Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity
Abstract
1. Introduction
2. Physicochemical and Structural Features of U. pinnatifida Polysaccharides
2.1. Fucoidan
2.2. Alginate
2.3. Laminarin
3. Extraction of U. pinnatifida Polysaccharides
4. Purification of U. pinnatifida Polysaccharides
4.1. Preliminary Purification
4.2. Chromatographic Purification
4.3. Economic and Environmental Considerations
4.4. Quality Control of UPPs
5. Biological Activity
5.1. Antioxidant and Free Radical Scavenging Activity
5.2. Immunomodulatory Activity
5.3. Anticancer Activity
5.4. Prebiotic Activity and Mucosal Barrier
5.5. Anti-Hyperglycemic Effects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Zhou, T.; Shabbir, I.; Choi, J.; Kim, Y.H.; Park, M.; Aweya, J.J.; Tan, K.; Zhong, S.; Cheong, K.L. Marine algal polysaccharides: Multifunctional bioactive ingredients for cosmetic formulations. Carbohydr. Polym. 2025, 353, 123276. [Google Scholar] [PubMed]
- Kee, P.E.; Phang, S.M.; Lan, J.C.-W.; Tan, J.S.; Khoo, K.S.; Chang, J.-S.; Ng, H.-S. Tropical Seaweeds as a Sustainable Resource Towards Circular Bioeconomy: Insights and Way Forward. Mol. Biotechnol. 2023, 1–18. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Extraction and characterization of bioactive compounds from brown seaweed (Undaria pinnatifida) sporophyll using two sequential green extraction techniques. Algal Res. 2024, 77, 103330. [Google Scholar]
- Nadeeshani, H.; Hassouna, A.; Lu, J. Proteins extracted from seaweed Undaria pinnatifida and their potential uses as foods and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 6187–6203. [Google Scholar] [CrossRef]
- Pedro, B.; Guedes, L.; André, R.; Gaspar, H.; Vaz, P.; Ascensão, L.; Melo, R.; Luísa Serralheiro, M. Undaria pinnatifida (U. pinnatifida) bioactivity: Antioxidant, gastro-intestinal motility, cholesterol biosynthesis and liver cell lines proteome. J. Funct. Foods 2021, 83, 104567. [Google Scholar]
- Li, G.; He, Y.; Liew, A.; Huang, C.; Song, B.; Jia, X.; Malairaj, S.; Zhong, S.; Cheong, K.-L. Dietary polysaccharides from dragon fruit pomace, a co-product of the fruit processing industry, exhibit therapeutic potential in high-fat diet-induced metabolic disorders. Food Res. Int. 2025, 203, 115818. [Google Scholar]
- Awanthi, M.G.G.; Umosa, M.; Yuguchi, Y.; Oku, H.; Kitahara, K.; Ito, M.; Tanaka, A.; Konishi, T. Fractionation and characterization of cell wall polysaccharides from the brown alga Cladosiphon okamuranus. Carbohydr. Res. 2023, 523, 108722. [Google Scholar]
- Jegadeshwari, B.; Rajaram, R. A critical review on pharmacological properties of sulfated polysaccharides from marine macroalgae. Carbohydr. Polym. 2024, 344, 122488. [Google Scholar]
- Lu, S.-Y.; Tan, K.; Zhong, S.; Cheong, K.-L. Marine algal polysaccharides as future potential constituents against non-alcoholic steatohepatitis. Int. J. Biol. Macromol. 2023, 250, 126247. [Google Scholar]
- Wu, X.; Huang, X.; Ma, W.; Li, M.; Wen, J.; Chen, C.; Liu, L.; Nie, S. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023, 14, 1387–1400. [Google Scholar] [PubMed]
- Chen, N.; Jiang, T.; Xu, J.; Xi, W.; Shang, E.; Xiao, P.; Duan, J.-A. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure–immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [PubMed]
- Lee, H.H.; Cho, Y.; Kim, G.H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X.; Guo, S.; Wang, Y.; Feng, D.; Dong, X.; Qi, H. Undaria pinnatifida gel inks for food 3D printing are developed based on the colloidal properties of Undaria pinnatifida slurry and protein/colloidal/starch substances. Int. J. Biol. Macromol. 2024, 261, 129788. [Google Scholar]
- Sharma, S.; Bhende, M. An overview: Non-toxic and eco-friendly polysaccharides—Its classification, properties, and diverse applications. Polym. Bull. 2024, 81, 12383–12429. [Google Scholar]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable Polysaccharides for Controlled Drug Delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar]
- Wang, M.; Cheong, K.-L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef]
- Li, D.; Chen, M.; Meng, X.; Sun, Y.; Liu, R.; Sun, T. Extraction, purification, structural characteristics, bioactivity and potential applications of polysaccharides from Avena sativa L.: A review. Int. J. Biol. Macromol. 2024, 265, 130891. [Google Scholar]
- Xue, H.; Li, P.; Bian, J.; Gao, Y.; Sang, Y.; Tan, J. Extraction, purification, structure, modification, and biological activity of traditional Chinese medicine polysaccharides: A review. Front. Nutr. 2022, 9, 1005181. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan From Undaria pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11, 00831. [Google Scholar]
- Cao, R.-A.; Lee, Y.; You, S. Water soluble sulfated-fucans with immune-enhancing properties from Ecklonia cava. Int. J. Biol. Macromol. 2014, 67, 303–311. [Google Scholar]
- Wang, C.-Y.; Chen, Y.-C. Extraction and characterization of fucoidan from six brown macroalgae. J. Mar. Sci. Technol. 2016, 24, 26. [Google Scholar]
- Yu, J.; Li, Q.; Wu, J.; Yang, X.; Yang, S.; Zhu, W.; Liu, Y.; Tang, W.; Nie, S.; Hassouna, A.; et al. Fucoidan Extracted From Sporophyll of Undaria pinnatifida Grown in Weihai, China—Chemical Composition and Comparison of Antioxidant Activity of Different Molecular Weight Fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar]
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.-L. Fucoidan-Derived Functional Oligosaccharides: Recent Developments, Preparation, and Potential Applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Zeng, J.; Luan, F.; Hu, J.; Liu, Y.; Zhang, X.; Qin, T.; Zhang, X.; Liu, R.; Zeng, N. Recent research advances in polysaccharides from Undaria pinnatifida: Isolation, structures, bioactivities, and applications. Int. J. Biol. Macromol. 2022, 206, 325–354. [Google Scholar]
- Wang, H.; Wen, J.; Ablimit, N.; Deng, K.; Wang, W.; Jiang, W. Degradation of Natural Undaria pinnatifida into Unsaturated Guluronic Acid Oligosaccharides by a Single Alginate Lyase. Mar. Drugs 2024, 22, 453. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-metal cation interactions: Macromolecular approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar]
- Du, L.; GhavamiNejad, A.; Yan, Z.-C.; Biswas, C.S.; Stadler, F.J. Effect of a functional polymer on the rheology and microstructure of sodium alginate. Carbohydr. Polym. 2018, 199, 58–67. [Google Scholar] [CrossRef]
- Park, E.-J.; Choi, J.-I. Melanogenesis inhibitory effect of low molecular weight fucoidan from Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 2213–2217. [Google Scholar] [CrossRef]
- Saqib, M.N.; Ahammed, S.; Liu, F.; Zhong, F. Customization of liquid-core sodium alginate beads by molecular engineering. Carbohydr. Polym. 2022, 284, 119047. [Google Scholar] [PubMed]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Li, J.-K.; Zhong, S. Preparation and Structure Characterization of High-Value Laminaria digitata Oligosaccharides. Front. Nutr. 2022, 9, 945804. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, S.C.; Pattathil, S.; Eberhard, S.; Hahn, M.G.; Popper, Z.A. β-1,3-Glucans are components of brown seaweed (Phaeophyceae) cell walls. Protoplasma 2017, 254, 997–1016. [Google Scholar] [CrossRef]
- Oda, M.; Tanabe, Y.; Noda, M.; Inaba, S.; Krayukhina, E.; Fukada, H.; Uchiyama, S. Structural and binding properties of laminarin revealed by analytical ultracentrifugation and calorimetric analyses. Carbohydr. Res. 2016, 431, 33–38. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar]
- Cheong, K.-L.; Liu, K.; Veeraperumal, S.; Jaikumar, G.; Sathuvan, M.; Liu, X.; Jia, X.; Zheng, P.; Jiang, H.; Cai, R.; et al. Functional Evaluation of Laminarin Butyl Esters: Antioxidant, Skin-Whitening, and Anti-Wrinkle Properties. Process Biochem. 2025, 152, 29–37. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar]
- Kapoor, D.U.; Sharma, H.; Maheshwari, R.; Pareek, A.; Gaur, M.; Prajapati, B.G.; Castro, G.R.; Thanawuth, K.; Suttiruengwong, S.; Sriamornsak, P. Konjac glucomannan: A comprehensive review of its extraction, health benefits, and pharmaceutical applications. Carbohydr. Polym. 2024, 339, 122266. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.; Liu, T.; Hu, Y.; Zheng, Q.; Wang, B.; Lin, H.; Li, X. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2016, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; You, D.-J.; Lee, K.-W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Kim, Y.-S.; Jeon, Y.-J.; Kim, S.-K. Enzyme-assisted extraction of bioactive compounds from seaweeds and microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Pang, Y.; Peng, Z.; Ding, K. An in-depth review: Unraveling the extraction, structure, bio-functionalities, target molecules, and applications of pectic polysaccharides. Carbohydr. Polym. 2024, 343, 122457. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Kim, E.-A.; Kang, S.I.; Yang, H.-W.; Ryu, B.; Wang, L.; Lee, J.-S.; Jeon, Y.-J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules 2020, 25, 2361. [Google Scholar] [CrossRef]
- Nonglait, D.L.; Gokhale, J.S. Review Insights on the Demand for Natural Pigments and Their Recovery by Emerging Microwave-Assisted Extraction (MAE). Food Bioprocess Technol. 2024, 17, 1681–1705. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [PubMed]
- Wang, D.; Wang, Y.; Zhao, W.; Pandiselvam, R.; Sun, X.; Guo, Y.; Su, D.; Xu, H. Effects of Penetrating Microwave Blanching on the Drying Characteristics, Physicochemical Properties, and Water Migration of Pleurotus eryngii: Observation of Cell Wall Polysaccharides and Microstructure. Food Bioprocess Technol. 2025, 18, 392–407. [Google Scholar] [CrossRef]
- Zhong, Q.-W.; Zhou, T.-S.; Qiu, W.-H.; Wang, Y.-K.; Xu, Q.-L.; Ke, S.-Z.; Wang, S.-J.; Jin, W.-H.; Chen, J.-W.; Zhang, H.-W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [PubMed]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous microwave-assisted step-by-step extraction of bioactive water-soluble materials and fucoidan from brown seaweed Undaria pinnatifida waste. Biomass Convers. Biorefinery 2024, 14, 7673–7682. [Google Scholar] [CrossRef]
- Martinez-Solano, K.C.; Garcia-Carrera, N.A.; Tejada-Ortigoza, V.; García-Cayuela, T.; Garcia-Amezquita, L.E. Ultrasound Application for the Extraction and Modification of Fiber-Rich By-Products. Food Eng. Rev. 2021, 13, 524–543. [Google Scholar]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar]
- Lee, J.-H.; Kim, J.-H.; Kim, S.-M.; Kim, J.-Y.; Kim, J.-H.; Eom, S.-J.; Kang, M.-C.; Song, K.-M. The Antioxidant Activity of Undaria pinnatifida Sporophyll Extract Obtained Using Ultrasonication: A Focus on Crude Polysaccharide Extraction Using Ethanol Precipitation. Antioxidants 2023, 12, 1904. [Google Scholar] [CrossRef]
- Song, K.-M.; Ha, S.J.; Lee, J.-E.; Kim, S.-H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT—Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar]
- Arrutia, F.; Adam, M.; Calvo-Carrascal, M.Á.; Mao, Y.; Binner, E. Development of a continuous-flow system for microwave-assisted extraction of pectin-derived oligosaccharides from food waste. Chem. Eng. J. 2020, 395, 125056. [Google Scholar]
- Eom, S.J.; Kim, Y.E.; Kim, J.-E.; Park, J.; Kim, Y.H.; Song, K.-M.; Lee, N.H. Production of Undaria pinnatifida sporophyll extract using pilot-scale ultrasound-assisted extraction: Extract characteristics and antioxidant and anti-inflammatory activities. Algal Res. 2020, 51, 102039. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Mohd Fauziee, N.A.; Chang, L.S.; Wan Mustapha, W.A.; Md Nor, A.R.; Lim, S.J. Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 2021, 167, 1135–1145. [Google Scholar] [PubMed]
- Hu, X.; Goff, H.D. Fractionation of polysaccharides by gradient non-solvent precipitation: A review. Trends Food Sci. Technol. 2018, 81, 108–115. [Google Scholar]
- Song, L.; Niu, Y.; Chen, R.; Ju, H.; Liu, Z.; Zhang, B.; Xie, W.; Gao, Y. A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida. Mar. Drugs 2024, 22, 316. [Google Scholar] [CrossRef]
- Hadidi, M.; Buckley, J.J.; Zydney, A.L. Ultrafiltration behavior of bacterial polysaccharides used in vaccines. J. Membr. Sci. 2015, 490, 294–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, J.; Zou, H.; Zhang, W.; Cheng, S.; Zhang, M.; Lin, H. The molecular weight-fouling matrix: A novel dissection of polysaccharide interactions in ultrafiltration processes. Sep. Purif. Technol. 2024, 345, 127340. [Google Scholar]
- Jia, R.-B.; Wu, J.; Li, Z.-R.; Ou, Z.-R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Comparison of physicochemical properties and antidiabetic effects of polysaccharides extracted from three seaweed species. Int. J. Biol. Macromol. 2020, 149, 81–92. [Google Scholar]
- Zheng, Y.; Yan, J.; Cao, C.; Liu, Y.; Yu, D.; Liang, X. Application of chromatography in purification and structural analysis of natural polysaccharides: A review. J. Sep. Sci. 2023, 46, 2300368. [Google Scholar]
- Zhang, X.; Liu, L.; Zhang, C.; Zhu, Y.; Zhao, H.; Zhao, L. Adsorption mechanisms of alginate oligosaccharides with specific degree of polymerizations on a novel ion-exchange resin: Experiments and simulations. Chem. Eng. J. 2023, 460, 141717. [Google Scholar]
- Kim, J.-H.; Kim, J.-H.; Lee, J.-H.; Eom, S.-J.; Lee, N.-H.; Lee, S.; Lim, T.-G.; Song, K.-M.; Kang, M.-C. Anti-Melanogenic Effects of a Polysaccharide Isolated from Undaria pinnatifida Sporophyll Extracts. Int. J. Mol. Sci. 2024, 25, 10624. [Google Scholar] [CrossRef]
- Huang, X.; Ai, C.; Yao, H.; Zhao, C.; Xiang, C.; Hong, T.; Xiao, J. Guideline for the extraction, isolation, purification, and structural characterization of polysaccharides from natural resources. eFood 2022, 3, e37. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, P. Structure, preparation, and biological activity of sulfated polysaccharides from the genus Caulerpa (Chlorophyta): A review. J. Appl. Phycol. 2023, 35, 3069–3085. [Google Scholar] [CrossRef]
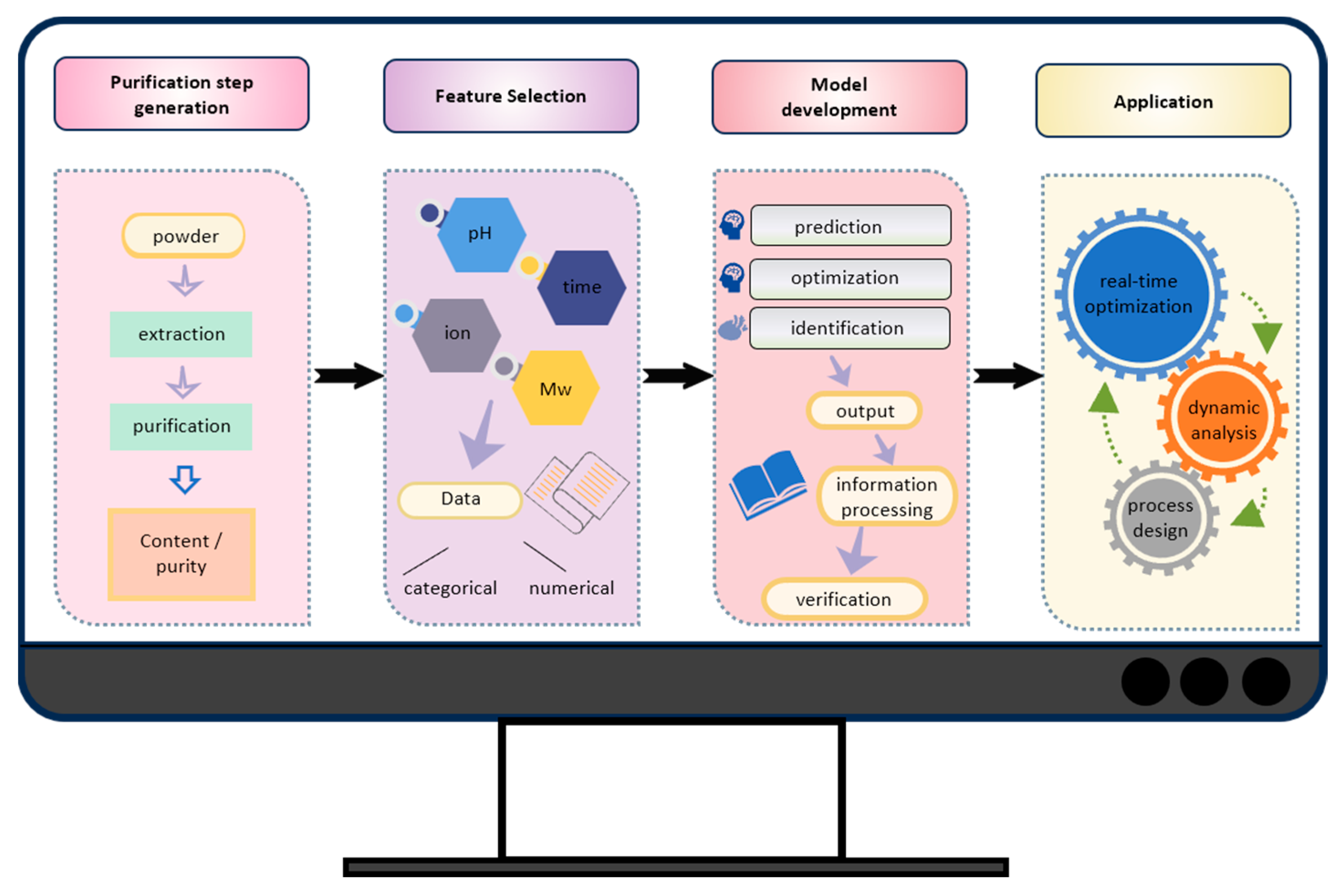
- Malashin, I.; Martysyuk, D.; Tynchenko, V.; Gantimurov, A.; Semikolenov, A.; Nelyub, V.; Borodulin, A. Machine Learning-Based Process Optimization in Biopolymer Manufacturing: A Review. Polymers 2024, 16, 3368. [Google Scholar] [CrossRef]
- Rabiya, R.; Sen, R. Artificial intelligence driven advanced optimization strategy vis-à-vis response surface optimization of production medium: Bacterial exopolysaccharide production as a case-study. Biochem. Eng. J. 2022, 178, 108271. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, H.; Fan, R.; Wan, Y.; Luo, J. Machine learning-based Bayesian optimization facilitates ultrafiltration process design for efficient protein purification. Sep. Purif. Technol. 2025, 363, 132122. [Google Scholar] [CrossRef]
- Deng, B.; Deng, Y.; Liu, M.; Chen, Y.; Wu, Q.; Guo, H. Integrated models for prediction and global factors sensitivity analysis of ultrafiltration (UF) membrane fouling: Statistics and machine learning approach. Sep. Purif. Technol. 2023, 313, 123326. [Google Scholar] [CrossRef]
- Ding, C.; Ierapetritou, M. Machine learning-based optimization of a multi-step ion exchange chromatography for ternary protein separation. Comput. Chem. Eng. 2024, 184, 108642. [Google Scholar] [CrossRef]
- Huang, J.; Su, J.; Chang, Q. Graph neural network and multi-agent reinforcement learning for machine-process-system integrated control to optimize production yield. J. Manuf. Syst. 2022, 64, 81–93. [Google Scholar] [CrossRef]
- Zhang, F.; Ran, C.; Zheng, J.; Ding, Y.; Chen, G. Polysaccharides obtained from bamboo shoots (Chimonobambusa quadrangularis) processing by-products: New insight into ethanol precipitation and characterization. Int. J. Biol. Macromol. 2018, 112, 951–960. [Google Scholar] [CrossRef]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in Extraction Methods to Recover Added-Value Compounds from Seaweeds: Sustainability and Functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Rajak, R.C.; Jacob, S.; Kim, B.S. A holistic zero waste biorefinery approach for macroalgal biomass utilization: A review. Sci. Total Environ. 2020, 716, 137067. [Google Scholar] [PubMed]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.A.; Priya, A.K.; Hawash, H.B.; Yap, P.-S. Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Razali, M.C.; Wahab, N.A.; Sunar, N.; Shamsudin, N.H. Existing Filtration Treatment on Drinking Water Process and Concerns Issues. Membranes 2023, 13, 285. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. Membrane diafiltration for enhanced purification of biologically active compounds from goji berries extracts. Sep. Purif. Technol. 2022, 282, 119991. [Google Scholar]
- Otitoju, T.A.; Kim, C.-H.; Ryu, M.; Park, J.; Kim, T.-K.; Yoo, Y.; Park, H.; Lee, J.-H.; Cho, Y.H. Exploring green solvents for the sustainable fabrication of bio-based polylactic acid membranes using nonsolvent-induced phase separation. J. Clean. Prod. 2024, 467, 142905. [Google Scholar]
- Wang, F.; Yong, M.; Yanhua, L.; Zhenggang, C.; Xiaoyan, Y.; Fuming, Z.; and Linhardt, R.J. A simple strategy for the separation and purification of water-soluble polysaccharides from the fresh Spirulina platensis. Sep. Sci. Technol. 2017, 52, 456–466. [Google Scholar]
- Rathore, A.S.; Kumar, D.; Kateja, N. Recent developments in chromatographic purification of biopharmaceuticals. Biotechnol. Lett. 2018, 40, 895–905. [Google Scholar]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, I.T.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23, 1065. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Food and Beverages Containing Algae and Derived Ingredients Launched in the Market from 2015 to 2019: A Front-of-Pack Labeling Perspective with a Special Focus on Spain. Foods 2021, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.; Yoo, B. Rheological properties of carboxymethyl cellulose–fucoidan mixture: Effect of fucoidan concentration and salt. Macromol. Res. 2025, 33, 175–184. [Google Scholar] [CrossRef]
- Nunes, A.; Azevedo, G.Z.; de Souza Dutra, F.; dos Santos, B.R.; Schneider, A.R.; Oliveira, E.R.; Moura, S.; Vianello, F.; Maraschin, M.; Lima, G.P.P. Uses and applications of the red seaweed Kappaphycus alvarezii: A systematic review. J. Appl. Phycol. 2024, 36, 3409–3450. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Xia, X.; Wong, I.N.; Chung, S.K.; Xu, B.; El-Seedi, H.R.; Wang, B.; Huang, R. Sulfated heteropolysaccharides from Undaria pinnatifida: Structural characterization and transcript-metabolite profiling of immunostimulatory effects on RAW264.7 cells. Food Chem. X 2022, 13, 100251. [Google Scholar] [CrossRef]
- Li, Z.-R.; Jia, R.-B.; Cai, X.; Luo, D.; Chen, C.; Zhao, M. Characterizations of food-derived ellagic acid-Undaria pinnatifida polysaccharides solid dispersion and its benefits on solubility, dispersity and biotransformation of ellagic acid. Food Chem. 2023, 413, 135530. [Google Scholar] [CrossRef]
- Ke, S.; Zhang, B.; Yu, Y.; Wang, S.; Jin, W.; Wu, J.; Chen, J.; Zhang, H.; Wei, B.; Wang, H. Structural characterization of sulfated galactofucan from Undaria pinnatifida and its effect on type 2 diabetic mice. J. Oceanol. Limnol. 2023, 41, 300–313. [Google Scholar] [CrossRef]
- Shi, F.-S.; Xie, Y.-H.; Yang, Y.-L.; Xu, L.-D.; Li, J.-J.; Wang, X.; Zhu, L.-Y.; Wang, W.-W.; Shen, P.-l.; Huang, Y.-W.; et al. Fucoidan from Ascophyllum nodosum and Undaria pinnatifida attenuate SARS-CoV-2 infection in vitro and in vivo by suppressing ACE2 and alleviating inflammation. Carbohydr. Polym. 2024, 332, 121884. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Cheong, K.-L.; Yu, B.; Chen, J.; Zhong, S. A Comprehensive Review of the Cardioprotective Effect of Marine Algae Polysaccharide on the Gut Microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.-M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Cui, Y.-L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Fan, M.; Sun, X.; Qian, Y.; Xu, Y.; Wang, D.; Cao, Y. Effects of metal ions in tea polysaccharides on their in vitro antioxidant activity and hypoglycemic activity. Int. J. Biol. Macromol. 2018, 113, 418–426. [Google Scholar]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar]
- Ajisaka, K.; Oyanagi, Y.; Miyazaki, T.; Suzuki, Y. Effect of the chelation of metal cation on the antioxidant activity of chondroitin sulfates. Biosci. Biotechnol. Biochem. 2016, 80, 1179–1185. [Google Scholar] [PubMed]
- Phull, A.-R.; Majid, M.; Haq, I.-u.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [PubMed]
- Silva, M.M.C.L.; dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar]
- Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin alleviates chromium toxicity in maize by modulation of cell wall polysaccharides biosynthesis, glutathione metabolism, and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef]
- Pinho, R.A.; Sepa-Kishi, D.M.; Bikopoulos, G.; Wu, M.V.; Uthayakumar, A.; Mohasses, A.; Hughes, M.C.; Perry, C.G.R.; Ceddia, R.B. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic. Biol. Med. 2017, 110, 381–389. [Google Scholar]
- Das, N.; Mandala, A.; Bhattacharjee, S.; Mukherjee, D.; Bandyopadhyay, D.; Roy, S.S. Dietary fat proportionately enhances oxidative stress and glucose intolerance followed by impaired expression of the genes associated with mitochondrial biogenesis. Food Funct. 2017, 8, 1577–1586. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Chen, X.-W.; Zheng, Y.-Y.; Ouyang, J.-M. Sulfated Undaria pinnatifida Polysaccharide Promotes Endocytosis of Nano-Calcium Oxalate Dihydrate by Repairing Subcellular Organelles in HK-2 Cells. Antioxidants 2023, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jia, J.; Zhang, C.; Zhang, P.; Song, S.; Ai, C. Undaria pinnatifida fucoidan ameliorates dietary fiber deficiency-induced inflammation and lipid abnormality by modulating mucosal microbiota and protecting intestinal barrier integrity. Int. J. Biol. Macromol. 2023, 247, 125724. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Makshakova, O. Multifaceted Computational Modeling in Glycoscience. Chem. Rev. 2022, 122, 15914–15970. [Google Scholar]
- Schmalhorst, P.S.; Deluweit, F.; Scherrers, R.; Heisenberg, C.-P.; Sikora, M. Overcoming the Limitations of the MARTINI Force Field in Simulations of Polysaccharides. J. Chem. Theory Comput. 2017, 13, 5039–5053. [Google Scholar]
- Shen, P.; Gu, Y.; Zhang, C.; Sun, C.; Qin, L.; Yu, C.; Qi, H. Metabolomic Approach for Characterization of Polyphenolic Compounds in Laminaria japonica, Undaria pinnatifida, Sargassum fusiforme and Ascophyllum nodosum. Foods 2021, 10, 192. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef]
- Capek, P.; Košťálová, Z. Isolation, chemical characterization and antioxidant activity of Prunus spinosa L. fruit phenolic polysaccharide-proteins. Carbohydr. Res. 2022, 515, 108547. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxidative Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides 2022, 3, 818–830. [Google Scholar] [CrossRef]
- Nam, J.; Kim, A.; Kim, K.; Moon, J.H.; Baig, J.; Phoo, M.; Moon, J.J.; Son, S. Engineered polysaccharides for controlling innate and adaptive immune responses. Nat. Rev. Bioeng. 2024, 2, 733–751. [Google Scholar] [CrossRef]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Lv, X.; Wang, Y.; Li, J.; Liu, Y.; Lu, W.; Yang, L.; Zhao, J.; Wang, F.; Zhang, L. Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int. J. Biol. Macromol. 2016, 93, 940–951. [Google Scholar] [PubMed]
- Zeng, F.; Li, Y.; Zhang, X.; Shen, L.; Zhao, X.; Beta, T.; Li, B.; Chen, R.; Huang, W. Immune regulation and inflammation inhibition of Arctium lappa L. polysaccharides by TLR4/NF-κB signaling pathway in cells. Int. J. Biol. Macromol. 2024, 254, 127700. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar]
- Liu, L.; Yang, X.; Yuan, P.; Cai, S.; Bao, J.; Zhao, Y.; Aimaier, A.; Aipire, A.; Lu, J.; Li, J. In Vitro and In Vivo Dendritic Cell Immune Stimulation Effect of Low Molecular Weight Fucoidan from New Zealand Undaria pinnatifida. Mar. Drugs 2022, 20, 197. [Google Scholar] [CrossRef]
- Hyun, G.H.; Cho, I.H.; Yang, Y.Y.; Jeong, D.-H.; Kang, Y.P.; Kim, Y.-S.; Lee, S.J.; Kwon, S.W. Mechanisms of interactions in pattern-recognition of common glycostructures across pectin-derived heteropolysaccharides by Toll-like receptor 4. Carbohydr. Polym. 2023, 314, 120921. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Balka, K.R.; De Nardo, D. Understanding early TLR signaling through the Myddosome. J. Leukoc. Biol. 2019, 105, 339–351. [Google Scholar]
- Kim, A.-Y.; Shim, H.-J.; Kim, S.Y.; Heo, S.; Youn, H.-S. Differential regulation of MyD88- and TRIF-dependent signaling pathways of Toll-like receptors by cardamonin. Int. Immunopharmacol. 2018, 64, 1–9. [Google Scholar] [PubMed]
- Múnera-Rodríguez, A.M.; Leiva-Castro, C.; Sobrino, F.; López-Enríquez, S.; Palomares, F. Sulforaphane-mediated immune regulation through inhibition of NF-kB and MAPK signaling pathways in human dendritic cells. Biomed. Pharmacother. 2024, 177, 117056. [Google Scholar]
- Kiddane, A.T.; Kim, G.-D. Anticancer and Immunomodulatory Effects of Polysaccharides. Nutr. Cancer 2021, 73, 2219–2231. [Google Scholar] [PubMed]
- Wu, J.; Li, H.; Wang, X.; Zhang, X.; Liu, W.; Wang, Y.; Zhang, Y.; Pan, H.; Wang, Q.; Han, Y. Effect of polysaccharide from Undaria pinnatifida on proliferation, migration and apoptosis of breast cancer cell MCF7. Int. J. Biol. Macromol. 2019, 121, 734–742. [Google Scholar]
- Yang, Y.; Zhang, Q.; Xu, Y.; Chen, G.; Qiu, Y. Sulfated Polysaccharide From Undaria Pinnatifida Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Ovarian Cancer via Suppressing the Hedgehog Signaling Pathway. Front. Mater. 2021, 8, 795061. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Synytsya, A.; Capek, P.; Lee, C.W.; Choi, J.W.; Cho, S.; Kim, W.J.; Park, Y.I. Low Molecular Weight Mannogalactofucans Derived from Undaria pinnatifida Induce Apoptotic Death of Human Prostate Cancer Cells In Vitro and In Vivo. Mar. Biotechnol. 2018, 20, 813–828. [Google Scholar]
- Li, Q.; Wang, X.; Wan, Y.; Hu, X.; Liu, J.; Wang, J. In vivo immunomodulatory activity offucoidan from brown alga Undaria pinnatifida in sarcoma 180-bearing mice. J. Funct. Foods 2023, 103, 105486. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar]
- Cheong, K.-L.; Liu, K.; Chen, W.; Zhong, S.; Tan, K. Recent progress in Porphyra haitanensis polysaccharides: Extraction, purification, structural insights, and their impact on gastrointestinal health and oxidative stress management. Food Chem. X 2024, 22, 101414. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Xie, X.-T.; Zhou, T.; Malairaj, S.; Veeraperumal, S.; Zhong, S.; Tan, K. Exploring the therapeutic potential of porphyran extracted from Porphyra haitanensis in the attenuation of DSS-induced intestinal inflammation. Int. J. Biol. Macromol. 2024, 271, 132578. [Google Scholar]
- Yu, B.; Wang, M.; Teng, B.; Veeraperumal, S.; Cheung, P.C.-K.; Zhong, S.; Cheong, K.-L. Partially Acid-Hydrolyzed Porphyran Improved Dextran Sulfate Sodium-Induced Acute Colitis by Modulation of Gut Microbiota and Enhancing the Mucosal Barrier. J. Agric. Food Chem. 2023, 71, 7299–7311. [Google Scholar] [PubMed]
- Li, L.; Wang, Y.; Yuan, J.; Liu, Z.; Ye, C.; Qin, S. Undaria pinnatifida improves obesity-related outcomes in association with gut microbiota and metabolomics modulation in high-fat diet-fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 10217–10231. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Sinisterra-Loaiza, L.; Mondragón-Portocarrero, A.; Ortiz-Viedma, J.; Cardelle-Cobas, A.; Abuín, C.M.F.; Cepeda, A. Potential prebiotic effect of two Atlantic whole brown seaweeds, Saccharina japonica and Undaria pinnatifida, using in vitro simulation of distal colonic fermentation. Front. Nutr. 2023, 10, 1170392. [Google Scholar]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar]
- Bandi, C.K.; Agrawal, A.; Chundawat, S.P.S. Carbohydrate-Active enZyme (CAZyme) enabled glycoengineering for a sweeter future. Curr. Opin. Biotechnol. 2020, 66, 283–291. [Google Scholar]
- Yang, C.; Dwan, C.; Wimmer, B.C.; Wilson, R.; Johnson, L.; Caruso, V. Fucoidan from Undaria pinnatifida Enhances Exercise Performance and Increases the Abundance of Beneficial Gut Bacteria in Mice. Mar. Drugs 2024, 22, 485. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Mo, C.; Lou, X.; Xue, J.; Shi, Z.; Zhao, Y.; Wang, F.; Chen, G. The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 2024, 16, 41. [Google Scholar]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Chen, Y.; Wang, Y.; Shi, N. Resistant starch and the gut microbiome: Exploring beneficial interactions and dietary impacts. Food Chem. X 2024, 21, 101118. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Kong, C.; Yang, M.; Yue, N.; Zhang, Y.; Tian, C.; Wei, D.; Shi, R.; Yao, J.; Wang, L.; Li, D. Restore Intestinal Barrier Integrity: An Approach for Inflammatory Bowel Disease Therapy. J. Inflamm. Res. 2024, 17, 5389–5413. [Google Scholar]
- Zhang, P.; Jia, J.; Jiang, P.; Zheng, W.; Li, X.; Song, S.; Ai, C. Polysaccharides from edible brown seaweed Undaria pinnatifida are effective against high-fat diet-induced obesity in mice through the modulation of intestinal microecology. Food Funct. 2022, 13, 2581–2593. [Google Scholar] [CrossRef]
- Yu, M.; Yu, B.; Chen, D. The effects of gut microbiota on appetite regulation and the underlying mechanisms. Gut Microbes 2024, 16, 2414796. [Google Scholar]
- Barakat, G.M.; Ramadan, W.; Assi, G.; Khoury, N.B.E. Satiety: A gut–brain–relationship. J. Physiol. Sci. 2024, 74, 11. [Google Scholar]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [PubMed]
- Meldrum, O.W.; Yakubov, G.E. Journey of dietary fiber along the gastrointestinal tract: Role of physical interactions, mucus, and biochemical transformations. Crit. Rev. Food Sci. Nutr. 2024, 1–29. [Google Scholar] [CrossRef]
- Zheng, W.; Jia, J.; Tang, S.; Song, S.; Ai, C. Undaria pinnatifida fucoidan contributes to anti-inflammation activity of Bacteroides in fiber-deficient mice via modulation of gut microbiota and protection of intestinal barrier integrity. Int. J. Biol. Macromol. 2023, 252, 126256. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Chen, L.; Lu, S.-Y.; Sabir, A.; Chen, J.; Wang, Z.; Veeraperumal, S.; Aweya, J.J.; Chen, X.-Q.; Zhong, S.; et al. Structure–function relationship of the brown seaweed Undaria pinnatifida laminaran: Protein kinase C-mediated mucus secretion and gut barrier restoration. Carbohydr. Polym. 2025, 358, 123525. [Google Scholar]
- Cui, H.; Wang, Z.; Liu, J.; Wang, Y.; Wang, Z.; Fu, J.; Wan, Z.; Li, R.; Li, Q.; Helen Fitton, J.; et al. Effects of a highly purified fucoidan from Undaria pinnatifida on growth performance and intestine health status of gibel carp Carassius auratus gibelio. Aquac. Nutr. 2020, 26, 47–59. [Google Scholar]
- Lai, H.-J.; Doan, H.T.; Lin, E.Y.; Chiu, Y.-L.; Cheng, Y.-K.; Lin, Y.-H.; Chiang, H.-S. Histones of Neutrophil Extracellular Traps Directly Disrupt the Permeability and Integrity of the Intestinal Epithelial Barrier. Inflamm. Bowel Dis. 2023, 29, 783–797. [Google Scholar]
- Hollander, D.; Kaunitz, J.D. The “Leaky Gut”: Tight Junctions but Loose Associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Drug Therapies for Diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar]
- Li, Z.-R.; Jia, R.-B.; Luo, D.; Lin, L.; Zheng, Q.; Zhao, M. The positive effects and underlying mechanisms of Undaria pinnatifida polysaccharides on type 2 diabetes mellitus in rats. Food Funct. 2021, 12, 11898–11912. [Google Scholar] [PubMed]
- Li, F.; Zeng, K.; Ming, J. Lowering glycemic levels via gastrointestinal tract factors: The roles of dietary fiber, polyphenols, and their combination. Crit. Rev. Food Sci. Nutr. 2025, 65, 575–611. [Google Scholar]
- Wei, B.; Wang, L.; Su, L.; Tao, X.; Chen, S.; Wu, J.; Xia, W. Structural characterization of slow digestion dextrin synthesized by a combination of α-glucosidase and cyclodextrin glucosyltransferase and its prebiotic potential on the gut microbiota in vitro. Food Chem. 2023, 426, 136554. [Google Scholar]
- Imai, M.; Kawakami, F.; Chiba, M.; Kanzaki, M.; Maruyama, H. Undaria pinnatifida (Wakame) Intake Ameliorates High-Fat Diet-Induced Glucose Intolerance via Promoting GLUT4 Expression and Membrane Translocation in Muscle. J. Nutr. Metab. 2023, 2023, 9774157. [Google Scholar]
- Animish, A.; Jayasri, M.A. A retrospective review of marine algae and the strategies employed for prospective diabetes management. Algal Res. 2023, 74, 103209. [Google Scholar]
- Sim, S.-Y.; Shin, Y.-E.; Kim, H.-K. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res. 2019, 65, 54–62. [Google Scholar] [CrossRef]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Ørgaard, A.; Holst, J.J. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia 2017, 60, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Wendt, A.; Eliasson, L. Pancreatic alpha cells and glucagon secretion: Novel functions and targets in glucose homeostasis. Curr. Opin. Pharmacol. 2022, 63, 102199. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Keane, K.N.; Carlessi, R.; Cruzat, V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol.-Cell Physiol. 2019, 317, C420–C433. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, K.-L.; Chen, W.; Wang, M.; Zhong, S.; Veeraperumal, S. Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity. Mar. Drugs 2025, 23, 163. https://doi.org/10.3390/md23040163
Cheong K-L, Chen W, Wang M, Zhong S, Veeraperumal S. Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity. Marine Drugs. 2025; 23(4):163. https://doi.org/10.3390/md23040163
Chicago/Turabian StyleCheong, Kit-Leong, Wenjie Chen, Min Wang, Saiyi Zhong, and Suresh Veeraperumal. 2025. "Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity" Marine Drugs 23, no. 4: 163. https://doi.org/10.3390/md23040163
APA StyleCheong, K.-L., Chen, W., Wang, M., Zhong, S., & Veeraperumal, S. (2025). Therapeutic Prospects of Undaria pinnatifida Polysaccharides: Extraction, Purification, and Functional Activity. Marine Drugs, 23(4), 163. https://doi.org/10.3390/md23040163








