | Chlorophyll a and b, β-carotene | Showed antioxidant activity by CUPRAC and ABTS assays. | Antioxidant activity | [245] |
| Hypnea musciformis—red algae | Fucoxanthin, chlorophyll a and b, β-carotene | Showed antioxidant activity by CUPRAC and ABTS assays. | Antioxidant activity | [245] |
| Cladostephus spongiosus—brown algae | Fucoxanthin, pheophytin-α, chlorophyll a | Showed antioxidant activity by CUPRAC and ABTS assays. | Antioxidant activity | [245] |
| Codium adhaerens—green algae | Fucoxanthin, pheophytin-α | Showed antioxidant activity by FRAP, DPPH, and ABTS assays. | Antioxidant activity | [246] |
| Pyropia yezoensis—red algae | R-phycoerythrin | Antioxidant activity by ABTS and FRAP assays and had significant cytotoxicity against Hep G2 cells | Antioxidant activity Antitumoral activity | [247] |
| Fucus virsoides—brown algae | Fucoxanthin, pheophytin-α | Proapoptotic activity for human cervical adenocarcinoma HeLa cells. | Antioxidant activity | [248] |
| Ceramium virgatum—red algae | Carotenoids, xanthophyll and β-carotene | Showed antioxidant activity by TEAC assay | Antioxidant activity | [249] |
| Ulva lactuca—green algae | Chlorophyll a and b | Showed antioxidant activity by TEAC assay | Antioxidant activity | [169] |
| Dictyota cervicornis (formerly Dictyota indica)—brown algae | Fucoxanthin | Strong antioxidant activity by FRAP assay | Antioxidant activity | [250] |
| Phyllariopsis brevipes (formerly Phyllaria reniformis)—brown algae | Fucoxanthin, pheophytin-α, | Antioxidant activity by DPPH assay | Antioxidant activity | [251] |
| Undaria pinnatifida—brown algae | Fucoxanthin | Showed protection from neurite breakage | Neurodegenerative diseases | [252] |
| Polyphenols | ||||
| Sargassum tenerrimum—brown algae | Polyphenol compound | Showed potential activity by TEAC, FRAP, H2O2, DPPH, and ABTS assays | Antioxidant activity | [227] |
| Dictyota dichotoma—brown algae | Protocatechuic, p-hydroxybenzoic, coumaric, and ferulic acid | Showed activity by FRAP, DPPH, ORAC assays | Antioxidant activity | [254] |
| Padina pavonica—brown algae | Protocatechuic, ferulic, p-hydroxy-benzoic acid | Showed activity by FRAP, DPPH, ORAC assays | Antioxidant activity | [254] |
| Padina boryana—brown algae | Polyphenolic compound | DPPH and FRAP assay | Antioxidant activity | [255] |
| Acanthophora spicifera—red algae | Polyphenolic compound: Velutin | DPPH and FRAP assay | Antioxidant activity | [255] |
| Gongolaria barbata—brown algae | Total phenolic content | DPPH radical scavenging activity and reducing power | Antioxidant activity | [256] |
| Gongolaria barbata—brown algae | Flavonoids | DPPH assay | Antioxidant activity | [257] |
| Gigartina acicularis—red algae | Flavonoids | DPPH assay | Antioxidant activity | [257] |
| Ecklonia radiata—brown algae | Phlorotannim | Inhibition of apoptosis induced by Aβ1–42 | Neuroprotective activity | [258] |
| Ecklonia radiata—brown algae | Eckol-type phlorotannins | Showed neuroprotective activity against the neurotoxic amyloid β-protein (Aβ1–42) in a neuronal PC-12 cell line in vitro experiment. | Neuroprotective activity | [259] |
| Eklonia cava—brown algae | Phloroglucinol | Decreases the amyloid β-peptide burden and pro-inflammatory cytokines in the hippocampus. | Neurodogenerative disease | [260] |
| Vitamins | ||||
| Odonthalia dentata—red algae | A, B1, B2, B3, B6, B9, C, and E | Increases the body’s immunity. Protects against oxidative stress. Involved in metabolic regulation processes. | Antioxidant activity | [261] |
| Caulerpa chemnitzia—green algae | Vitamin C | Strengthening the immune system. Involvement in the cell regeneration process | Antioxidant activity | [262] |
4.3. Antimicrobial Activity
4.4. Anti-Inflammatory Activity
4.5. Cardioprotective and ACE Inhibitory Activity
| Type of Seaweed | Bioactive Metabolites/Compounds | Mechanism of Action | Biological Activity | References |
|---|---|---|---|---|
| Antimicrobial activity | ||||
| Terpenoides | ||||
| Dictyota dichotoma—brown algae | Diterpenes as dictyols. | Have effects on cell viability in murine macrophage cell line RAW 264.7 | Antimicrobial potential | [114] |
| Kappapycus alvarezii—red algae | Purified terpenoids fractions | Minimum inhibitory concentration (MIC) value was 1.5 mg/mL against S. mutans. Minimal bactericidal concentration (MBC) value was 3.0 mg/mL | Antimicrobial activity | [263] |
| Gracillaria dura—red algae | hexadecanoic acid methyl ester, n-hexadecenoic acid, 11-octadecanoic acid, and phytol | Minimum inhibitory concentration (MIC) value was 0.065 mg/mL against S. mutans. Minimal bactericidal concentration (MBC) value was 0.12 mg/mL | Antimicrobial activity | [263] |
| Ulva lactuca—green algae | Sesquiterpenoid as neophytadiene | Showed excellent inhibitory effects with the maximum activity (by diffusion) against E. coli, K. pneumonia, and S. typhi | Antimicrobial activity | [267] |
| Stypopodium zonale—brown algae | Atomaric acid and 4-acetoxydolastane, secundary metabolites, | Anti-HSV-2 activity with low cytotoxicity, inactivated 90% of the viral particle. | Anti-Herpes simplex virus | [268] |
| Canistrocarpus cervicornis—brown algae | Atomaric acid and 4-acetoxydolastane | Anti-HSV-2 activity with low cytotoxicity | Anti-Herpes simplex virus | [269] |
| Canistrocarpus cervicornis—brown algae | Dolastane-type diterpenoids | For Chikungunya virus, the compound was able to inhibit around 90% of the virus infectivity and for Zika virus, the effects were at approximately 64% | Anti-viral activity on Zika and Chikungunya viruses | [269] |
| Pigments | ||||
| Sargassum angustifolium—brown algae | Fucoxanthin | Showed antimicrobial activity against S. aureus by diffusion method | Antimicrobial activity | [270] |
| Gongolaria indica—brown algae | Fucoxanthin | Significant inhibition zone against E. Coli and S. aureus. | Antimicrobial activity | [270] |
| Polyphenols | ||||
| Padina pavonica—brown algae | Protocatechuic acid; p-hdroxybenzoic acid; p-coumaric acid; t-ferulic acid; o-coumaric acid | Inhibition zone: B. subtilis: 12.7 ± 0.6 mm; P. aeruginosa: 15.7 ± 2.1 mm; S. aureus: 10.3 ± 1.5 mm and C. albicans: 10 ± 0.9 mm | Antimicrobial activity | [254] |
| Anti-inflammatory activity | ||||
| Polysaccharides | ||||
| Sargassum autumnale—brown algae | Fucoidan | Down-regulation of iNOS and COX2 and signaling pathways (NF-κB and MAPK). | Anti-inflammatory activity | [271] |
| Sargassum siliquastrum—brown algae | Fucoidan | Down-regulated the expression of inflammatory mediators (NO, PGE2, iNOS, COX-2), and pro-inflammatory cytokines via regulating MAPK and NF-κB. | Anti-inflammatory activity | [272] |
| Sargassum confusum—brown algae | Fucoidan | Reducing the expression of inflammatory mediators through regulation of NF-κB and MAPKs signaling pathways via activating Nrf2/HO-1 signaling. | Anti-inflammatory activity | [273] |
| Cystoseira crinita—brown algae | Fucoidan | Decreases IL-1β production. | Anti-inflammatory activity | [274] |
| Sargassum swartzii—brown algae | Sulfated Polysaccharide (fucoidan) | Inhibition of inflammatory mediators and pro-inflammatory cytokines. | Anti-inflammatory activity | [275] |
| Codium fragile—green algae | Sulfated polysaccharides | Decreases cell death and the generation of NO and ROS. | Anti-inflammatory activity | [276] |
| Saccharina japonica—brown algae | Sulfated galactofucan | Decreases cell death and the generation of NO and ROS. | Anti-inflammatory activity | [277] |
| Sargassum binderi—brown algae | Sulfated polysaccharides | Decreases LPS-induced cell death and NO production. | Anti-inflammatory activity | [278] |
| Sargassum fulvellum—brown algae | Sulfated polysaccharides | Decreases cell death and the generation of NO and ROS. | Anti-inflammatory activity | [279] |
| Saccharina japonica—brown algae | Sulfated polysaccharides | Decreases cell death and the generation of NO and ROS. | Anti-inflammatory activity | [280] |
| Fatty acids | ||||
| Fucus spiralis—brown algae | EPA and octadecatetraenoic acid | Showed dose-dependent effect on murine macrophage RAW 264.7 cell line, | Anti-inflammatory activity | [120] |
| Undaria pinnatifida—brown algae | SA, EPA | SA: IC50 values of 160 µg on ear for edema, 314 µg on ear for erythema, 235 µg on ear for blood flow. EPA: IC50 values of 230 µg on ear for edema, 462 µg on ear for erythema, 236 µg on ear for blood flow. | Anti-inflammatory activity | [120] |
| Palmaria palmata—red algae | Palmitic acid, Oleic acid, and EPA | Inhibition of the COX-2 enzyme | Anti-inflammatory activity | [117] |
| Gracilaria gracilis—red algae | SFA, MUFA, PUFA, HUFA, omega-3, omega-6 | Inhibition of the COX-2 enzyme | Anti-inflammatory activity | [118] |
| Undaria pinnatifida—brown algae | SFA, MUFA, PUFA, HUFA, omega-3, omega-6 | Inhibition of the COX-2 enzyme | Anti-inflammatory activity | [118] |
| Ulva lactuca—green algae | SFA, MUFA, PUFA, omega-3, omega-6 | Inhibition of the COX-2 enzyme | Anti-inflammatory activity | [281] |
| Ulva intestinalis—green algae | SFA, MUFA, and PUFA | Inhibition of the COX-2 enzyme | Anti-inflammatory activity | [282] |
| Pigments | ||||
| Sargassum fusiformis—brown algae | Fucoxanthin, pheophytin-α, chlorophyll-a, β-carotene | Inhibiting production of prostaglandin E2 (PGE2), cyclooxygenase-2, interleukin (IL)-1β, and IL-6 from exposed HaCaT keratinocytes | Anti-inflammatory activity | [283] |
| Cardioprotective activity andACE inhibitory activity | ||||
| Polysaccharides | ||||
| Sargassum wightii—brown algae | Sulfated polygalacto-pyranosyl-fucopyranan | The formation of hydrogen bonds with Zn2+ and other amino acid residues by the electronegative functionalities can lead to effective inhibition of ACE. | Antihipertensive activity | [235] |
| Fucus vesiculosus—brown algae | Fucoidan | Decreases lipid levels and the carotid atherosclerotic plaque formation. | Cardioprotective activity | [284] |
| Fatty acids | ||||
| Alaria esculenta—brown algae | Palmitic acid, Oleic acid and EPA | Showed high content of linoleic acid which indicates potential activity on coronary heart disease | Coronary hearth diseases, | [117] |
| Palmaria palmata—red algae | Palmitic acid, Oleic acid and EPA | Inhibition of the COX-2 enzyme | Coronary hearth disease | [117] |
| Undaria pinnatifida—brown algae | SFA, MUFA, PUFA, HUFA, omega-3, omega-6 | Inhibition of the COX-2 enzyme | Cardioprotective activity | [118] |
| Gracilaria gracilis—red algae | SFA, MUFA, PUFA, HUFA | Inhibition of the COX-2 enzyme | Cardioprotective activity | [118] |
| Ulva lactuca—green algae | SFA, MUFA, PUFA | Inhibition of the COX-2 enzyme | Cardioprotective activity | [281] |
| Ulva intestinalis—green algae | SFA, MUFA, and PUFA | Inhibition of the COX-2 enzyme | Cardioprotective activity | [282] |
| Curdiea racovitzae—red algae | SFA, MUFA, PUFA | Inhibition of the COX-2 enzyme | Cardioprotective activity | [282] |
| Proteins | ||||
| Sphaerococcus coronopifolius—red algae | Protein hydrolysates with MW 300–1800 Da | ACE inhibitory activity IC50 = 160.32 µM; ACE inhibitory activity IC50 = 656.15 µM; | ACE inhibitory activity | [130] |
| Gelidium spinosum—red algae | Protein hydrolysates with MW 300–1800 Da (Fractions F1–F10) | F4: ACE inhibitory activity IC50 = 149.35 µM; F6: ACE inhibitory activity IC50 = 656.15 µM; | ACE inhibitory activity | [130] |
| Palmaria palmata—red algae | Protein hydrolysates | Positive role in glucose transport, increasing glucose uptake | ACE inhibitory activity | [286] |
| Mazzaella japonica—red algae | Protein sequences | Significant IC50 values were found in sequence IY from the peptide chain | ACE inhibitory activity | [287] |
| Porphyra dioica—red algae | Peptides sequences | TYIA: ACE inhibitory activity and YLVA: DPP-IV inhibitory activity | ACE inhibitory activity | [288] |
| Undaria pinnatifida—brown algae | Peptide | ACE inhibitory activity with IC50 = 225.87 μM | Antihypertensive activity | [133] |
| Sargassum mcclurei—brown algae | Peptide sequence | activity on endothelin-1 suppressing capacity for ACE inhibitory activity | Antihypertensive activity | [144] |
| Ulva intestinalis—green algae | Peptide sequences | Sequences: FGMPLDR: ACE inhibitory and MELVLR: ACE inhibitory | Antihypertensive activity | [289] |
| Pigments | ||||
| Sargassum wightii—brown algae | Fucoxanthin | Showed inhibition of ACE with half maximal inhibitory value | ACE inhibitory activity | [290] |
4.6. Antidiabetic Activity
4.7. Activities in the Treatment of Metabolic Diseases
4.8. Anticoagulant Activity
4.9. Neuroprotective Activity and Alzheimer’s Disease (AD)
4.10. Antiprotozoal Activity
4.11. Bone Deficiencies
4.12. Malnutrition
5. Nutraceutical Applications
5.1. Anti-Nutritional Compounds
5.2. Nutraceutical Applications of Marine Algae Products
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SFE | Supercritical Fluid Extraction | HPLC | High-performance liquid chromatography |
| SWE | Subcritical Water Extraction | CG-MS | Gas Chromatography coupled with mass spectrometry |
| SFE | Supercritical Fluid Extraction | GP | Glycoproteins |
| PSE | Pressurized Solvents Extraction | AGPs | Arabinogalactan proteins |
| MAE | Microwave-Assisted Extraction | PBPs | Phycobiliproteins |
| EAE | Enzyme-Assisted Extraction | TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction | TFC | Total flavonoid content |
| CSE | Conventional Solvent Extraction | DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SLE | Solid–Liquid Extraction | ABTS | 2,2′-azino-bis(3-ethylbenzothi-azoline-6-sulfonic acid |
| LLE | Liquid–Liquid Extraction | TEAC | Trolox equivalent antioxidant capacity |
| CSE | Conventional solvent extraction | FRAP | Ferric reducing ability of plasm |
| MAPs | Marine algae Polysaccharides | AAS | Atomic absorption spectroscopy |
| AF | Fatty acids | ICP-MS | Inductively coupled plasma mass spectrometry. |
| MUFAs | Monounsaturated fatty acids | SFAs | Saturated fatty acids |
| PUFAs | Polyunsaturated fatty acids | EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid | ACE inhibitors | Angiotensin-converting enzyme (ACE) inhibitors |
References
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Miranda, J.M.; Del Carmen Mondragon, A.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential use of marine seaweeds as prebiotics: A review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef] [PubMed]
- Din, N.A.S.; Mohd Alayudin, A.S.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Cadar, E.; Axinte, E.R.; Amzoiu, M.; Jurja, S.; Cherim, M. Preliminary study on the marine algae from the romanian Black Sea coast. J. Sci. Arts 2019, 4, 989–1000. [Google Scholar]
- Ferrara, L. Seaweeds: A Food for Our Future. J. Food Chem. Nanotechnol. 2020, 6, 56–64. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The Global Satus of Seaweed Production, Trade and Utilization; Pub. FAO Report Global Seaweed 2018; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Volume 124, pp. 1–77. Available online: https://orbit.dtu.dk/en/publications/the-global-status-of-seaweed-production-trade-and-utilization (accessed on 24 February 2025).
- Embling, R.; Neilson, L.; Randall, T.; Mellor, C.; Lee, D.M.; Wilkinson, L.L. Edible seaweeds as an alternative to animal-based proteins in the UK: Identifying product beliefs and consumer traits as drivers of consumer acceptability for macroalgae. Food Qual. Prefer. 2022, 100, 104613–104623. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Cadar, E.; Sirbu, R.; Negreanu-Pirjol, B.S.; Ionescu, A.-M.; Negreanu-Pirjol, T. Heavy metals bioaccumulation capacity on marine algae biomass from romanian Black Sea coast. Rev. Chim. 2019, 70, 3065–3072. [Google Scholar] [CrossRef]
- Cherim, M.; Sirbu, R.; Tomescu, A.; Popa, M.F.; Cadar, E. Comparative studies on the physico-chemical characteristics of biomaterials with collagen from calf and fish skins from Black Sea. Mater. Plast. 2019, 56, 179–185. [Google Scholar] [CrossRef]
- Sirbu, R.; Mustafa, A.; Tomescu, A.; Stanciu, G.; Cadar, E. Rheological and microbiological study on biocomposites with marine chitosan polymers from Black Sea stone crabs used in medical therapy of tissue regeneration. Mater. Plast. 2019, 56, 148–155. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. An Emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Cai, J. Global Status of Seaweed Production, Trade and Utilization; Food and Agriculture Organisation of United Nations: Rome, Italy, 2021; pp. 1–18. Available online: https://www.competecaribbean.org/wp-content/uploads/2021/05/Global-status-of-seaweed-production-trade-and-utilization-Junning-Cai-FAO.pdf (accessed on 24 February 2025).
- FAO. Fishery and Aquaculture Statistics. Global Production Statistics 1950–2019. In FAO Fisheries Division. FishStaJ—Software for Fishery and Aquaculture Statistical Time Series. 2021. Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj/en (accessed on 1 September 2022).
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown, Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Chao Zhao, C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef]
- Moga, M.A.; Dima, L.; Balan, A.; Blidaru, A.; Dimienescu, O.G.; Podasca, C.; Toma, S. Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review. Int. J. Mol. Sci. 2021, 22, 629. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Arain, M.A.; Ali Fazlani, S.; Marghazani, I.B.; Umar, M.; Soomro, J.; Bhutto, Z.A.; Soomro, F.; Noreldin, A.E.; Alagawany, M. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives; Food Safety and Quality Series No. 13; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/fishery/en/publication/289063 (accessed on 24 February 2025).
- Park, E.; Yu, H.; Lim, J.-H.; Choi, J.H.; Park, K.J.; Lee, J. Seaweed metabolomics: A review on its nutrients, bioactive compounds and changes in climate change. Food Res. Int. 2023, 163, 112221. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Caf, F.N.; Özdemira, N.S.; Yılmazb, O.; Durucanc, F.; Akd, I. Fatty acid and lipophilic vitamin composition of seaweeds from Antalya and Çanakkale (Turkey). Grasas Aceites 2019, 70, e312. [Google Scholar] [CrossRef]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.; Nabavid, S.M.; Daglia, M.; et al. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Foods Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Cadar, E.; Cherim, M. Studies on the physico-chemical characteristics of the marine algae ecosystem from the Romanian Black Sea. J. Sci. Arts 2018, 3, 717–726. [Google Scholar]
- Gaspar, R.; Fonseca, R.; Pereira, L. Illustrated Guide to the Macroalgae of Buarcos Bay, Figueira da Foz, Portugal, 1st ed.; MARE UC, DCV, FCT: Coimbra, Portugal, 2020; pp. 3–129. [Google Scholar] [CrossRef]
- Veluchamy, C.; Palaniswamy, R. A Review on Marine Algae and its Applications. Asian J. Pharm. Clin. Res. 2020, 13, 21–27. [Google Scholar] [CrossRef]
- Kennedy, J. Marine Algae: The 3 Types of Seaweed. 2019. Available online: https://www.thoughtco.com/types-of-marinealgae-2291975 (accessed on 24 February 2025).
- Sirbu, R.; Negreanu-Pirjol, T.; Mirea, M.; Negreanu-Pirjol, B.S. Bioactive Compounds from Three Green Algae Species along Romanian Black Sea Coast with Therapeutically Properties. Eur. J. Nat. Sci. Med. 2020, 3, 67–86. [Google Scholar] [CrossRef]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of Antioxidant and Antimicrobial Activity in Relation to Total Phenolic Content of Green Algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A.; Chauhan, O.P.; Patel, M.K.; Shokralla, S.; El-Abedin, T.K.Z.; Elansary, H.O.; Mahmoud, E.A. Antioxidant, Scavenging, Reducing, and Anti-Proliferative Activities of Selected Tropical Brown Seaweeds Confirm the Nutraceutical Potential of Spatoglossum asperum. Foods 2021, 10, 2482. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Badar, S.N.; Mohammad, M.; Emdadi, Z.; Yaakob, Z. Algae and their growth requirements for bioenergy: A review. Biofuels 2021, 12, 307–325. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C. An introduction to farming and biomass utilisation of marine macroalgae. Phycologia 2019, 58, 443–445. [Google Scholar] [CrossRef]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods 2024, 13, 269. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Simirgiotis, M. Cochayuyo (Durvillaea incurvata) extracts: Their impact on starch breakdown and antioxidant activity in pasta during in vitro digestion. Foods 2023, 12, 3326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.; Mathimani, T.; Sudhakar, M.P.; Rajendran, K.; Nizami, A.-S.; Brindhadevi, K.; Pugazhendhi, A. A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew. Sustain. Energy Rev. 2021, 138, 110649. [Google Scholar] [CrossRef]
- Suthar, P.; Gajaria, T.K.; Reddy, C.R.K. Production of quality seaweed biomass through nutrient optimization for the sustainable land-based cultivation. Algal Res. 2019, 42, 101583. [Google Scholar] [CrossRef]
- Hwang, E.K.; Park, C.S. Seaweed cultivation and utilization of Korea. Algae 2020, 35, 107–121. [Google Scholar] [CrossRef]
- Obando, J.M.C.; Cunha dos Santos, T.; Martins, R.C.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Current and promising applications of seaweed culture in laboratory conditions. Aquaculture 2022, 560, 738596. [Google Scholar] [CrossRef]
- Regal, A.L.; Alves, V.; Gomes, R.; Matos, J.; Bandarra, N.M.; Afonso, C.; Cardoso, C. Drying process, storage conditions, and time alter the biochemical composition and bioactivity of the anti-greenhouse seaweed Asparagopsis taxiformis. Eur. Food Res. Technol. 2020, 246, 781–793. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Oerbekke, A.; Cvijetinovic, S.; Wahlstrom, N.; Harrysson, H.; Steinhagen, S.; Kinnby, A.; White, J.; Edlund, U. Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3255–3263. [Google Scholar] [CrossRef]
- Alvarez-Gomez, F.; Korbee, N.; Figueroa, F.L. Effects of UV Radiation on Photosynthesis, Antioxidant Capacity and the Accumulation of Bioactive Compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Sobuj, M.K.A.; Islam, M.A.; Islam, M.S.; Islam, M.M.; Mahmud, Y.; Rafiquzzaman, S.M. Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci. Rep. 2021, 11, 19082. [Google Scholar] [CrossRef]
- Mansur, A.A.; Brown, M.T.; Billington, R.A. The cytotoxic activity of extracts of the brown alga Cystoseira tamariscifolia (Hudson) Papenfuss, against cancer cell lines changes seasonally. J. Appl. Phycol. 2020, 32, 2419–2429. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown alga Durvillaea antarctica. Dry. Technol. 2019, 38, 1915–1928. [Google Scholar] [CrossRef]
- Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Mar. Drugs 2022, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Negreanu-Pirjol, T.; Sirbu, R.; Dragan, A.-M.L.; Negreanu-Pirjol, B.-S.; Axente, E.R.; Ionescu, A.-M. Biocompounds from Green Algae of Romanian Black Sea Coast as Potential Nutraceuticals. Processes 2023, 11, 1750. [Google Scholar] [CrossRef]
- Amlani, M.; Yetgin, S. Seaweeds: Bioactive Components and Properties, Potential Risk Factors, Uses, Extraction and Purification Methods. Mar. Sci. Technol. Bull. 2022, 11, 9–31. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Sirbu, R.; Stanciu, G.; Cadar, E.; Tomescu, A.; Cherim, M. Validation of a Quantitative Analysis Method for Collagen Extracted from Grey mullet Marine Fish. Rev. Chim. 2019, 70, 835–842. [Google Scholar] [CrossRef]
- Choudhary, B.; Khandwal, D.; Gupta, N.K.; Patel, J.; Mishra, A. Nutrient composition, physicobiochemical analyses, oxidative stability and antinutritional assessment of abundant tropical seaweeds from the Arabian Sea. Plants 2023, 12, 2302. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Subramani, K.; Shanmugam, M.; Seedevi, P.; Park, S.; Alfarhan, A.H.; Rajagopal, R.; Balasubramanian, B. A comparison of nutritional value of underexploited edible seaweeds with recommended dietary allowances. J. King Saud Univ. 2020, 32, 1206–1211. [Google Scholar] [CrossRef]
- Metin, C.; Baygar, T. Determination of nutritional composition of Enteromorpha intestinalis and investigation of its usage as food. Ege J. Fish. Aq. Sci. 2018, 35, 7–14. [Google Scholar] [CrossRef]
- Cadar, E. Research and Development of Semi-Solid Pharmaceutical Systems Based on Marine Resources. Ph.D. Thesis, IOSUD Carol Davila UMF, Bucharest, Romania, 2017. [Google Scholar]
- Premarathna, A.D.; Tuvikene, R.; Fernando, P.H.P. Comparative analysis of proximate compositions, mineral and functional chemical groups of 15 different seaweed species. Sci. Rep. 2022, 12, 19610. [Google Scholar] [CrossRef]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria edulis and Gracilaria corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [PubMed]
- Farghl, A.A.M.; Al-Hasawi, Z.M.; El-Sheekh, M.M. Assessment of Antioxidant Capacity and Phytochemical Composition of Brown and Red Seaweeds Sampled off Red Sea Coast. Appl. Sci. 2021, 11, 11079. [Google Scholar] [CrossRef]
- Praiboon, J.; Palakas, S.; Noiraksa, T. Seasonal variation in nutritional composition and anti-proliferative activity of brown seaweed, Sargassum oligocystum. J. Appl. Phycol. 2018, 30, 101–111. [Google Scholar] [CrossRef]
- Ilyas, Z.; Ali Redha, A.; Wu, Y.S. Nutritional and Health Benefits of the Brown Seaweed Himanthalia elongata. Plant Foods Hum. Nutr. 2023, 78, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Fouda, W.A.; Ibrahim, W.; Ellamie, A.M.; Ramadan, G. Biochemical and mineral composition of six brown seaweeds collected from Red Sea at Hurghada Coast. Indian J. Mar. Sci. 2019, 48, 484–491. [Google Scholar]
- Ullah, M.R.; Akhter, M.; Khan, A.B.S.; Yasmin, F.; Hasan, M.M.; Bosu, A.; Haque, M.A.; Islam, M.S.; Islam, M.A.; Mahmud, Y. Nutritional composition and phenolic contents of Gracilariopsis longissima, Padina tetrastromatica and Ulva intestinalis from the Bay of Bengal, Bangladesh coast. Heliyon 2024, 10, e31128. [Google Scholar] [CrossRef]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Review on Seaweeds and Seaweed-Derived Polysaccharides: Nutrition, Chemistry, Bioactivities, and Applications. Food Rev. Int. 2024, 40, 1312–1347. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Kulshreshtha, G.; Humayun, S.; Darko, C.N.S.; Rjabovs, V.; Hammami, R.; Critchley, A.T.; Tuvikene, R.; Hincke, H.T. Polysaccharides from red seaweeds: Effect of extraction methods on physicochemical characteristics and antioxidant activities. Food Hydrocoll. 2024, 147, 109307. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Kermanshahi-pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, E.A.; Kang, S.I.; Yang, H.W.; Ryu, B.; Wang, L.; Lee, J.S.; Jeon, Y.J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules 2020, 25, 2361. [Google Scholar] [CrossRef]
- Yao, W.Z.; Veeraperumal, S.; Qiu, H.-M.; Chen, X.-Q.; Cheong, K.L. Anti-cancer effects of Porphyra haitanensis polysaccharides on human colon cancer cells via cell cycle arrest and apoptosis without causing adverse effects in vitro. 3 Biotech 2020, 10, 386. [Google Scholar] [CrossRef]
- Akter, A.; Khairul, M.; Sobuj, A.; Islam, S.; Chakroborty, K.; Tasnim, N.; Ayon, M.H.; Hossain, F.; Rafiquzzaman, S.M. Seaweed polysaccharides: Sources, structure and biomedical applications with special emphasis on antiviral potentials. Future Foods 2024, 10, 100440. [Google Scholar] [CrossRef]
- Udo, T.; Gopinath Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Fu, X.; Gao, X.; Xu, J. Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Dragan, A.M.L.; Sirbu, R.; Cadar, E. Valuable Bioactive Compounds Extracted from Ceramium rubrum on the Romanian Seaside with Medical Interest. Eur. J. Nat. Sci. Med. 2022, 5, 64–74. [Google Scholar] [CrossRef]
- Shao, Z.; Duan, D. The Cell Wall Polysaccharides Biosynthesis in Seaweeds: A Molecular Perspective. Front. Plant Sci. 2022, 13, 902823. [Google Scholar] [CrossRef]
- Wang, S.H.; Huang, C.Y.; Chen, C.Y.; Chang, C.C.; Huang, C.Y.; Dong, C.D.; Chang, J.S. Structure and Biological Activity Analysis of Fucoidan Isolated from Sargassum siliquosum. ACS Omega 2020, 5, 32447–32455. [Google Scholar] [CrossRef]
- Dragan, A.M.L.; Sirbu, R.; Cadar, E. Brown Seaweeds from Black Sea Coast as an Important Source of Bioactive Compouns of Interest for Human Health. Eur. J. Nat. Sci. Med. 2023, 6, 100–113. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular Characteristics and Antioxidant Activity of Laminarin Extracted from the Seaweed Species Laminaria hyperborea, Using Hydrothermal-Assisted Extraction and a Multi-Step Purification Procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of Alginate Molecular Weight and M/G Ratio in Beads Properties Foreseeing the Protection of Probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef]
- Pengyan, Z.; Chang, L.; Zhanru, S.; Fuli, L.; Jianting, Y.; Delin, D. Genome-Wide Transcriptome Profiling and Characterization of Mannuronan C5-Epimerases in Saccharina Japonica. Algal Res. 2021, 60, 102491. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Luiten, C.A.; Carnachan, S.M.; Daines, A.M.; Kidgell, J.T.; Hinkley, S.F.R.; Praeger, C.; Martinez, M.A.; Sargison, L.; Magnusson, M. Structural Characterization of Ulvans Extracted from Blade Ulva ohnoi and Filamentous (Ulva tepida and Ulva prolifera) Species of Cultivated Ulva. Int. J. Biol. Macromol. 2022, 194, 571–579. [Google Scholar] [CrossRef]
- Li, Q.; Hu, F.; Zhu, B.; Ni, F.; Yao, Z. Insights into Ulvan lyase: Review of source, biochemical characteristics, structure and catalytic mechanism. Crit. Rev. Biotechnol. 2020, 40, 432–441. [Google Scholar] [CrossRef]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guegan, J.P.; Jeftić, J.; Benvegnu, T. Extracted Ulvans from green seaweeds Ulva linza of Lebanese origin and amphiphilic derivatives: Evaluation of their physico-chemical and rheological properties. J. Appl. Phycol. 2019, 31, 1931–1946. [Google Scholar] [CrossRef]
- Ciancia, M.; Ferna’ndez, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides From Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Gomaa, M.; Al-Badaani, A.A.; Hifney, A.F.; Adam, M.S. Utilization of Cellulose and Ulvan from the Green Seaweed Ulva lactuca in the Development of Composite Edible Films with Natural Antioxidant Properties. J. Appl. Phycol. 2022, 34, 2615–2626. [Google Scholar] [CrossRef]
- Firdayanti, L.; Yanti, R.; Rahayu, E.S.; Hidayat, C. Carrageenan extraction from red seaweed (Kappaphycopsis cottonii) using the bead mill method. Algal Res. 2023, 69, 102906. [Google Scholar] [CrossRef]
- Martín-del-Campo, A.; Fermín-Jiménez, J.A.; Fernández-Escamilla, V.V. Improved extraction of carrageenan from red seaweed (Chondracantus canaliculatus) using ultrasound-assisted methods and evaluation of the yield, physicochemical properties and functional groups. Food Sci. Biotechnol. 2021, 30, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Heriyanto, H.; Kustiningsih, I.; Sari, D.K. The effect of temperature and time of extraction on the quality of Semi Refined Carrageenan (SRC). MATEC Web Conf. 2018, 154, 01034. [Google Scholar] [CrossRef]
- Lebbar, S.; Fanuel, M.; Le Gall, S.; Falourd, X.; Ropartz, D.; Bressollier, P.; Gloaguen, V.; Faugeron-Girard, C. Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides. Molecules 2018, 23, 3364. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.; Ni, H.; Hong, Q.; Lin, K.; Xiao, A. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019, 87, 530–540. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Ultrasound-assisted extraction of sulfated polysaccharide from Nizamuddinia zanardinii: Process optimization, structural characterization, and biological properties. J. Food Process. Eng. 2018, 42, e12979. [Google Scholar] [CrossRef]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Kumar, K.A. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Montes, L.; Gisbert, M.; Hinojosa, I.; Sineiro, J.; Moreira, R. Impact of drying on the sodium alginate obtained after polyphenols ultrasound-assisted extraction from Ascophyllum nodosum seaweeds. Carbohydr. Polym. 2021, 272, 118455. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.M.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and Characterization of Alginate Extracted from Brown Seaweed Collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and Characterization of Alginate from an Edible Brown Seaweed (Cystoseira barbata) Harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef]
- Malvis Romero, A.; Picado Morales, J.J.; Klose, L.; Liese, A. Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata. Molecules 2023, 28, 6781. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Cikoš, A.M.; Jurin, M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Update on Monoterpenes from Red Macroalgae: Isolation, Analysis, and Bioactivity. Mar. Drugs 2019, 17, 537. [Google Scholar] [CrossRef]
- Polzin, J.; Rorrer, G.L. Selective production of the acyclic monoterpene β-myrcene by microplantlet suspension cultures of the macrophytic marine red alga Ochtodes secundiramea under nutrient perfusion cultivation with bromide-free medium. Algal Res. 2018, 36, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the marine algae of the genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef]
- Rajamani, K.; Balasubramanian, T.; Thirugnanasambandan, S.S. Bioassay-guided isolation of triterpene from brown alga Padina boergesenii possess anti-inflammatory and anti-angiogenic potential with kinetic inhibition of β-carotene linoleate system. LWT 2018, 93, 549–555. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Preparative separation of three terpenoids from edible brown algae Sargassum fusiforme by high-speed countercurrent chromatography combined with preparative high-performance liquid chromatography. Algal Res. 2021, 59, 102449. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Attia, E.Z.; Saber, H.; Saber, A.A.; Bringmann, G.; Abdelmohsen, U.R. The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules 2022, 27, 672. [Google Scholar] [CrossRef]
- Santos, J.P.; Guihéneuf, F.; Fleming, G.; Chow, F.; Stengel, D.B. Temporal stability in lipid classes and fatty acid profiles of three seaweed species from the north-eastern coast of Brazil. Algal Res. 2019, 41, 101572. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Foseid, L.; Natvik, I.; Devle, H.; Ekeberg, D. Identification of fatty acids in fractionated lipid extracts from Palmaria palmata, Alaria esculenta and Saccharina latissimi by off-line SPE GC-MS. J. Appl. Phycol. 2020, 32, 4251–4262. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Bases, E.A.; El-Shenody, R.A.; El Shafay, S.M. Lipid extraction from some seaweeds and evaluation of its biodiesel production. Biocatal. Agric. Biotechnol. 2021, 35, 102087. [Google Scholar] [CrossRef]
- Jaworowska, A.; Murtaza, A. Seaweed derived Lipids sre a potential Anti-Inflammatory agent: A Review. Int. J. Environ. Res. Public Health 2023, 20, 730. [Google Scholar] [CrossRef]
- Kord, A.; Cherif, Y.F.; Amiali, M.; Mustapha, M.A.; Benfares, R.; Soumia, B.; Belfadel, O. Fatty acids composition of Cystoseira sauvageauana and Laurencia pinnatifida collected from the algerian coast. Acta Period. Technol. 2019, 50, 113–122. [Google Scholar] [CrossRef]
- Rodríguez-González, I.; Diaz-Reinos, B.; Domínguez, H. Intensification Strategies for the Extraction of Polyunsaturated Fatty Acids and Other Lipophilic Fractions from Seaweeds. Food Bioprocess Technol. 2022, 15, 978–997. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Hosokawa, M.; Miyashita, K. Variation in lipid components from 15 species of tropical and temperate seaweeds. Mar. Drugs 2019, 17, 630. [Google Scholar] [CrossRef]
- Pangestuti, R.; Haq, M.; Rahmadi, P.; Chun, B.S. Nutritional value and biofunctionalities of two edible green seaweeds (Ulva lactuca and Caulerpa racemosa) from Indonesia by subcritical water hydrolysis. Mar. Drugs 2021, 19, 578. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Domingues, M.R. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Al-Adilah, H.; Al-Sharrah, T.K.; Al-Bader, D.; Ebel, R.; Küpper, F.C.; Kumari, P. Assessment of Arabian Gulf seaweeds from Kuwait as sources of nutritionally important polyunsaturated fatty acids (PUFAs). Foods 2021, 10, 2442. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Current knowledge and challenges in extraction, characterization and bioactivity of seaweed protein and seaweed-derived proteins, Chapter Nine. In Advances in Botanical Research; Bourgougnon, N., Ed.; Elsevier: Amsterdam, The Netherland, 2020; Volume 95, pp. 289–326. [Google Scholar] [CrossRef]
- Dhaouafi, J.; Romdhania, M.; Deracinoisa, B.; Flahauta, C.; Nedjara, N.; Baltic, R. Fractionation and identification of bioactive peptides from red macroalgae protein hydrolysates: In silico analysis and in vitro bioactivities. Biocat. Agric. Biotechnol. 2024, 58, 103211. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. Seaweed proteins. In Proteins in Food Processing; Yada, Y.R., Ed.; Elsevier: Amsterdam The Netherlands, 2018; Volume 2, pp. 245–262. [Google Scholar] [CrossRef]
- Rawiwan, P.; Peng, Y.; Paramayuda, Y.G.P.B.; Quek, S.Y. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Feng, X.; Liao, D.; Sun, L.; Wu, S.; Lan, P.; Wang, Z.; Li, C.; Zhou, Q.; Lu, Y.; Lan, X. Affinity Purification of Angiotensin Converting Enzyme Inhibitory Peptides from Wakame (Undaria pinnatifida) Using Immobilized ACE on Magnetic Metal Organic Frameworks. Mar. Drugs 2021, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, E.S.; Ünüvar, Ö.C.; Aydın, M. Identification of alternative oxidase encoding genes in Caulerpa cylindracea by de novo RNA-Seq assembly analysis. Mar. Genom. 2019, 46, 41–48. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidant 2022, 11, 176. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.M.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- O’Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of protein from four different seaweeds using three different physical pre-treatment strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Sonchaeng, U.; Wongphan, P.; Pan-utai, W.; Paopun, Y.; Kansandee, W.; Satmalee, P.; Tamtin, M.; Kosawatpat, P.; Harnkarnsujarit, N. Preparation and Characterization of Novel Green Seaweed Films from Ulva rigida. Polymers 2023, 15, 3342. [Google Scholar] [CrossRef]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Teixeira, C.; Abreu, H.; Silva, J.; Costas, B.; Kiron, V.; Valente, L.M.P. Nutritional value, antimicrobial and antioxidant activities of micro- and macroalgae, single or blended, unravel their potential use for aquafeeds. J. Appl. Phycol. 2021, 33, 3507–3518. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the protein fraction with high antioxidant activity from red seaweed industrial solid residue after agar extraction by subcritical water treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a novel ACE-inhibitory peptide from Sargassum maclurei in hypertension and reduction of intracellular endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef]
- Reynolds, D.; Caminiti, J.; Edmundson, S.; Gao, S.; Wick, M.; Huesemann, M. Seaweed proteins are nutritionally valuable components in the human diet. Am. J. Clin. Nutr. 2022, 116, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Afraz, M.A.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.-A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L.A. Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Mondal, S.; Seo, H.; Dae Lee, K.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkwik, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, R.; Coutinho, J.A.; Faustino, M.A.F.; Neves, M.G.P.; Pinto, D.C.; Ventura, S.P. Recovery of pigments from Ulva rigida. Sep. Purif. Technol. 2021, 255, 117723. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Brain-Isasi, S.; Correa, S.; Amado-Hinojosa, J.; Buschmann, A.H.; Camus, C.; Lienqueo, M.E. Combined extraction methodology for simultaneous recovery of phycobiliproteins and agar from the red alga Gracilaria chilensis. CJ Bird, McLachlan & EC Oliveira. Algal Res. 2022, 67, 102821. [Google Scholar] [CrossRef]
- Ktari, L.; Mdallel, C.; Aoun, B.; Chebil Ajjabi, L.; Sadok, S. Fucoxanthin and Phenolic Contents of Six Dictyotales From the Tunisian Coasts With an Emphasis for a Green Extraction Using a Supercritical CO2 Method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef] [PubMed]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An Analysis of the Nutritional and Health Values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two Chlorophyta Collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Sridhar, A.; Kari, Z.A.; Téllez-Isaías, G.; Ramasamy, T. Evaluation of Phytochemical Screening, Pigment Content, In Vitro Antioxidant, Antibacterial Potential and GC-MS Metabolite Profiling of Green Seaweed Caulerpa racemosa. Mar. Drugs 2023, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Amin, N.A.; Sani, M.S.A.; Fadzillah, N.A.; Jamaludin, M.A. Carotenoid and Chlorophyll Profiles in Five Species of Malaysian Seaweed as Potential Halal Active Pharmaceutical Ingredient (API). Int. J. Adv. Sci. Eng. Inf. Techol. 2018, 8, 1610–1616. [Google Scholar]
- Babadi, F.E.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extractionby liquefied dimethyl ether. Food Bioprod. Process. 2020, 123, 296–303. [Google Scholar] [CrossRef]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Mohd Fairulnizal, M.N.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Garcia-Perez, P.; Carreira-Casais, A.; Silva, A.; Simal-Gandara, J.; Prieto, M.A. A HPLC -DAD method for identifying and estimating the content of fucoxanthin, β-carotene and chlorophyll a in brown algal extracts. Food Chem. Adv. 2022, 1, 100095. [Google Scholar] [CrossRef]
- Negreanu-Pîrjol, T.; Sîrbu, R.; Mirea, M.; Negreanu-Pîrjol, B.S. Antioxidant Activity Correlated with Chlorophyll Pigments and Magnesium Content of some Green Seaweeds. Eur. J. Nat. Sci. Med. 2020, 3, 87–96. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Sørensen, A.D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Rodríguez, P.A.; Esquivel-Solis, H.; Murillo-Álvarez, J.I.; Ascencio, F.; Campa-Córdova, Á.I.; Angulo, C. Biosprospecting potential of kelp (Laminariales, Phaeophyceae) from Baja California Peninsula: Phenolic content, antioxidant properties, anti-inflammatory, and cell viability. J. Appl. Phycol. 2019, 31, 3115–3129. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Lajili, S.; Elkaibi, M.A.; Ben Salem, Y.; Abdelhamid, A.; Muller, C.D.; Majdoub, H.; Kraiem, J.; Bouraoui, A. Optimized extraction, preliminary characterization and evaluation of the in vitro anticancer activity of phlorotannin-rich fraction from the Brown Seaweed, Cystoseira sedoides. J. Aquat. Food Prod. Technol. 2019, 28, 892–909. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Catarino, M.; Silva, A.; Mateus, N.; Cardoso, S. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drug 2019, 17, 162. [Google Scholar] [CrossRef]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. (TrAC) Trends Anal. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound–microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-Assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 2018, 53, 1711–1723. [Google Scholar] [CrossRef]
- Týskiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. (TrAC) Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- Wekre, M.E.; Kåsin, K.; Underhaug, J.; Holmelid, B.; Jordheim, M. Quantification of Polyphenols in Seaweeds: A Case Study of Ulva intestinalis. Antioxidants 2019, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Dimova, D.; Dobreva, D.; Panayotova, V.; Makedonski, L. DPPH Antiradical Activity and Total Phenolic Content of Methanol and Ethanol Extracts from Macroalgae (Ulva rigida) and Microalgae (Chlorella). Scr. Sci. Pharm. 2019, 6, 37–41. [Google Scholar] [CrossRef]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef]
- Sanger, G.; Rarung, L.K.; Kaseger, B.E.; Assa, J.R.; Agustin, A.T. Phenolic content and antioxidant activities of five seaweeds from North Sulawesi, Indonesia. AACL Bioflux 2019, 12, 2041–2050. [Google Scholar]
- Sasadara, M.M.V.; Wirawan, I.G.P. Effect of extraction solvent on total phenolic content, total flavonoid content, and antioxidant activity of Bulung sangu (Gracilaria sp.) Seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 712, 012005. [Google Scholar] [CrossRef]
- Sobuj, M.K.A.; Islam, A.; Haque, A.; Islam, M.; Alam, J.; Rafquzzaman, S.M. Evaluation of bioactive chemical composition, phenolic, and antioxidant profling of diferent crude extracts of Sargassum coriifolium and Hypnea pannosa seaweeds. J. Food Measurem. Charact. 2021, 15, 1653–1665. [Google Scholar] [CrossRef]
- El Shafay, S.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, antidiabetic, anti-inflammatory and anticancer potential of some seaweed extracts. Food Sci. Technol. 2022, 42, e20521. [Google Scholar] [CrossRef]
- Hmani, I.; Ktari, L.; Ismail, A.; M’dallel, C.; El Bour, M. Assessment of the antioxidant and antibacterial properties of red algae (Rhodophyta) from the north coast of Tunisia. Euro-Mediterr. J. Environ. Integr. 2021, 6, 13. [Google Scholar] [CrossRef]
- Nursid, N.; Khatulistiani, T.S.; Noviendri, D.; Hapsari, F.; Hardiyati, T. Total phenolic content, antioxidant activity and tyrosinase inhibitor from marine red algae extract collected from Kupang, East Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2020, 493, 012013. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef] [PubMed]
- Siangu, B.N.; Sauda, S.; John, M.K.; Njue, W.M. Antioxidant activity, total phenolic and flavonoid content of selected Kenyan medicinal plants, sea algae and medicinal wild mushrooms. Afr. J. Pure Appl. Chem. 2019, 13, 43–48. [Google Scholar] [CrossRef]
- Cadar, E.; Sirbu, R.; Ibram, A.; Ionescu, A.M. Evaluation of Total Phenolic Content in Relation to Antioxidant Activity of Brown Algae Cystoseira barbata from Black Sea. Rev. Chim. 2019, 70, 2684–2689. [Google Scholar] [CrossRef]
- Subbiah, V.; Duan, X.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative study on the effect of different drying techniques on phenolic compounds in Australian beach-cast brown seaweeds. Algal Res. 2023, 72, 103140. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jouini, M.; Amor, H.B.H.; Mzoughi, Z.; Dridi, M.; Said, R.B.; Bouraoui, A. Pytochemical analysis and evaluation of the antioxidant, anti-inflammatory, and antinociceptive potential of phlorotannin-rich fractions from three Mediterranean brown seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef]
- Bekah, D.; Thakoor, A.D.; Ramanjooloo, A.; Phul, I.C.; Botte, S.; Roy, P.; Oogarah, P.; Curpen, S.; Goonoo, N.; Bolton, J.; et al. Vitamins, minerals and heavy metals profiling of seaweeds from Mauritius and Rodrigues for food security. J. Food Comp. Anal. 2023, 115, 104909. [Google Scholar] [CrossRef]
- Lovander, M.D.; Lyon, J.D.; Parr, D.L.; Wang, J.; Parke, B.; Leddy, J. Critical Review—Electrochemical Properties of 13 Vitamins: A, Critical Review and Assessment. J. Electrochem. Soc. 2018, 165, 18–49. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Washington, DC, USA, 2019. [Google Scholar]
- Susanti, D.; Ruslan, F.S.; Shukor, M.I.; Nor, N.M.; Aminudin, N.I.; Taher, M.; Khotib, J. Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design. Molecules 2022, 27, 4459. [Google Scholar] [CrossRef] [PubMed]
- Chandra-Hioe, M.V.; Xu, H.; Arcot, J. The Efficiency of Ultrasonic-Assisted Extraction of Cyanocobalamin Is Greater than Heat Extraction. Heliyon 2020, 6, e03059. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Wahab, A.; Nahiduzzaman, M.; Mohiuddin, M.; Iqbal, M.Z.; Shakil, A.; Mamun, A.A.; Khan, S.R.; Wong, L.; Asaduzzaman, M. Seaweed farming for food and nutritional security, climate change mitigation and adaptation, and women empowerment: A review. Aquac. Fish. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Ryzhik, I.V.; Klindukh, M.P.; Dobychina, E.O. The B-group vitamins in the red alga Palmaria palmata (Barents Sea): Composition, seasonal changes and influence of abiotic factors. Algal Res. 2021, 59, 102473. [Google Scholar] [CrossRef]
- Dragomir, R.E.; Toader, D.O.; Gheoca Mutu, D.E.; Dogaru, I.A.; Răducu, L.; Tomescu, L.C.; Moleriu, L.C.; Bordianu, A.; Petre, I.; Stănculescu, R. Consequences of Maternal Vitamin D Deficiency on Newborn Health. Life 2024, 14, 714. [Google Scholar] [CrossRef]
- Soares, C.; Švarc-Gajić, J.; Oliva-Teles, M.T.; Pinto, E.; Nastić, N.; Savić, S.; Almeida, A.; Delerue-Matos, C. Mineral Composition of Subcritical Water Extracts of Saccorhiza Polyschides, a Brown Seaweed Used as Fertilizer in the North of Portugal. J. Mar. Sci. Eng. 2020, 8, 244. [Google Scholar] [CrossRef]
- Admassu, H.; Abera, T.; Abraha, B.; Yang, R.; Zhao, W. Proximate, mineral and amino acid composition of dried laver (Porphyra spp.) seaweed. J. Artif. Intell. Res. 2018, 6, 149–154. [Google Scholar]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 1592–1607. [Google Scholar] [CrossRef]
- Díaz, O.; Tapia, Y.; Muñoz, O.; Montoro, R.; Velez, D.; Almela, C. Total and inorganic arsenic concentrations in different species of economically important algae harvested from coastal zones of Chile. Food Chem. Toxicol. 2012, 50, 744–749. [Google Scholar] [CrossRef]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive exploration of marine algae diversity, bioactive compounds, health benefits, regulatory issues, and food and drug applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- Silva, M.; Avni, D.; Varela, J.; Barreira, L. The Ocean’s Pharmacy: Health Discoveries in Marine Algae. Molecules 2024, 29, 1900. [Google Scholar] [CrossRef] [PubMed]
- Mabate, B.; Daub, C.D.; Pletschke, B.I.; Edkins, A.L. Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells. Mar. Drugs 2023, 21, 203. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown Algae-Derived Fucoidan Exerts Oxidative Stress-Dependent Antiproliferation on Oral Cancer Cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Y.; Liu, S.; Shao, Z.; Chu, X.; Mao, W. Immunomodulatory Activity In Vitro and In Vivo of a Sulfated Polysaccharide with Novel Structure from the Green Alga Ulva conglobata Kjellman. Mar. Drugs 2022, 20, 447. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. [Google Scholar] [CrossRef]
- Bellan, D.L.; Biscaia, S.M.P.; Rossi, G.R.; Cristal, A.M.; Gonçalves, J.P.; Oliveira, C.C.; Simas, F.F.; Sabry, D.A.; Rocha, H.A.O.; Franco, C.R.C.; et al. Green does not always mean go: A sulfated galactan from Codium isthmocladum green seaweed reduces melanoma metastasis through direct regulation of malignancy features. Carbohydr. Polym. 2020, 250, 116869. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, G.; Wu, D.; Liu, D.; You, L.; Hogger, P.; Simal-Gandara, J.; Wang, M.; da Costa, J.G.M.; Marunaka, Y.; et al. The algal polysaccharide ulvan suppresses growth of hepatoma cells. Food Front. 2020, 1, 83–101. [Google Scholar] [CrossRef]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. 2020, 108, 254–266. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.; Kim, S.C.; You, S.; Lee, C.W.; Shin, J.; Park, Y.I. Glucuronorhamnoxylan from Capsosiphon fulvescens inhibits the growth of HT29 human colon cancer cells in vitro and in vivo via induction of apoptotic cell death. Int. J. Biol. Macromol. 2019, 124, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Mendes Marques, M.L.; Presa, F.B.; Viana, R.L.S.; Costa, M.S.S.P.; Amorim, M.O.R.; Bellan, D.L.; Alves, M.G.C.F.; Costa, L.S.; Trindade, E.S.; Rocha, H.A.O. Anti-Thrombin, Anti-Adhesive, Anti-Migratory, and Anti-Proliferative Activities of Sulfated Galactans from the Tropical Green Seaweed, Udotea flabellum. Mar. Drugs 2019, 17, 5. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.S.; Raja, A.B.; Deshpande, M.N.; Chakrapani, I.S.; Priyadarsini, A.I.; Anbazhagan, M.; Sampath, V. New record of Dictyota bartayresiana, a marine brown algal species revealed from rich seaweed diversity area of south India. J. Coast. Life Med. 2023, 11, 825–830. [Google Scholar]
- Wang, J.J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Mahendran, S.; Sankaralingam, S.; Sethupathi, S.M. Evaluation of antioxidant and cytotoxicity activities of polyphenol extracted from brown seaweed Sargassum tenerrimum biomass. Biomass Conv. Bioref. 2024, 14, 2063–2069. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.-S.; Tomescu, C.L.; Ionescu, A.-M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hamzeh, A.; Tabarsa, M.; Cravotto, G. Cellular antioxidant and emulsifying activities of fucoidan extracted from Nizamuddinia zanardinii using different green extraction methods. J. Food Process. Preserv. 2022, 46, e17238. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K. Isolation, Characterization, and Antioxidant Activity Evaluation of a Fucoidan from an Enzymatic Digest of the Edible Seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Jeon, Y.J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K. Pharmacological potential of sulphated polygalacto-pyranosyl-fucopyranan from the brown seaweed Sargassum wightii. J. Appl. Phycol. 2018, 30, 1971–1988. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Fu, S.; Shen, Z.; Zong, W.; Xia, Z.; Zhan, Z.; Jiang, X. Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice. Mar. Drugs 2021, 19, 539. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.Y.; Ding, L.P.; Liang, H.; Tu, P.F.; Zhang, Q.Y. Antioxidant terpenoids from the red alga Laurencia tristicha. Nat. Prod. Res. 2020, 35, 5048–5054. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Sun, X. Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2585–2596. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Cadar, E.; Pesterau, A.-M.; Sirbu, R.; Negreanu-Pirjol, B.S.; Tomescu, C.L. Jellyfishes—Significant Marine Resources with Potential in the Wound-Healing Process: A Review. Mar. Drugs 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Pesterau, A.-M.; Prasacu, I.; Ionescu, A.-M.; Pascale, C.; Dragan, A.-M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Dharani, G.; Paramasivam, A. Evaluation of antimicrobial, antioxidant and cytotoxicity potential of R-phycoerythrin extracted from Gracilaria corticata seaweed. Curr. Res. Green Sustain. Chem. 2023, 6, 100352. [Google Scholar] [CrossRef]
- Yalçın, S.; Karakaş, Ö.; Okudan, E.Ş.; Başkan, K.S.; Çekiç, S.D.; Apak, R. HPLC detection and antioxidant capacity determination of brown, red and green algal pigments in seaweed extracts. J. Chromatogr. Sci. 2021, 59, 325–337. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less Polar Compounds and Targeted Antioxidant Potential (In Vitro and In Vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef]
- Ulagesan, S.; Nam, T.-J.; Choi, Y.-H. Extraction and Purification of R-Phycoerythrin Alpha Subunit from the Marine Red Algae Pyropia yezoensis and Its Biological Activities. Molecules 2021, 26, 6479. [Google Scholar] [CrossRef]
- Jerković, I.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects In Vitro and In Vivo (Zebrafish Model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef]
- Negreanu-Pîrjol, T.; Negreanu-Pîrjol, B.S.; Popoviciu, D.R.; Roncea, F.N. Preliminary Data Regarding Pharmaceutical Forms Type Gels Based on Marine Algae Extracts with Antioxidant Activity. Eur. J. Nat. Sci. Med. 2021, 4, 55–65. [Google Scholar] [CrossRef]
- Karkhaneh, Y.M.; Seyed, H.M.; Mashinchian, M.A.; Ghassempour, A.R. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, persian gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Ghaliaoui, N.; Mokrane, H.; Hazzit, M.; Hadjadj, M.; Otmani, F.S.; Touati, S.; Seridi, H. Impact of freezing and drying preprocessing on pigments extraction from the brown seaweed Phyllaria reniformis collected in algerian coast. Carphatian J. Food Sci. Technol. 2020, 12, 81–94. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.; Moon, I.; Hong, Y.K. Neuroprotective effects of fucoxanthin and its derivative fucoxanthinol from the phaeophyte Undaria pinnatifida attenuate oxidative stress in hippocampal neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Fu, Y.; Jiao, H.; Sun, J.; Okoye, C.O.; Zhang, H.; Li, Y.; Lu, X.; Wang, Q.; Liu, J. Structure-activity relationships of bioactive polysaccharides extracted from macroalgae towards biomedical application: A review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Šimat, V.; Botić, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Čagalj, M.; Skroza, D. Bioactive Phenolic Metabolites from Adriatic Brown Algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef]
- Hassan, S.; Hamed, S.; Almuhayawi, M.; Hozzin, W.; Selim, S.; AbdElgawad, H. Bioactivity of Ellagic Acid and Velutin: Two Phenolic Compounds Isolated from Marine Algae. Egypt. J. Bot. 2021, 61, 219–231. [Google Scholar] [CrossRef]
- Alkhalaf, M.I. Chemical composition, antioxidant, anti-inflammatory and cytotoxic effects of Chondrus crispus species of red algae collected from the Red Sea along the shores of Jeddah city. J. King Saud Univ.–Sci. 2021, 33, 101210. [Google Scholar] [CrossRef]
- Ak, I.; Turker, G. Antioxidant Activity of Five Seaweed Extracts. New Know. J. Sci. 2018, 7, 149–155. [Google Scholar]
- Alghazwi, M.; Charoensiddhi, S.; Smid, S.; Zhang, W. Impact of Ecklonia radiata Extracts on the Neuroprotective Activities against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. J. Funct. Foods 2020, 68, 103893. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Begbie, A.J.; Pukala, T.L.; Smid, S.D. Ecklonia radiata Extract Containing Eckol Protects Neuronal Cells against Aß1-42evoked Toxicity and Reduces Aggregate Density. Food Funct. 2020, 11, 6509–6516. [Google Scholar] [CrossRef]
- Yang, E.J.; Mahmood, U.; Kim, H.; Choi, M.; Choi, Y.; Lee, J.P.; Cho, J.Y.; Hyun, J.W.; Kim, Y.S.; Chang, M.J.; et al. Phloroglucinol Ameliorates Cognitive Impairments by Reducing the Amyloid β Peptide Burden and Pro-Inflammatory Cytokines in the Hippocampus of 5XFAD Mice. Free Radic. Biol. Med. 2018, 126, 221–234. [Google Scholar] [CrossRef]
- Le, A.T.; Prabhu, N.S.; Almoallim, H.; Awad Alahmadi, T. Assessment of nutraceutical value, physicochemical, and anti-inflammatory profile of Odonthalia floccose and Odonthalia dentata. Environ. Res. 2024, 259, 119487. [Google Scholar] [CrossRef]
- Subramoni, M.; Kumar, S.; Abraham, J. Nutritional content of selected macroalgae of the south-west coast of India. Egypt. J. Phycol. 2023, 24, 161–193. [Google Scholar] [CrossRef]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Bactericidal Potentiality of Purified Terpenoid Extracts from the Selected Sea Weeds and its Mode of Action. J. Trop. Life Sci. 2020, 10, 197–205. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Chapter 8—Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Perspective paper Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Anjali, K.P.; Sangeetha, B.M.; Devi, G.; Raghunathan, R.; Dutta, S. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine-a comparative study. J. Phytochem. Phatobiol. 2019, 200, 111622. [Google Scholar] [CrossRef]
- da Graça Pedrosa de Macena, L.; dos Santos Corrêa Amorim, L.; Francisco Corrêa de Souza e Souza, K.; Dantas Pereira, L.; Cirne dos Santos, C.C.; de Souza Barros, C.; Nunes de Palmer Paixão, I.C. Antiviral activity of terpenes isolated from marine brown seaweeds against herpes simplex virus type 2. Nat. Prod. Res. 2023, 39, 712–717. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; de Souza Barros, C.; de Oliveira, M.C. In vitro Studies on The Inhibition of Replication of Zika and Chikungunya Viruses by Dolastane Isolated from Seaweed Canistrocarpus cervicornis. Sci. Rep. 2020, 10, 8263. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-assisted extraction of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae. J. Food Process. Preserv. 2021, 45, e15929. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Lee, H.G.; Nagahawatta, D.P.; Jayawardhana, H.; Song, K.M.; Choi, Y.S.; Jeon, Y.J.; Kang, M.C. Fucoidan from Sargassum autumnale Inhibits Potential Inflammatory Responses via NF-kappaB and MAPK Pathway Suppression in Lipopolysaccharide-Induced RAW264.7 Macrophages. Mar. Drugs 2023, 21, 374. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Kim, K.N.; Oh, J.Y.; Ahn, G. The Anti-Inflammatory Effect of Low MolecularWeight Fucoidan from Sargassum siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via Inhibiting NFkappaB/MAPK Signaling Pathways. Mar. Drugs 2023, 21, 347. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Han, E.J.; Oh, G.W.; Jung, W.K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-alpha/IFN-gamma-Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinie, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural Characterization and In Vivo Anti-Inflammatory Activity of Fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Nagahawatta, D.P.; Lee, H.G.; Lu, Y.A.; Vaas, A.; Abeytunga, D.T.U.; Nanayakkara, C.M.; Lee, D.S.; Jeon, Y.J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via BlockingTLR/NF-Kappab Signal Transduction. Mar. Drugs 2020, 18, 601. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.G.; Huang, C.; Oh, J.Y.; Fu, X.; Wang, K.; Ahn, G.; Xu, J.; Gao, X.; Jeon, Y.J. Anti-Inflammatory Effect of Sulfated Polysaccharides Isolated from Codium fragile In Vitro in RAW 264.7 Macrophages and In Vivo in Zebrafish. Mar. Drugs 2022, 20, 391. [Google Scholar] [CrossRef]
- Chen, X.; Ni, L.; Fu, X.; Wang, L.; Duan, D.; Huang, L.; Xu, J.; Gao, X. Molecular Mechanism of Anti-Inflammatory Activities of a Novel Sulfated Galactofucan from Saccharina japonica. Mar. Drugs 2021, 19, 430. [Google Scholar] [CrossRef]
- Je, J.G.; Lee, H.G.; Fernando, K.H.N.; Jeon, Y.J.; Ryu, B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.J. In Vitro and In Vivo Anti-Inflammatory Effects of SulfatedPolysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Mar. Drugs 2021, 19, 277. [Google Scholar] [CrossRef]
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-kappaB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. [Google Scholar] [CrossRef]
- Pereira, T.; Horta, A.; Barroso, S.; Mendes, S.; Gil, M.M. Study of the Seasonal Variations of the Fatty Acid Profiles of Selected Macroalgae. Molecules 2021, 26, 5807. [Google Scholar] [CrossRef]
- Berneira, L.; da Silva, C.; Poletti, T. Evaluation of the volatile composition and fatty acid profile of seven Antarctic macroalgae. J. Appl. Phycol. 2020, 32, 3319–3329. [Google Scholar] [CrossRef]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Yu, J.B.; Kang, M.C.; Jeon, Y.J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 6, 349–358. [Google Scholar] [CrossRef]
- Cheng, Y.; Pan, X.; Wang, J.; Li, X.; Yang, S.; Yin, R.; Ma, A.; Zhu, X. Fucoidan Inhibits NLRP3 Inflammasome Activation by Enhancing p62/SQSTM1-Dependent Selective Autophagy to Alleviate Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 3186306. [Google Scholar] [CrossRef]
- Kumagai, Y.; Toji, K.; Katsukura, S.; Morikawa, R.; Uji, T.; Yasui, H.; Shimizu, T.; Kishimura, H. Characterization of ACE Inhibitory Peptides Prepared from Pyropia pseudolinearis Protein. Mar. Drugs 2021, 19, 200. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Harnedy-Rothwell, P.A.; Lafferty, R.A. Macroalgal protein hydrolysates from Palmaria palmata influence the ‘incretin effect’ in vitro via DPP-4 inhibition and upregulation of insulin, GLP-1 and GIP secretion. Eur. J. Nutr. 2021, 60, 4439–4452. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide Identification from a Porphyra Dioica Protein Hydrolysate with Antioxidant, Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, F.; Yan, Y.; Jin, J.; Quan, Z.; Tong, H.; Du, J. Fucoidan derived from Sargassum pallidum alleviates metabolism disorders associated with improvement of cardiac injury and oxidative stress in diabetic mice. Phytother. Res. 2023, 37, 4210–4223. [Google Scholar] [CrossRef]
- Thambi, A.; Chakraborty, K. A novel anti-hyperglycemic sulfated pyruvylated polysaccharide from marine macroalga Hydropuntia edulis. Nat. Prod. Res. 2023, 37, 2987–2999. [Google Scholar] [CrossRef]
- Xavier, J.; Jose, J. Study of mineral and nutritional composition of some seaweeds found along the coast of Gulf of Mannar, India. Plant Sci. Today 2020, 7, 631–637. [Google Scholar] [CrossRef]
- de Carvalho, M.M.; Noseda, M.D.; Juliana, C.C.; Dallagnol, J.C.C.; Ferreira, L.G.; Ducatti, D.R.B.; Gonçalves, A.G.; de Freitas, R.A.; Duarte, M.E.R. Conformational analysis of ulvans from Ulva fasciata and their anticoagulant polycarboxylic derivatives. Int. J. Bio. Macromol. 2020, 162, 599–608. [Google Scholar] [CrossRef]
- Chagas, S.F.D.; Lima, G.C.; dos Santos, V.I.N.; Costa, L.E.C.; de Sousa, W.M. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Liu, S.; Yu, H.; Li, R.; Wang, X. Preparation of low molecular weight Sargassum fusiforme polysaccharide and its anticoagulant activity. J. Oceanol. Limnol. 2018, 36, 882–891. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid_production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Mohan, E.H.; Madhusudan, S.; Baskaran, R. The sea lettuce Ulva sensu lato: Future food with health-promoting bioactivities. Algal Res. 2023, 71, 103069. [Google Scholar] [CrossRef]
- Koseki, K.; Yoshimura, R.; Ido, K.; Katsuura, K.; Bito, T.; Watanabe, F. Determination of Vitamin B12 and Folate Compounds in Commercially Available Edible Seaweed Products. Front. Biosci. 2023, 15, 10. [Google Scholar] [CrossRef]
- Ownsworth, E.; Selby, D.; Ottley, C.J.; Unsworth, E.; Raab, A.; Feldmann, J.; Bücker, P. Tracing the natural and anthropogenic influence on the trace elemental chemistry of estuarine macroseaweeds and the implications for human consumption. Sci. Total Environ. 2019, 685, 259–272. [Google Scholar] [CrossRef]
- Cadar, E.; Mustafa, A.; Tomescu, A.; Cherim, M. Studies Regarding Polluting Agents in Black Sea Algae. J. Sci. Arts 2018, 42, 255–264. [Google Scholar]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Speranza, L.G. Algae as food in Europe: An overview of species diversity and their application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Belkacemi, L.; Belalia, M.; Djendara, A.; Bouhadda, Y. Antioxidant and antibacterial activities and dentification of bioactive compounds of various extracts of Caulerpa racemosa from Algerian coast. Asian Pac. J. Trop. Biomed. 2020, 10, 87–94. [Google Scholar] [CrossRef]
- Geranpour, M.; Seid, E.A.; Jafari, M. Recent advances in the spray drying encapsulation of essential fatty acids and functional oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Cornish, L. Those curious and delicious seaweeds: A fascinating voyage from biology to gastronomy. Phycologia 2019, 58, 578–579. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Barot, M.; Nirmal Kumar, J.I.; Kumar, R.N. An Evaluation of the Nutritional Composition of Seaweeds as Potential Source of Food and Feed. Natl. Acad. Sci. Lett. 2019, 42, 459–464. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]

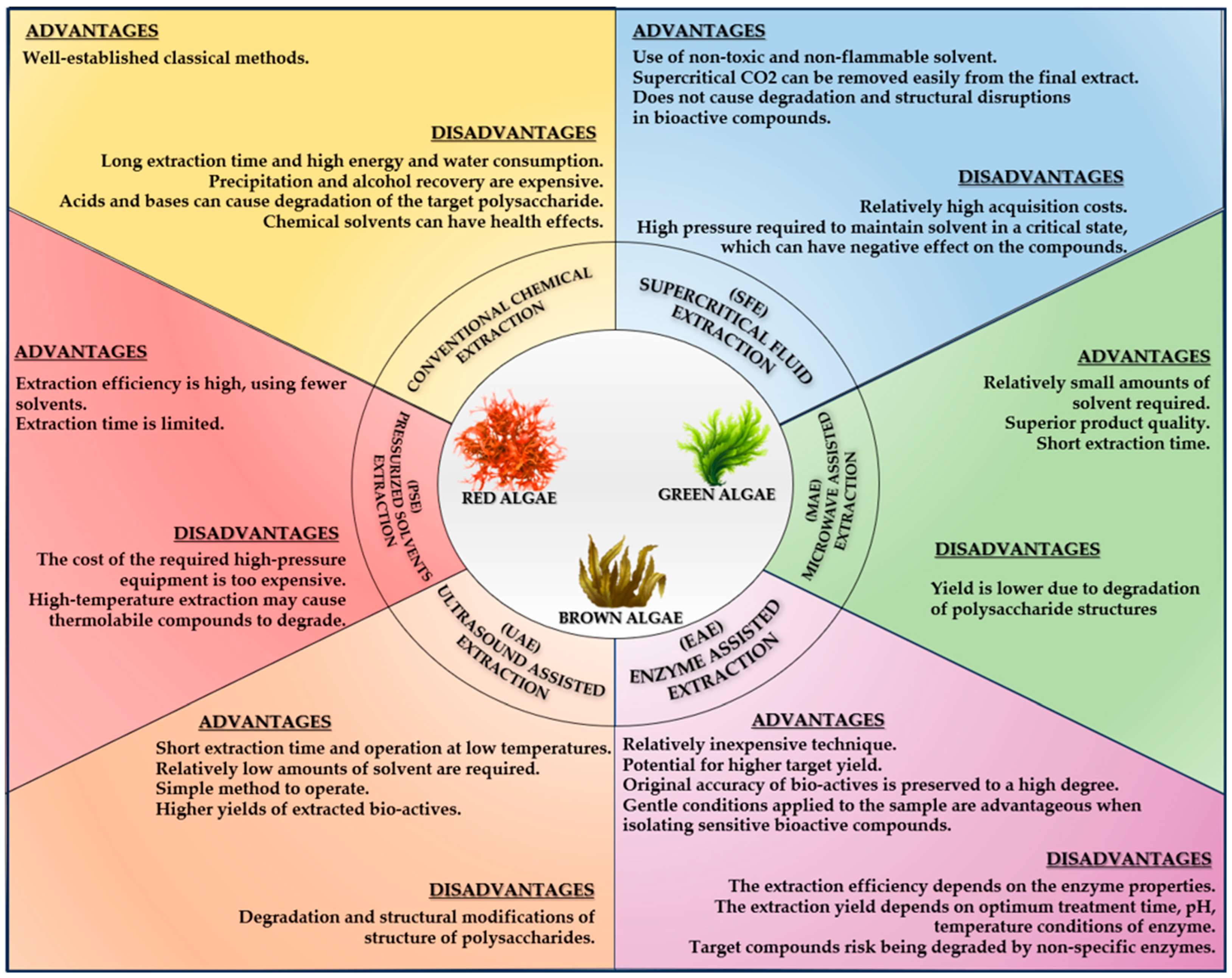
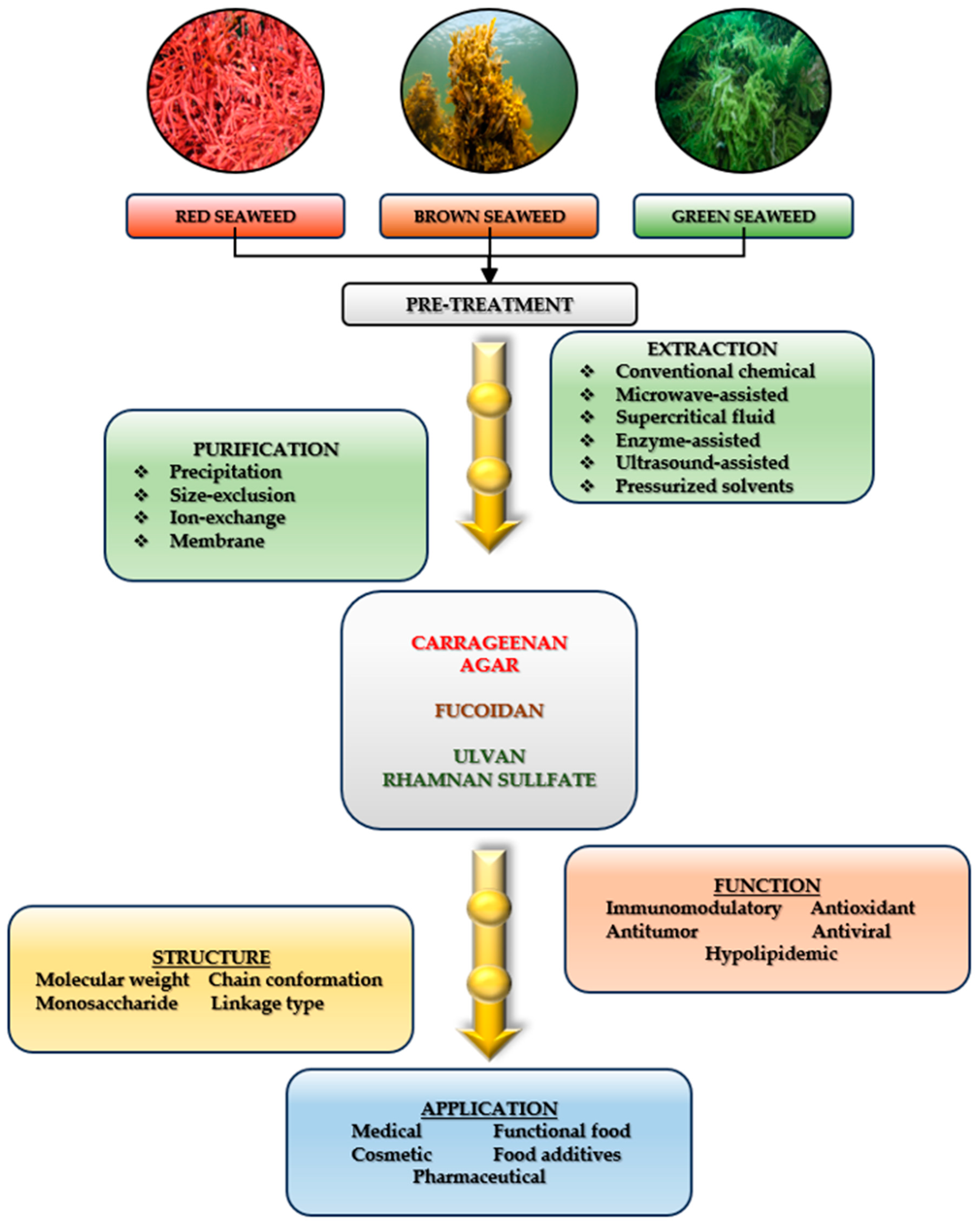




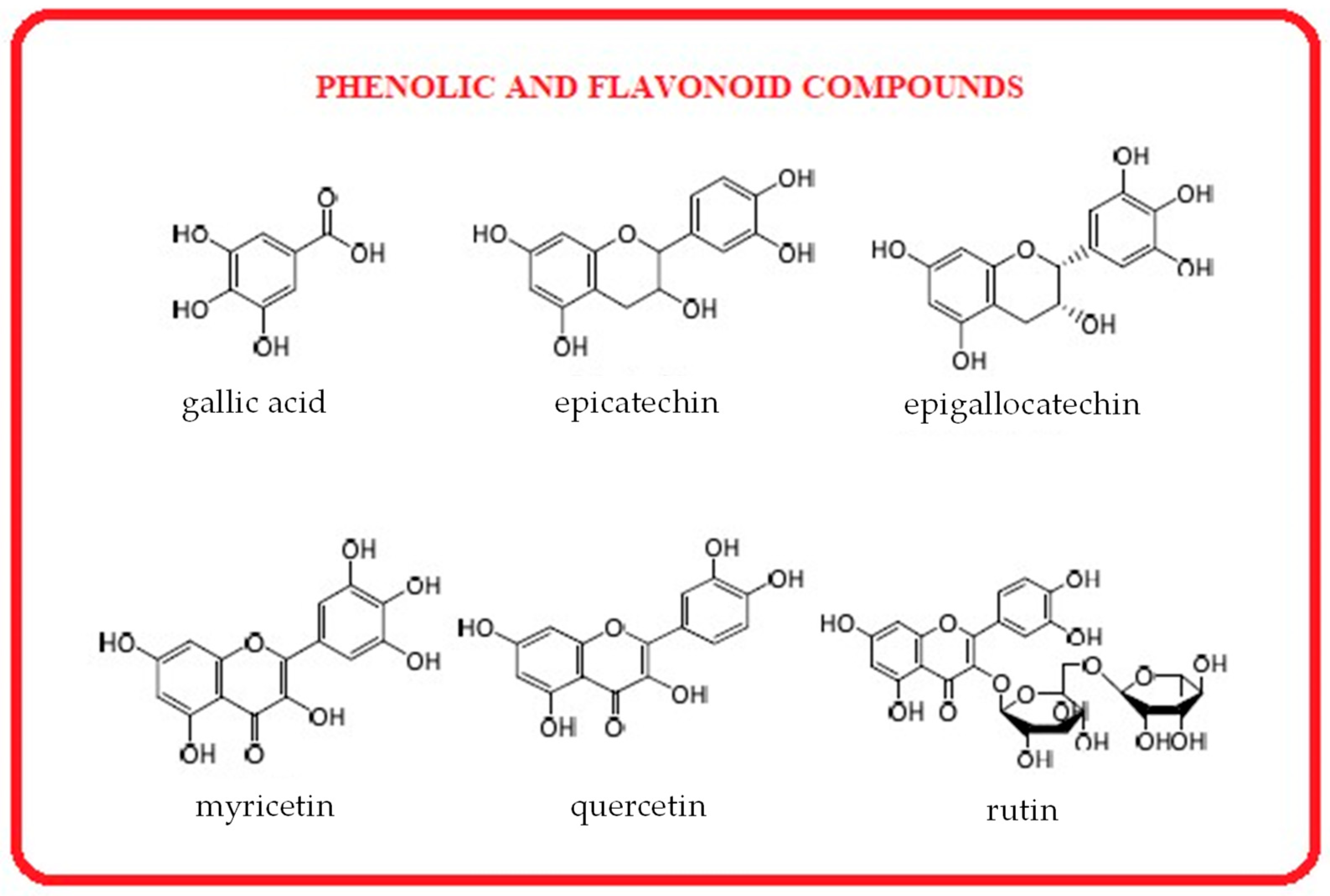


| Green algae | ||||||||||||
| Ulva lactuca | Ulva lactuca | Ulva rigida | Ulva lactuca (as Ulva fasciata) | Cladophora vagabunda | Cladophora vagabunda | Acrosiphonia orientalis | Caulerpa scalpelliformis | Caulerpa lentillifera | Ulva intestinalis (as Enteromorpha intestinalis) | Ulva flexuosa (as Enteromorpha flexuosa) | Ulva intestinalis (as Enteromorpha intestinalis) | |
| Black Sea | Arabian Sea | Atlantic waters | Indian waters | Black Sea | Black Sea | Arabian Sea | Arabian Sea | Atlantic waters | Black Sea | Indian waters | Gulf Gökova Aegean Sea | |
| Moisture (%) | 10.85 ± 0.26 | 25.0 ± 1.0 | - | - | 5.71 ± 0.92 | 11.98 ± 0.84 | 19 ± 2.0 | 20.0 ± 1.0 | - | 11.98 ± 0.84 | - | 12.14 ± 1.11% |
| Ash (%) | 23.62 ± 0.59 | 16.0 ± 2.0 | 28.6 | 27.0 ± 0.024 | 26.38 ± 0.31 | 24.63 ± 0.84 | 22 ± 2.0 | 15.0 ± 2.0 | 24–37 | 25.65 ± 0.98 | 32.2 ± 0.92 | 14.81 ± 0.23 |
| Sulphates (%) | 70.46 ± 1.87 | - | - | - | 67.92 ± 0.53 | 68.65 ± 1.78 | - | - | - | 67.68 ± 1.63 | - | - |
| Nitrogen (%) | 2.26 ± 0.48 | 66.0 ± 4.0 | - | - | 2.45 ± 0.02 | 2.39 ± 0.26 | 41 ± 3.0 | 51.0 ± 2.0 | - | 2.18 ± 0.39 | - | - |
| Protein (%) | 14.13 ± 0.85 | 6.0 ± 1.0 | 18–19 | 22.7 ± 0.22 | 15.43 ± 0.36 | 14.94 ± 0.92 | 7 ± 0.2 | 6.0 ± 1.0 | 10–13 | 13.63 ± 0.96 | 17.29 ± 1.24 | 13.42 ± 1.31 |
| Lipid (%) | 2.78 ± 0.69 | 1.0 ± 0.5 | 0.9–2.0 | 0.89 ± 0.12 | 3.85 ± 0.47 | 2.86 ± 0.75 | 3.0 ± 0.2 | 4.0 ± 0.5 | 0.86–1.11 | 1.72 ± 0.56 | 0.76 ± 0.24 | 1.31 ± 0.5 |
| Carbohydrates (%) | 58.36 ± 1.64 | 56.0 ± 2.0 | 43–56 | 32.0 ± 0.04 | 48.45 ± 0.5 | 62.37 ± 1.74 | 16 ± 2.0 | 23 ± 2.0 | 38–59 | 60.68 ± 1.36 | 30.1 ± 0.18 | 58.03 ± 2.31 |
| Total dietary fiber (%) | 60.56 ± 1.1 | 11.0 ± 0.9 | 38–41 | - | 61.56 ± 1.5 | 63.35 ± 1.24 | 27.0 ± 0.5 | 24.0 ± 2.2 | 33 | 59.66 ± 1.95 | - | 52.36 ± 3.26 |
| References | [49] | [53] | [54] | [55] | [30] | [49] | [53] | [53] | [54] | [49] | [55] | [56] |
| Red algae | ||||||||||||
| Halymenia porphyriformis | Palmaria palmata | Porphyra umbilicalis | Acanthophora spicifera | Gracilaria edulis | Ceramium virgatum (as Ceramium rubrum) | Jania pedunculata var. adhaerens (as Jania adhaerens) | Gracilaria corticata | Scinaia carnosa | Gracilaria edulis | Gracilaria corticata | Laurencia obtusa | |
| Arabian Sea | Atlantic waters | Atlantic waters | Indian waters | Indian waters | Black Sea | Sri Lanka coastal area | Sri Lanka coastal area | Arabian Sea | Southeast coast of India | Southeast coast of India | Red sea coast | |
| Moisture (%) | 19.0 ± 1.1 | - | - | - | - | 11.01 ± 0.13 | 92.96 ± 0.27 | 96.32 ± 0.02 | 21.0 ± 2.0 | 10.40 ± 0.69 | 8.40 ± 0.65 | - |
| Ash (%) | 17.0 ± 1.2 | 12–37 | 12 | 21.0 ± 0.08 | 22.8 ± 0.04 | 13.83 ± 1.68 | 05.01 ± 0.01 | 07.15 ± 0.01 | 46.0 ± 2.0 | 7.36 ± 0.39 | 8.10 ± 0.49 | - |
| Sulphates (%) | - | - | - | - | - | 75.16 ± 1.56 | - | - | - | - | - | - |
| Nitrogen (%) | 75. 0 ± 8.0 | - | - | - | - | 3.19 ± 0.41 | - | - | 45.0 ± 8.0 | - | - | - |
| Protein (%) | 3.0 ± 1.0 | 8–35 | 29–39 | 20.2 ± 0.12 | 18.04 ± 0.03 | 19.94 ± 2.56 | 29.47 ± 0.15 | 28.70 ± 0.46 | 2.0 ± 0.1 | 25.29 ± 0.67 | 22.84 ± 0.87 | 5.41 ± 0.11 |
| Lipid (%) | 1.0 ± 0.4 | 0.7–3 | 0.3 | 0.48 ± 0.04 | 0.72 ± 0.04 | 3.43 ± 0.25 | 1.52 ± 0.08 | 1.66 ± 0.18 | 3.0 ± 1.0 | 4.76 ± 0.73 | 7.07 ± 0.33 | 3.04 ± 0.12 |
| Carbohydrate (%) | 22 ± 1.0 | 46–56 | 43.0 | 26.2 ± 0.02 | 24.8 ± 0.12 | 51.90 ± 4.35 | - | - | 30.0 ± 1.0 | 4.71 ± 0.60 | 8.30 ± 0.49 | 20.17 ± 0.1 |
| Total dietary fiber (%) | 3.0 ± 0.2 | 29–46 | 29–35 | - | - | - | 56.81 ± 0.38 | 59.15 ± 0.76 | 5.0 ± 0.5 | - | - | - |
| References | [53] | [54] | [54] | [55] | [55] | [57] | [58] | [58] | [53] | [59] | [59] | [60] |
| Brown algae | ||||||||||||
| Sargassum linearifolium | Fucus vesiculosus | Laminaria digitata | Undaria pinnatifida | Saccharina latissima | Padina gymnospora | Gongolaria barbata (as Cystoseira barbata) | Sargassum ilicifolium | Sargassum polycystum | Sargassum oligocystum | Himanthalia elongata | Sargassum asperifolium | |
| Arabian Sea | Atlantic waters | Atlantic waters | Atlantic warers | Atlantic waters | Indian waters | Black Sea | Sri Lanka coastal area | Sri Lanka coastal area | Indo-West Pacific area | North-eastern Atlantic coast | Red Sea at Hurghada Coast | |
| Moisture (%) | 14.0 ± 1.0 | - | - | - | - | - | 9.27 ± 0.42 | 95.92 ± 0.37 | 92.58 ± 0.32 | - | - | 88.08 |
| Ash (%) | 24 ± 2.0 | 14–30 | 38 | 26–40 | 34.78 | 23.2 ± 0.03 | 17.63 ± 1.73 | 13.15 ± 0.41 | 18.48 ± 0.21 | 21.91 ± 0.28 | - | 19.60 |
| Sulphates (%) | - | - | - | - | - | - | - | - | - | - | - | - |
| Nitrogen (%) | 56 ± 3.0 | - | - | - | - | - | - | - | - | - | - | - |
| Protein (%) | 5 ± 1.0 | 3–14 | 8.15 | 12–23 | 6–6.26 | 12.07 ± 0.78 | 14.13 ± 2.11 | 28.02 ± 0.68 | 16.15 ± 0.33 | 9.26 ± 0.16 | 5.4 ± 0.46 | 3.50 |
| Lipid (%) | 7 ± 0.5 | 1.9 | 1.9 | 1.05–45 | 0.5–1.1 | 1.4 ± 0.82 | 1.03 ± 0.54 | 4.45 ± 0.12 | 4.50 ± 0.21 | 3.51 ± 0.21 | 17.06 ± 1.50 | 0.17 |
| Carbohydrate (%) | 53.0 ± 3.0 | 46.8 | 48 | 45–51 | 52–61 | 28.0 ± 0.12 | 58.05 ± 0.72 | - | - | 52.06 | 26.3 | 39.25 |
| Total dietary fiber (%) | 12 ± 0.5 | 43–59 | 37 | 16–51 | 30 | - | - | 51.46 ± 0.53 | 54.49 ± 0.95 | - | 53.3 ± 3.5 | - |
| References | [53] | [54] | [54] | [54] | [54] | [55] | [57] | [58] | [58] | [61] | [62] | [63] |
| Polysaccharides | Species | Yield | Method | References |
|---|---|---|---|---|
| Carrageenans | Kappaphycopsis cottonii (formerly Eucheuma cottonii)—red algae | 67.86% | Bead mill extraction | [91] |
| Chondracantus canaliculatus—red algae | 45.05% | UAE—Ultrasound-assisted extraction | [92] | |
| Euchema cottonii—red algae | 30.20% | Conventional extraction method of semi-refined carrageenan | [93] | |
| Gelidium corneum (formerly Gelidium sesquipedale)—red algae | 8.4% | Solubilization in hot water and alkali treatment | [94] | |
| Agars | Gelidium corneum (formerly Gelidium sesquipedale)—red algae | 10–12% | Sonication with hot water treatment | [95] |
| Gracilariopsis lemaneiformis—red algae | 12.32% | Alkaline extraction | [96] | |
| Gracilariopsis lemaneiformis—red algae | 2.52% | EAE—Enzyme extraction | [96] | |
| Gracilariopsis lemaneiformis—red algae | 5.33% | EA—Enzyme-assisted extraction | [96] | |
| Nizamuddinia zanardinii—brown algae | 3.51% | UA—Ultrasound-assisted extraction | [97] | |
| Fucus distichus subsp. evanescens (formerly Fucus evanescens)—brown algae | 4.44% | UAE—Ultrasound-assisted extraction | [98] | |
| Fucoidans | Nizamuddinia zanardinii—brown algae | Alcalase, cellulase, flavourzyme, viscozyme, hot water 10–15% | EAE—Enzyme-assisted extraction | [99] |
| Sargassum fusiforme—brown algae | 11.24% | Conventional extraction with dilute hydrochloric acid | [100] | |
| Sargassum wightii—brown algae | 14.61% | UAE—Ultrasound-assisted extraction | [101] | |
| Alginates | Ascophyllum nodosum—brown algae | 18.3–23.7% | UAE and Conventional method (HCl) | [102] |
| Turbinaria triquetra—brown algae | 22.2% | Conventional method (formaldehyde) | [103] | |
| Gongolaria barbata—brown algae | 19% | Conventional method (HCl) | [104] | |
| Ulva fenestrata—green algae | 9.03% | EAE–Enzyme assisted extraction | [105] | |
| Ulva fenestrata—green algae | 8.65% | UAE–Ultrasound assisted extraction | [105] | |
| Ulvans | Ulva fenestrata—green algae | 17.92% | U-EAE—combined ultrasound with enzymatic extraction | [105] |
| Ulva lactuca—green algae | 36.4% | Conventional extraction (strong acid produces higher extraction yields) | [106] | |
| Ulva prolifera—green algae | 36.38% | Microwave-assisted hydrothermal extraction | [107] | |
| Ulva intestinalis—green algae | 12% | Conventional extraction (ethanol) | [108] |
| Green Algae | Red Algae | Brown Algae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Algae | Ulva rigida | Ulva rigida | Palmaria palmata | Chondrus crispus | Porphyra dioica | Gracilaria gracilis | Gelidium corneum | Fucus spiralis | Ascophylum nodosum | Undaria pinnatifida | Sargassum mcclurei |
| Proteins (% dw) | 5.67 | 9.6 | 12.5 | 35.2 | 28.7 | 18.7 | 21 | 11.8 | 9.4 | 16.5 | 8.4 |
| Essential amino acids (EAAs) (%) | - | 40.8 | 37.7 | 40.9 | 39.8 | 45.6 | 44.1 | 38.7 | 39.2 | 37.2 | 27.8 |
| Arginine (Arg) | 0.7 | 6 | 6 | 6.5 | 2.3 | 1.4 | - | 1.5 | 1.7 | 2.7 | 3.8 |
| Cysteine (Cys) | 0.7 | 2.9 | 2.1 | 0.7 | - | - | - | - | - | - | 3.5 |
| Glutamic acid (Glu) | 1.4 | - | 15.5 | 12.1 | 3.1 | 12.5 | 1.6 | 7.2 | 7.2 | 7.6 | 29.7 |
| Glycine (Gly) | 1 | 6 | 5.8 | 5.2 | 1.8 | 1.3 | 0.8 | - | - | 0.2 | 4.2 |
| Histidine (His) | 0.2 | - | 4.6 | 2.1 | 0.6 | 0.1 | 0.3 | 1.6 | 1.1 | 1.4 | 1.3 |
| Isoleucine (Ile) | 0.2 | 4.4 | 3.6 | 4 | - | 0.9 | 0.9 | 1.9 | 1.6 | 2 | 3.7 |
| Leucine (Leu) | 0.7 | 7.8 | 5.9 | 6.9 | 2.2 | 1.2 | 1.6 | - | 2.3 | 3 | 6.2 |
| Lysine (Lys) | 0.5 | 4.7 | 5.6 | 5.3 | 2.2 | 1.3 | 1.2 | 3.7 | 3.3 | 2.8 | 4.1 |
| Hydroxylysine (Hyl) | - | - | 2.7 | - | - | - | - | - | - | - | - |
| Methionine (Met) | 0.2 | 1.3 | - | 3.3 | 0.5 | 0.3 | 0.1 | 0.2 | 0.4 | 0.7 | 1.3 |
| Phenylalanine (Phe) | 0.6 | 5.7 | 3.8 | 4.3 | 1.1 | 0.9 | 1 | 1.2 | 1.2 | 1.7 | 4 |
| Proline (Pro) | 0.6 | 4.4 | 4.4 | 5.6 | 0.9 | 0.9 | 1.5 | - | - | - | 3.9 |
| Hydroxiproline (Hyp) | - | 1 | - | - | - | - | - | 1.8 | 1.6 | 0.9 | - |
| Threonine (Thr) | 0.5 | 4.8 | 4.7 | 5.5 | 1.2 | 1 | 0.7 | 2.7 | 1.9 | 2.4 | 3.6 |
| Valine (Val) | 0.3 | 6.8 | 6.1 | 6.2 | 1.2 | 1 | 1.4 | 2.2 | 1.9 | 2.5 | - |
| Alanine (Ala) | 1 | 8.4 | 6.3 | 7.5 | 3 | 1.2 | 1.9 | 0.7 | 1.5 | 3.4 | 7.9 |
| Aspartic acid (Asp) | 2 | 12.5 | 10.2 | 12 | 3.3 | 2.1 | 2 | 5.2 | 4.1 | 4.3 | 8.2 |
| Serine (Ser) | 0.8 | 5.5 | 5 | 5.1 | 1.6 | 1.2 | 0.8 | 5.5 | - | 5.8 | - |
| References | [140] | [141] | [138] | [138] | [141] | [142] | [143] | [139] | [139] | [139] | [144] |
| Typ of Algae | Region | Total Clorophyll mg/g; mg/L *; µg/g ** | Clorophyll-a mg/g; mg/L *; µg/g ** | Clorophyll-b mg/g; μg/g ** | Total Carotenoids mg/g; mg/L *; μg/g ** | β-Carotene mg/g; g/100 g *; mg/100 g **; µg/g *** | Fucoxanthin mg/g; mg/100 g *; µg/g ** | Astaxanthin mg/100 g | Zeaxanthin mg/100 g; µg/g * | Lutein mg/100 g; mg/g; *; µg/g** | References |
| Green algae | |||||||||||
| Ulva lactuca (as Ulva fasciata) | Black Sea, Romanian coast | 23.23 ± 0.67 | 19.16 ± 2.69 | 4.07 ± 0.36 | 9.97 ± 0.85 | - | - | - | - | - | [30] |
| Ulva lactuca (as Ulva fasciata) | Black Sea, Romanian coast | 35.37 ± 1.7 | 26.95 ± 1.5 | 8.42 ± 1.56 | 16.25 ± 1.3 | - | - | - | - | - | [49] |
| Ulva lactuca (as Ulva fasciata) | Saurashtra Coast, India | 14.00 ± 0.11 * | 8.16 ± 2.69 * | 4.97 ± 0.85 | 0.80 ± 0.02 * | - | - | - | - | - | [53] |
| Ulva fasciata | Indian waters | 3.49 ± 0.62 | 2.09 ± 0.15 | 1.4 ± 0.46 | 0.60 ± 0.06 | 0.37 ± 0.02 | - | - | - | - | [55] |
| Ulva fasciata | Coastal area of Philippines | 6.82 | 2.18 ± 0.74 | 4.64 ± 0.6 | - | 0.72 ± 0.00 | - | - | - | - | [161] |
| Ulva intestinalis | Black Sea, Romanian coast | 20.97 ± 1.67 | 16.74 ± 1.65 | 4.25 ± 0.45 | 12.73 ± 1.32 | - | - | - | - | - | [30] |
| Ulva intestinalis | Black Sea, Romanian coast | 30.51 ± 1.82 | 23.56 ± 1.88 | 6.95 ± 1.6 | 15.98 ± 1.98 | - | - | - | - | - | [49] |
| Ulva flexuosa | Indian waters | 3.14 ± 0.09 | 1.90 ± 0.25 | 1.24 ± 0.06 | 0.49 ± 0.12 | 0.32 ± 0.04 | - | - | - | - | [55] |
| Cladophora vagabunda | Black Sea, Romanian coast | 43.67 ± 1.97 | 24.13 ± 2.57 | 19.54 ± 1.55 | 13.90 ± 0.42 | - | - | - | - | - | [30] |
| Cladophora vagabunda | Black Sea, Romanian coast | 41.64 ± 1.52 | 29.25 ± 1.56 | 12.39 ± 1.35 | 17.66 ± 1.56 | - | - | - | - | - | [49] |
| Acrosiphonia orientalis | Saurashtra Coast, India | 7.00 ± 0.05 * | - | - | 11.00 ± 0.01 * | - | - | - | - | - | [53] |
| Caulerpa scalpelliformis | Saurashtra Coast, India | 3.00 ± 0.05 * | - | - | 8.00 ± 0.01 * | - | - | - | - | - | [53] |
| Caulerpa racemosa | Coastal area of Philippines | 123.58 | 42.15 ± 0.21 | 81.42 ± 0.24 | - | 17.26 ± 1.88 | - | - | - | - | [161] |
| Caulerpa racemosa | Indian waters | 21.09 ± 0.60 | 10.14 ± 0.13 | 11.12 ± 0.57 | 11.45 ± 0.59 | - | - | - | - | - | [162] |
| Caulerpa racemosa | Indonesian Coast | - | - | - | - | 20.50 ± 0.10 * | 1.40 ± 0.01 * | 4.60 ± 0.10 | 4.70 ± 0.01 | 1.50 ± 0.50 | [163] |
| Caulerpa lentillifera | Malaysian waters | 7.29 | 3.32 | 3.97 | 63.47 | 10.7 | - | - | 21.30 * | 21.13 ** | [164] |
| Chlorococcum infusionum (as Chlorococcum humicola) (green microalga) | Thailand Coast | 10.01 ± 0.13 | 5.90 ± 0.15 | 4.11 ± 0.03 | 2.01 ± 0.16 | - | - | - | - | 0.59 ± 0.12 | [165] |
| Red algae | |||||||||||
| Scinaia carnosa | Saurashtra Coast, India | 1.50 ± 0.01 * | - | - | 0.70 ± 0.01 * | - | - | - | - | - | [53] |
| Halymenia porphyriformis | Saurashtra Coast, India | 7.00 ± 0.05 * | - | - | 0.20 ± 0.01 * | - | - | - | - | - | [53] |
| Gracilaria corticata | Indian waters | - | - | - | - | 4.13 ± 0.07 *** | 6.06 ± 0.05 ** | - | 0.65 ± 0.04 * | 0.26 ± 0.05 ** | [166] |
| Gracilaria corticata | Southeast coast of India | - | 8.96 ± 0.39 ** | 7.74 ± 0.33 ** | 12.82 ± 0.50 ** | - | - | - | - | - | [59] |
| Eucheuma denticulatum | Malaysian waters | - | - | - | - | 4.7 ± 0.1 ** | 4.0 ± 0.0 * | 3.0 ± 0.0 | 21.3 ± 0.1 | 87.7 ± 0.1 | [167] |
| Gracilaria edulis | Indian waters | 0.79 ± 0.05 | 0.66 ± 0.26 | 0.13 ± 0.08 | 0.13 ± 0.02 | 0.11 ± 0.02 | - | - | - | - | [55] |
| Gracilaria edulis | Southeast coast of India | - | 17.14 ± 0.55 ** | 8.44 ± 0.63 ** | 2.99 ± 0.56 ** | - | - | - | - | - | [59] |
| Acanthophora spicifera | Indian waters | 1.41 ± 0.62 | 1.17 ± 0.18 | 0.24 ± 0.02 | 0.32 ± 0.12 | 0.24 ± 0.04 | - | - | - | - | [55] |
| Kappaphycus striatus | Malaysian waters | 4.52 | 3.41 | 1.1 | 57.02 | 7.59 | - | - | 4.4 * | 38.6 ** | [164] |
| Gracilaria tikvahiae | Malaysian waters | 2.97 | 2.55 | 0.42 | 25.13 | 3.05 | - | - | 4.15 * | 8.86 ** | [164] |
| Brown algae | |||||||||||
| Laminaria saccharina | Galician coastline from Spain | - | 0.67 | - | - | 0.07 | 9.54 | - | - | - | [16] |
| Iyengaria stellata | Saurashtra Coast, India | 1.50 ± 0.02 * | - | - | 0.7 ± 0.01* | - | - | - | - | - | [53] |
| Sargassum linearifolium | Saurashtra Coast, India | 34.00 ± 0.27 * | - | - | 3.0 ± 0.1 * | - | - | - | - | - | [53] |
| Undaria pinnatifida | Galician coastline from Spain | - | 1.58 | - | - | 0.3 | 6.15 | - | - | - | [168] |
| Padina gymnospora | Indian waters | 2.13 ± 0.43 | 1.75 ± 0.42 | 0.38 ± 0.04 | 0.78 ± 0.08 | 0.48 ± 0.23 | [55] | ||||
| Himanthalia elongate | Atlantic North Coast | 168.2 ± 15.0 ** | 67.6 ± 3.2 ** | - | 2.9 ± 0.3 ** | - | 2.79 ± 0.31 ** | - | - | - | [153] |
| Laminaria ochroleuca | Atlantic North Coast | 235.3 ± 15.4 ** | 183.5 ± 14.8 ** | 14.1 ± 0.5 ** | 27.0 ± 2.4 ** | - | 14.21 ± 0.31 ** | - | - | - | [153] |
| Undaria pinnatifida | Atlantic North Coast | 574.1 ± 33.2 ** | 321.3 ± 19.2 ** | - | 54.6 ± 1.3 ** | - | 26.81 ± 0.79 ** | - | - | - | [153] |
| Padina pavonica | Malaysian water | 7.51 | 3.4 | - | 100.89 | 9.14 | - | - | 10.87 * | 7.21 ** | [164] |
| Green Algae | |||||||||||||
| Algae | Ulva lactuca | Ulva lactuca | Ulva intestinalis | Ulva intestinalis | Ulva intestinalis | Ulva rigida | Chaetomorpha linum | Chaetomorpha sp. | Halimeda macroloba | Cladophora vagabunda | Cladophora vagabunda | Caulerpa scalpelliformis | Acrosiphonia orientalis |
| Region | Black Sea Romania | Arabian Sea | Black Sea | Black Sea Bulgaria | Western coast of Norway | Black Sea Bulgaria | Black Sea Coast Bulgaria | Arabian Gulf | Indonesian waters | Black Sea Romania | Black Sea Romania | Arabian Sea | Arabian Sea |
| TFC mg CE /100 g d.w. * mg QE/g d.w. ** | 15.6 ± 1.65 * | 56 ± 9 ** | 13.1 ± 1.68 * | - | - | - | - | 189.14 ± 0.99 ** | - | - | 12.3 ± 1.78 * | 25.0 ± 5.0 ** | 277 ± 3.0 ** |
| TPC mg GAE/100 g d.w. * mg GAE/g d.w. ** μgGAE/g d.w. *** | 416.6 ± 1.56 * | 285.5 ± 0.6 ** | 412.5 ± 1.26 * | 512.8 ± 23.5 * | 11.3 ± 1.4 ** | 32.80 ± 2.16 *** | 403.9 ± 16.4 * | 21.92 ± 0.43 ** | 186.80 ± 15.54 *** | 356.8 ± 0.3 * | 409.8 ± 1.68 * | 26.0 ± 1.0 * | 107 ± 1.0 ** |
| References | [49] | [53] | [49] | [184] | [185] | [186] | [184] | [187] | [188] | [30] | [49] | [53] | [53] |
| Red Algae | |||||||||||||
| Algae | Scinaia carnosa | Halymenia porphyriformis | Laurencia obtusa | Gracilaria sp. | Hypnea pannosa | Jania rubens | Ellisolandia elongata | Gracilaria gracilis | Asparagopsis armata | Chondrus crispus | Gracilaria verrucosa | Gracilaria edulis | Eucheuma denticulatum |
| Region | Arabian Sea | Arabian Sea | Red Sea Coast | Bali Coast | Saint Martin Island, Bangladesh | Egyptian waters | Egyptian waters | North coast of Tunisia | North coast of Tunisia | Red Sea Coast | Kupang, East Nusa Tenggara | Northwestern coast of Sri Lanka | Kenyan South Coast |
| TF C mgQE/g d.w. * mgCE/ g d.w. ** μg/g *** | 95.0 ± 1.5 * | 18.0 ± 1.0 * | 4.78 ± 0.05 ** | 45.933 ± 0.56 * | 43.12 ± 0.98 * | 173.7 ± 6.8 * | 69.7 ± 2.5 * | - | 464 ± 0.63 ** | 202.66 ± 3.05 *** | - | 541.02 ± 51.84 *** | 9.36 ± 0.12 * |
| TPC mg. GAE/g d.w. * μgGAE/g d.w. ** | 31.0 ± 1.0 * | 10.0 ± 1.0 * | 7.83 ± 0.14 * | 36.273 ± 0.2 * | 89.89 ± 1.13 * | 176.7 ± 6.9 * | 22.9 ± 3.8 * | 19.29 ± 1.8 * | 14.95 ± 0.5 * | 12.38 ± 2.31 ** | 11.27 * | 1007.81 ± 54.21 ** | 146.15 ± 1.11 * |
| References | [53] | [53] | [60] | [189] | [190] | [191] | [191] | [192] | [192] | [193] | [194] | [195] | [196] |
| Brown Algae | |||||||||||||
| Algae | Gongolaria barbata (as C. barbata) | Iyengaria stellata | Sargassum linearifolium | Sargassum oligocystum | Himanthalia elongata | Sargassum asperifolium | Ericaria crinita | S. odonto-carpum (as S.coriifolium) | Padina pavonica | Taoria atomaria | Phyllospora comosa | Ecklonia radiata | Cladostephus spongiosum |
| Region | Black Sea | Arabian Sea | Arabian Sea | Indo-West Pacific Ocean area | North-eastern Atlantic Ocean | Red Sea at Hurghada Coast | Black Sea Coast Bulgaria | Saint Martin Island, Bangladesh | Egyptian waters | Egyptian waters | Australian Beach Coast | Australian Beach Coast | Mediterranean waters, Tunisia coast |
| TFC mg CE/g d.w. * | - | 39 ± 4.0 * | 200 ± 18.0 * | - | 31.9 ± 2.65 * | - | - | 58.29 ± 1.19 * | 206.7 ± 4.7 * | 374.1 ± 27.41 * | 0.22 ± 0.01 * | 0.03 ± 0.01 * | - |
| TPC mg. GAE/100 g d.w. * ppm ** | 358.6 ± 1.85 * | 61 ± 1.0 * | 61 ± 2.0 * | 1.55 ± 0.11 * | 52.7 ± 1.93 * | 141.9 ** | 2662.4 ± 54.2 * | 128.56 ± 0.59 * | 152.5 ± 8.8 * | 157.3 ± 5.9 * | 3.01 ± 0.15 * | 0.52 ± 0.05 * | 10.91 * |
| References | [196] | [53] | [53] | [61] | [62] | [63] | [184] | [190] | [191] | [191] | [197] | [197] | [198] |
| Green algae | |||||||||||
| Algae | Ulva lactuca | Ulva intestinalis | Cladophora vagabunda | Caulerpa scalpelliformis | Acrosiphonia orientalis | Ulva lactuca | Ulva rigida | Caulerpa lentillifera | Ulva lactuca | Ulva flexuosa | Ulva intestinalis |
| Region | Black Sea | Black Sea | Black Sea | Arabian Sea | Arabian Sea | Arabian Sea | Atlantic waters | Atlantic waters | Indian waters | Indian waters | Gulf Gökova Aegean Sea |
| Minerals (Inorganic compounds) | |||||||||||
| Na, mg/kg d.w. | 825 ± 1.6 | 793.31 ± 1.20 | 853.15 ± 0.89 | 600 ± 110 | 1400 ± 125 | 2000 ± 100 | 1595 | 8917 | 20.12 ± 0.02 | 13.2 ± 0.8 | - |
| K, mg/100 g d.w. | 1120.54 ± 1.03 | 1230.56 ± 1.65 | 985.64 ± 2.03 | 9300 ± 250 | 4400 ± 120 | 3000 ± 220 | 1561 | 700–1142 | 27.2 ± 1.02 | 22.32 ± 1.08 | 1052.70 |
| Ca, mg/100 g d.w. | 1790.35 ± 2.55 | 1604.15 ± 2.96 | 1720.64 ± 2.87 | 44 ± 7.0 | 270 ± 30 | 62 ± 20 | 524 | 780–1874 | 740 ± 0.28 | 712 ± 0.04 | 15,977 |
| Mg, mg/100 g d.w. | 95.26 ± 1.05 | 90.87 ± 0.96 | 93.45 ± 0.91 | 800 ± 100 | 1400 ± 100 | - | 2094 | 630–1650 | 420 ± 0.02 | 436 ± 0.24 | 90.87 ± 0.96 |
| Fe, mg/100 g d.w. | 524.25 ± 0.64 | 490.36 ± 1.56 | 565.35 ± 1.05 | 0.5 ± 0.01 | 2.0 ± 0.01 | 0.40 ± 0.01 | - | - | 47 ± 0.04 | 40 ± 0.28 | 338.70 |
| Zn, mg100 g d.w.; µg/100 g d.w. * | 21.62 ± 0.65 | 24.74 ± 0.86 | 20.26 ± 0.85 | 2.0 ± 0.01 | 2.2 ± 0.01 | 4.00 ± 0.01 | - | - | 2.34 ± 0.48 * | 1.518 ± 0.81 * | - |
| I (iodine content); mg/100 g); µg/100 g d.w. * | - | - | - | 4.0 ± 1.0 | 15.0 ± 1.0 | 30 ± 11 | - | - | 38.89 ± 1.08 * | 42.03 ± 1.02 * | - |
| References | [49] | [49] | [49] | [53] | [53] | [53] | [54] | [54] | [55] | [55] | [56] |
| Red algae | |||||||||||
| Algae | Scinaia carnosa | Halymenia porphyriformis | Palmaria palmata | Porphyra umbilicalis | Acanthophora spicifera | Gracilaria edulis | Jania pedunculata | Gracilaria corticata | Gracilaria edulis | Gracilaria corticata | Laurencia obtusa |
| Region | Arabian Sea | Arabian Sea | Atlantic waters | Atlantic waters | Indian waters | Indian waters | Sri Lanka coastal area | Sri Lanka coastal area | Southeast coast of India | Southeast coast of India | Red sea coast |
| Minerals (Inorganic compounds) | |||||||||||
| Na; mg/100 g d.w. | 1400 ± 70 | 2700 ± 30 | 1600–2500 | 940 | 36.08 ± 1.08 | 32.03 ± 0.28 | 86.23 | 67.06 | - | - | 102.55 ± 0.03 |
| K; mg/100 g d.w. | 25.2 ± 2.2 | 5800 ± 50.0 | 7000–9000 | 2030 | 52.08 ± 0.22 | 52.12 ± 0.07 | 121.61 | 125.82 | - | - | 870.38 ± 0.13 |
| Ca; mg/100 g d.w. | 70.0 ± 10.0 | 85.0 ± 15.0 | 560–1200 | 330 | 430 ± 0.14 | 410 ± 0.08 | 181.64 | 176.05 | - | - | 845.35 ± 0.11 |
| Mg; mg/100 g d.w. | 4000 ± 80 | 2200 ± 80 | 170–610 | 370 | 480 ± 1.02 | 580 ± 0.98 | 60.02 | 58.54 | 8.956 ± 0.77 | 46.32 ± 8.87 | 101.2 ± 0.13 |
| Fe; mg/100 g d.w. | - | 1.0 ± 0.01 | - | - | 52 ± 0.24 | 72 ± 0.24 | 73.97 | 49.48 | 55.736 ± 0.57 | 107.24 ± 20.9 | - |
| Zn; mg/100 g d.w.; µg/100 g d.w. * | 2.2 ± 0.01 | 3.00 ± 0.01 | - | - | 4.08 ± 0.28 * | 5.21 ± 0.24 * | 70.94 | 8.61 | 4.273 ± 2.12 | 3.152 ± 0.69 | 4.6 ± 0.05 |
| I (iodin content); mg/100 g; µg/100 g d.w. * | 2.0 ± 0.2 | 2.0 ± 0.1 | - | - | 64.8 ± 0.12 * | 72.2 ± 0.08 | - | - | - | - | - |
| References | [53] | [53] | [54] | [54] | [55] | [55] | [58] | [58] | [59] | [59] | [60] |
| Brown algae | |||||||||||
| Algae | Iyengaria stellata | Sargassum linearifolium | Fucus vesiculosus | Laminaria digitata | Saccharina latissima | Padina gymnospora | Sargassum ilicifolium | Sargassum polycystem | Himanthalia elongata | Sargassum oligocystum | Sargassum asperifolium |
| Region | Arabian Sea | Arabian Sea | Atlantic waters | Atlantic waters | Atlantic Waters | Indian waters | Sri Lanka coastal area | Sri Lanka coastal area | North-eastern Atlantic area | Indo-West Pacific area | Red Sea Coast |
| Minerals (Inorganic compounds) | |||||||||||
| Na; mg/100 g d.w. | 11,000 ± 250 | 7000 ± 130 | 2450–5469 | 3818 | 2620 | 36.36 ± 0.18 | 64.55 | 80.87 | 25.805 ± 7924 | 4.09 ± 0.13 | - |
| K; mg/100 g d.w. | 11,700 ± 400 | 6800 ± 190 | 2500–4322 | 11.5–79 | 4330 | 30.02 ± 0.17 | 127.47 | 127.66 | 57.480 ± 19.976 | 38.57 ± 8.57 | 12 |
| Ca; mg/100 g d.w. | 820 ± 35 | 300 ± 20 | 725–938 | 1005 | 810 | 820 ± 0.34 | 198.15 | 187.43 | 3469 ± 1526 | 30.95 ± 1.11 | 15.200 |
| Mg; mg/100 g d.w. | 1700 ± 110 | 900 ± 75 | 670–994 | 659 | 715 | 780 ± 0.08 | 84.73 | 86.38 | 3537 ± 1497 | 6.40 ± 0.13 | 5.778 |
| Fe; mg/100 g d.w. | 6.00 ± 0.12 | 0.30 ± 0.01 | - | - | - | 14.8 ± 0.32 | 48.50 | 128.46 | 17.8 ± 3.3 | 416.95 ± 4.24 | 0.802 |
| Zn; mg/100 g d.w.; µg/100 g d.w. *; | 2.60 ± 1.203 | 2.5 ± 1.1 | - | - | - | 4.19 ± 0.08 * | 4.58 | 5.76 | 21.3 ± 13 | 21.84 ± 4.04 | 0.316 |
| I (iodin content); mg100/g; µg/100 g d.w. *; | 8.0 ± 1.0 | 41.0 ± 2.0 | - | - | - | 46.2 ± 1.03 * | - | - | - | 3.79 ± 0.03 | - |
| References | [53] | [53] | [54] | [54] | [54] | [55] | [58] | [58] | [61] | [60] | [63] |
| Type of Seaweed | Bioactive Metabolites/Compounds | Mechanism of Action | Biological Activity | References |
|---|---|---|---|---|
| Antidiabetic activity | ||||
| Polysaccharides | ||||
| Sargassum pallidum—brown algae | Fucoidan | Decreases lipid peroxidation. Reduces the activation of NF-κB signaling pathway. | Antidiabetic activity | [291] |
| Gracilaria edulis—red algae | Sulphated pyruvylated polysaccharide | Anti-hyperglycemic effect. Activities against type II transmembrane serine exopeptidase DPP-IV and carbolytic enzyme bundles. | Antidiabetic activity | [292] |
| Sargassum wightii—brown algae | Sulfated polysaccharide | Sulfated polygalacto-pyranosyl-fucopyranan could function as a potential pharmacophore lead against inflammation, type 2 diabetes. | Antidiabetic activity | [235] |
| Metabolic Deseases | ||||
| Fatty acids | ||||
| Undaria pinnatifida—brown algae | SFA, MUFA, PUFA, HUFA, | Inhibition of the COX-2 enzyme | Metabolic diseases | [118] |
| Ulva intestinalis—green algae | SFA, MUFA, and PUFA | Inhibition of the COX-2 enzyme | Metabolic diseases | [282] |
| Curdiea racovitzae—red algae | SFA, MUFA, PUFA | Inhibition of the COX-2 enzyme | Metabolic diseases | [282] |
| Minerals | ||||
| Valoniopsis pachynema—green algae | Iron | Iron is vital because it is used in the production of hemoglobin and myoglobin. The iron content was high in V. pachynema. | Metabolic activities | [293] |
| Gelidium spinosum (as Gelidium latifolium)—red algae | Zinc | Zinc is associated with metabolism and immune function. Zinc is involved in the repairing the body cells. | Metabolic activities | [293] |
| Anticoagulant Activity | ||||
| Polysaccharides | ||||
| Udotea flabellum—green algae | Sulfated galactan | Inhibited B16-F10 cell adhesion, migration, and proliferation. | Anticoagulant activity | [223] |
| Ulva lactuca (as Ulva fasciata)—green algae | Polycarboxyl ulvans | Anticoagulant activity by increasing the carboxyl groups. | Anticoagulant activity | [294] |
| Gelidiella acerosa—red algae | Sulfated polysaccharides | For anticoagulant, antiplatelet and antithrombotic activities, the mechanism of action is mainly due to the chemical structure of the isolated polysaccharide. | Anticoagulant, antiplatelet activity | [295] |
| Sargassum fusiforme—brown algae | Polysaccharides with low MW | These low MW polysaccharides possessed anticoagulant activity in the intrinsic, extrinsic, and common coagulation pathways. | Anticoagulant activity | [296] |
| Neuroprotective Activity and Alzheimer’s Disease (AD) | ||||
| Pigments | ||||
| Macroalgae | Carotenoids | The potent antioxidant properties of carotenoids may explain the neuroprotective effects of carotenoids by inhibiting neuroinflammation and activating autophagy. | Neuroprotective activity | [297] |
| Polysaccharides | ||||
| Macroalgae | Polysaccharides | Seaweed polysaccharides reduced lipid peroxidation and erythrocyte hemolysis. | Alzheimer’s disease | [298] |
| Ecklonia cava—brown algae | Fucoidan | Polysaccharide extracts inhibit BACE-1 protease, resulting in decreased amyloid-beta. | Alzheimer’s disease | [299] |
| Fatty acids | ||||
| Sargassum fusiforme | Fatty acids extract | Derived lipid extract to AD mice significantly improved short-term memory and reduced hippocampal Aβ plaque load by 81% | Alzheimer’s disease | [300] |
| Antiprotozoal Activitaty | ||||
| Polyphenolic compound | ||||
| Padina boryana—brown algae | Ellagic acid | Activity against Trypzanosoma cruzi showed a value of IC50 = 9.2 ± 0.87 µg/mL and against Leishmania donovani, showed IC50 = 8.87 ± 2.3 µg/mL | Antiprotozoal activity | [255] |
| Bone Deficiencies | ||||
| Minerals | ||||
| Valoniopsis pachynema—green algae | Calcium | Calcium to improve bone density | Bone deficiencies | [293] |
| Ulva lactuca (as Ulva fasciata)—green algae | Ca, Mg, Na, K, and Fe | Supplementing the body with exogenous daily intake. Calcium to improve bone density | Bone deficiencies | [301] |
| Malnutrition | ||||
| Vitamins | ||||
| Saccharina japonica—brown algae | Vitamin B9, B12 | Supplementing daily intake | Malnutrition | [302] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadar, E.; Popescu, A.; Dragan, A.-M.-L.; Pesterau, A.-M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. https://doi.org/10.3390/md23040152
Cadar E, Popescu A, Dragan A-M-L, Pesterau A-M, Pascale C, Anuta V, Prasacu I, Velescu BS, Tomescu CL, Bogdan-Andreescu CF, et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Marine Drugs. 2025; 23(4):152. https://doi.org/10.3390/md23040152
Chicago/Turabian StyleCadar, Emin, Antoanela Popescu, Ana-Maria-Laura Dragan, Ana-Maria Pesterau, Carolina Pascale, Valentina Anuta, Irina Prasacu, Bruno Stefan Velescu, Cezar Laurentiu Tomescu, Claudia Florina Bogdan-Andreescu, and et al. 2025. "Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review" Marine Drugs 23, no. 4: 152. https://doi.org/10.3390/md23040152
APA StyleCadar, E., Popescu, A., Dragan, A.-M.-L., Pesterau, A.-M., Pascale, C., Anuta, V., Prasacu, I., Velescu, B. S., Tomescu, C. L., Bogdan-Andreescu, C. F., Sirbu, R., & Ionescu, A.-M. (2025). Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Marine Drugs, 23(4), 152. https://doi.org/10.3390/md23040152










