Unveiling the Anti-Aging Potential of Marine Natural Bioproducts
Abstract
1. Introduction
2. Aging
3. Anti-Aging Compounds
3.1. Polyunsaturated Fatty Acids
3.2. Vitamins
3.3. Trace Elements and Minerals
3.4. Polyphenols
| Compound Types | Compounds | Example (Molecular Formula, Structure) | Modes of Anti-Aging Action [REF] | Sources (REF) |
|---|---|---|---|---|
| Polyunsaturated fatty acids (PUFAs) | Omega-3 PUFA Omega-6 PUFA | Eicosapentaenoic acid (C2OH30O2, 20:5)  | Maintaining the in vivo redox homeostasis; by lowering oxidative stress and reducing telomere shortening; by down-regulating the antioncogene expression [67]. | Macroalgae [62] Microalgae [59,108] |
| Vitamins | Vitamins A, B, C, D, E | Vitamin A (C20H30O) 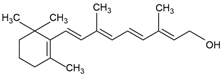 | Antioxidative; modulating the gut microbiota; improving inetene function; neuroprotective effect; enhancing fat metabolism [50] | Macroalgae [21] Microalgae [29] |
| Trace elements (TEs) and minerals | Zinc, copper, selenium, sodium, potassium, calcium | Zn, Cu, Se, Na, K, Ca | Maintaining the in vivo cellular homeostasis; metalloenzymes [36] | Macroalgae [87,88] Microalgae [74] |
| Polyphenols (PPs) | Flavonoids, phlorotannins, phenolic acids, stilbenes, lignans | Phlorotannin: phloroglucinol (C6H6O3) 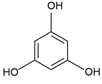 | Antioxidant; anti-inflammatory; anticancer properties [91] | Macroalgae [99,100,105,106] Microalgae [107,109] |
| Mycosporine-like amino acids (MAAs) | Mycosporine glycine, shinorine, porphyra-334, mycosporine- 2-glycine, palythine | MAA direct precursor: 4-deoxygadusol (C8H12O5) 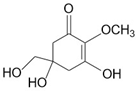 | UV-absorbing property (max range 310–360 nm); antioxidative; anti-inflammatory; anti-adipogenic [51,110] | Macroalgae [111,112,113,114] Microalgae [115,116,117,118] |
| Marine algal polysaccharides (MAPs) | Alginate, carrageenan, fucoidan, ulvan, and laminarin | Fucoidan (C6H9O3SO3)n  | Antioxidants; anti-inflammation; antitumor [119,120] | Macroalgae [53,121] Microalgae [122,123] |
3.5. Amino Acids
3.6. Polysaccharides
| Compound Name (Molecular Formula; Structure) | Source of the Compounds [Reference] | UV-Absorbing Maximum (λmax) |
|---|---|---|
Shinorine (C13H20N2O8)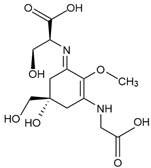 | Macroalgae Red seaweeds [111,112,114,130,142,145,147,165,166,167] Rhodymenia spp., Acanthophora spicifera Gelidium corneum, Georgiella confluens; Gelidium amansii, Gracilaria confervoides, Gracilaria sp., Bostrychia scorpioides, Porphyra dioica Brown seaweeds [111,113,168] Ecklonia radiata, Dictyota bartayresii, Dictyosiphon foeniculaceus, Pilayella littoralis, Ecklonia radiata, Halopteris scoparia, Hydroclathrus clathratus, Sargassum oligocystum Green seaweeds [111] Prasiola crispa Microalgae [153,169,170,171] Chlamydomonas nivalis, Cyclops abyssorum tatricus, Alexandrium sp., Chlamydomonas hedleyi, Gloeodinium viscum, Gymnodinium catenatum, Acetabularia mediterranea Other MAA sources Cyanobacteria [117,172,173,174] Nostoc commune, Aphanothece halophytica, Lyngbya sp. Fungi and Animals [112,116,175] Ascochyta pisi, Knufia cryptophialidica, Emiliania huxleyi; Gymnodinium linucheae Corals, Sea Anemones, Jellyfish | 333 nm |
Porphyra-334 (C14H22N2O8) | 334 nm | |
Mycosporine-glycine (C10H15NO6) | 310 nm | |
Mycosporine-2-glycine (C12H18N2O7)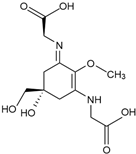 | 332 nm | |
Palythine (C10H16N2O5) | 320 nm |
4. In Vitro, Ex Vivo, and In Vivo Experimental Assessments of Natural Products for Anti-Aging Properties
5. Proteomics for Natural Product Discovery
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C.; Sousa Lobo, J.M.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Fedele, G.; Donatti, C.I.; Bornacelly, I.; Hole, D.G. Nature-dependent people: Mapping human direct use of nature for basic needs across the tropics. Glob. Environ. Change 2021, 71, 102368. [Google Scholar] [CrossRef]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2024, 29, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Vrushali Bothare. Anti-Aging Products Market Size. In Market Research Report; Straits Research: Pune, India, 2024; Available online: https://straitsresearch.com/report/anti-aging-products-market (accessed on 19 January 2025).
- Andrade, L.F.; Hernandez, L.E.; Mashoudy, K.D.; Lalama, M.J.; Saaraswat, M.; Scheinkman, R.J.; Hu, S. A Cost-Based Analysis of Anti-aging Products Across Four Major United States Retailers. Cureus 2023, 15, e46596. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health; WHO Food Additives Series; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Rosic, N.; Remond, C.; Mello-Athayde, M.A. Differential impact of heat stress on reef-building corals under different light conditions. Mar. Environ. Res. 2020, 158, 104947. [Google Scholar] [CrossRef]
- Ewere, E.E.; Rosic, N.; Bayer, P.E.; Ngangbam, A.; Edwards, D.; Kelaher, B.P.; Mamo, L.T.; Benkendorff, K. Marine heatwaves have minimal influence on the quality of adult Sydney rock oyster flesh. Sci. Total Environ. 2021, 795, 148846. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing carbon footprint via microalgae as a biological capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Ralph, P.J.; Pernice, M. Save the planet with green industries using algae. PLoS Biol. 2023, 21, e3002061. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.; Delamare-Deboutteville, J.; Dove, S. Heat stress in symbiotic dinoflagellates: Implications on oxidative stress and cellular changes. Sci. Total Environ. 2024, 944, 173916. [Google Scholar] [CrossRef] [PubMed]
- Schaum, C.E.; Collins, S. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141486. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T.; Bender-Champ, D.; Kubicek, A.; van der Zande, R.; Achlatis, M.; Hoegh-Guldberg, O.; Dove, S.G. The Dynamics of Coral-Algal Interactions in Space and Time on the Southern Great Barrier Reef. Front. Mar. Sci. 2018, 5, 181. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, D.; Saini, R.; Suhag, R.; Thakur, D. Cultivating blue food proteins: Innovating next-generation ingredients from macro and microalgae. Biocatal. Agric. Biotechnol. 2024, 60, 103278. [Google Scholar] [CrossRef]
- Adarshan, S.; Sree, V.S.S.; Muthuramalingam, P.; Nambiar, K.S.; Sevanan, M.; Satish, L.; Venkidasamy, B.; Jeelani, P.G.; Shin, H. Understanding Macroalgae: A Comprehensive Exploration of Nutraceutical, Pharmaceutical, and Omics Dimensions. Plants 2023, 13, 113. [Google Scholar] [CrossRef]
- Rosic, N.; Thornber, C. Biotechnological Potential of Macroalgae during Seasonal Blooms for Sustainable Production of UV-Absorbing Compounds. Mar. Drugs 2023, 21, 633. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; González-Hernández, J.C. Vitamins from microalgae. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: New York, NY, USA, 2023; Chapter 10; p. 111. [Google Scholar]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Martinez, A. Minerals and trace elements in microalgal biomass. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: New York, NY, USA, 2023; Chapter 9; p. 103. [Google Scholar]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Ross, I.L.; Kruse, O.; Hankamer, B. Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wu, B.; Chen, L. Application of Marine Microbial Natural Products in Cosmetics. Front. Microbiol. 2022, 13, 892505. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Su, Y.; Jia, D.; Zeng, Z.; Yang, Y.; Wei, D.; Lu, H.; Cao, Y. Experimentally validated oxidative stress-associated prognostic signatures describe the immune landscape and predict the drug response and prognosis of SKCM. Front. Immunol. 2024, 15, 1387316. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.; Hass, R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol. Chem. 2008, 389, 211–220. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Luo, J.; Ganesan, K.; Xu, B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. J. Fungi 2024, 10, 215. [Google Scholar] [CrossRef]
- Passarino, G.; De Rango, F.; Montesanto, A. Human longevity: Genetics or Lifestyle? It takes two to tango. Immun. Ageing 2016, 13, 12. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Morris, B.J.; Willcox, B.J.; Donlon, T.A. Genetic and epigenetic regulation of human aging and longevity. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1718–1744. [Google Scholar] [CrossRef]
- Navarro, C.; Salazar, J.; Díaz, M.P.; Chacin, M.; Santeliz, R.; Vera, I.; D’Marco, L.; Parra, H.; Bernal, M.C.; Castro, A.; et al. Intrinsic and environmental basis of aging: A narrative review. Heliyon 2023, 9, e18239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 2, 4172. [Google Scholar] [CrossRef]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Moreira, B.R.; Vega, J.; Sisa, A.D.A.; Bernal, J.S.B.; Abdala-Díaz, R.T.; Maraschin, M.; Figueroa, F.L.; Bonomi-Barufi, J. Antioxidant and anti-photoaging properties of red marine macroalgae: Screening of bioactive molecules for cosmeceutical applications. Algal Res. 2022, 68, 102893. [Google Scholar] [CrossRef]
- Elhady, S.S.; Habib, E.S.; Abdelhameed, R.F.A.; Goda, M.S.; Hazem, R.M.; Mehanna, E.T.; Helal, M.A.; Hosny, K.M.; Diri, R.M.; Hassanean, H.A.; et al. Anticancer Effects of New Ceramides Isolated from the Red Sea Red Algae Hypnea musciformis in a Model of Ehrlich Ascites Carcinoma: LC-HRMS Analysis Profile and Molecular Modeling. Mar. Drugs 2022, 20, 63. [Google Scholar] [CrossRef]
- Begolli, R.; Chatziangelou, M.; Samiotaki, M.; Goutas, A.; Barda, S.; Goutzourelas, N.; Kevrekidis, D.P.; Malea, P.; Trachana, V.; Liu, M.; et al. Transcriptome and proteome analysis reveals the anti-cancer properties of Hypnea musciformis marine macroalga extract in liver and intestinal cancer cells. Hum. Genom. 2023, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Ponte, J.M.S.; Seca, A.M.L.; Barreto, M.C. Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition. Molecules 2022, 27, 1787. [Google Scholar] [CrossRef] [PubMed]
- Ridoutt, B.; Lehnert, S.A.; Denman, S.; Charmley, E.; Kinley, R.; Dominik, S. Potential GHG emission benefits of Asparagopsis taxiformis feed supplement in Australian beef cattle feedlots. J. Clean. Prod. 2022, 337, 130499. [Google Scholar] [CrossRef]
- Şimşek, H.; Uçar, A. Polyunsaturated fatty acids as a nutraceutical for age-related neurodegenerative diseases: Current knowledge and future directions. Clin. Nutr. Open Sci. 2024, 56, 65–73. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical Sources of Very Long-Chain Polyunsaturated Fatty Acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Cong, W. Enzyme-Assisted Aqueous Extraction of Lipid from Microalgae. J. Agric. Food Chem. 2012, 60, 11771–11776. [Google Scholar] [CrossRef]
- Ramesh Kumar, B.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of n-6:n-3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Chen, X.; Jiao, J.; Zhang, Y. Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget 2017, 8, 7301–7314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, R.; Cheng, J.; Ye, J.; Qiu, Y.; Huang, S.; Li, M.; Liu, Z.; Pang, J.; Zhang, X.; et al. Effects and Mechanisms of Polyunsaturated Fatty Acids on Age-Related Musculoskeletal Diseases: Sarcopenia, Osteoporosis, and Osteoarthritis—A Narrative Review. Nutrients 2024, 16, 3130. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R. Molecular Mechanism and Pathogenesis of Sarcopenia: An Overview. Int. J. Mol. Sci. 2021, 22, 3032. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-J.; Cui, X.-S.; Shin, S.-H. Increased Omega-3 Fatty Acid Intake Is Associated with Low Grip Strength in Elderly Korean Females. Nutrients 2022, 14, 2374. [Google Scholar] [CrossRef]
- Dupont, J.; Wauters, E.; Dedeyne, L.; Vercauteren, L.; Amini, N.; Lapauw, L.; Matthys, C.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are dietary intake and nutritional status of specific polyunsaturated fatty acids correlated with sarcopenia outcomes in community-dwelling older adults with sarcopenia?—Exploratory results from ENHANce. BMC Geriatr. 2023, 23, 272. [Google Scholar] [CrossRef]
- Ghzaiel, I.; Zarrouk, A.; Nury, T.; Libergoli, M.; Florio, F.; Hammouda, S.; Ménétrier, F.; Avoscan, L.; Yammine, A.; Samadi, M.; et al. Antioxidant Properties and Cytoprotective Effect of Pistacia lentiscus L. Seed Oil against 7β-Hydroxycholesterol-Induced Toxicity in C2C12 Myoblasts: Reduction in Oxidative Stress, Mitochondrial and Peroxisomal Dysfunctions and Attenuation of Cell Death. Antioxidants 2021, 10, 1772. [Google Scholar] [CrossRef]
- Kerdiles, O.; Layé, S.; Calon, F. Omega-3 polyunsaturated fatty acids and brain health: Preclinical evidence for the prevention of neurodegenerative diseases. Trends Food Sci. Technol. 2017, 69, 203–213. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. 1990, 5, 259–263. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Bennis, I.; El Arroussi, H. Recent advances in microalgae-based vitamin D metabolome: Biosynthesis, and production. Bioresour. Technol. 2024, 407, 131078. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.; Alcantar-Rivera, B.; Meléndez-Sánchez, E.R.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldivar, R.; Martínez-Ruiz, M. Effects of UV and UV-vis Irradiation on the Production of Microalgae and Macroalgae: New Alternatives to Produce Photobioprotectors and Biomedical Compounds. Molecules 2022, 27, 5334. [Google Scholar] [CrossRef]
- De Roeck-Holtzhauer, Y.; Quere, I.; Claire, C. Vitamin analysis of five planktonic microalgae and one macroalga. J. Appl. Phycol. 1991, 3, 259–264. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.P.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- Kendler, B.S. Carnitine: An overview of its role in preventive medicine. Prev. Med. 1986, 15, 373–390. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Li, L.; Ou, Z.L. Carnitine promotes recovery from oxidative stress and extends lifespan in C. elegans. Aging 2020, 13, 813–830. [Google Scholar] [CrossRef]
- La Fata, G.; van Vliet, N.; Barnhoorn, S.; Brandt, R.M.C.; Etheve, S.; Chenal, E.; Grunenwald, C.; Seifert, N.; Weber, P.; Hoeijmakers, J.H.J.; et al. Vitamin E Supplementation Reduces Cellular Loss in the Brain of a Premature Aging Mouse Model. J. Prev. Alzheimers Dis. 2017, 4, 226–235. [Google Scholar]
- Martín-Martínez, A.; Sánchez-Marzo, N.; Martínez-Casanova, D.; Abarquero-Cerezo, M.; Herranz-López, M.; Barrajón-Catalán, E.; Matabuena-Yzaguirre, M. High global antioxidant protection and stimulation of the collagen synthesis of new anti-aging product containing an optimized active mix. J. Cosmet. Dermatol. 2022, 21, 3993–4000. [Google Scholar] [CrossRef]
- Baudry, J.; Kopp, J.F.; Boeing, H.; Kipp, A.P.; Schwerdtle, T.; Schulze, M.B. Changes of trace element status during aging: Results of the EPIC-Potsdam cohort study. Eur. J. Nutr. 2020, 59, 3045–3058. [Google Scholar] [CrossRef]
- Ahuja, K.; Lio, P. The role of trace elements in dermatology: A systematic review. J. Integr. Dermatol. 2023. Available online: https://www.jintegrativederm.org/article/73228-the-role-of-trace-elements-in-dermatology-a-systematic-review (accessed on 19 January 2025).
- Kazeminejad, A.; Hajheydari, Z.; Taghian, S.S.; Gholizadeh, N. Serum zinc, selenium, and vitamin D levels in patients with acne vulgaris: A case–control study. J. Cosmet. Dermatol. 2024, 23, 4249–4254. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, G.; Casini, I.; Lucarini, M.; Sánchez del Pulgar, J.; Aguzzi, A.; Caproni, R.; Gabrielli, P.; Lombardi-Boccia, G. Chemical characterization and nutritional evaluation of microalgal biomass from large-scale production: A comparative study of five species. Eur. Food Res. Technol. 2020, 246, 323–332. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef]
- Zhor, C.; Wafaa, L.; Ghzaiel, I.; Kessas, K.; Zarrouk, A.; Ksila, M.; Ghrairi, T.; Latruffe, N.; Masmoudi-Kouki, O.; El Midaoui, A.; et al. Effects of polyphenols and their metabolites on age-related diseases. Biochem. Pharmacol. 2023, 214, 115674. [Google Scholar] [CrossRef]
- Halm, H.; Lüder, U.H.; Wiencke, C. Induction of phlorotannins through mechanical wounding and radiation conditions in the brown macroalga Laminaria hyperborea. Eur. J. Phycol. 2011, 46, 16–26. [Google Scholar] [CrossRef]
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and Antioxidant Responses Upon Short-term Exposure to UV Radiation and Elevated Temperature in Three South Pacific Kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Role of Marine Nutraceuticals in Cardiovascular Health. In Sustained Energy for Enhanced Human Functions and Activity; Bagchi, D., Ed.; Academic Press: New York, NY, USA, 2017; p. 273. [Google Scholar]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.E.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, M.I.; Abou-Dobara, M.I.; Ibrahim, H.A.H.; Abdulhafith, S.A.; Okbah, M.A. Polyphenols in selected marine algae and aromatic herbs with antimicrobial properties: A comparative study. Egypt. J. Aquat. Res. 2024, 50, 71–77. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar. Drugs 2023, 21, 261. [Google Scholar] [CrossRef]
- Sharma, G.; John, J. Identification of polyphenols using UPLC-QTOF MS/MS, in-vitro photoprotective and antiaging activities of brown macroalga Padina tetrastromatica. Algal Res. 2023, 75, 103255. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Chapter Twelve—Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: New York, NY, USA, 2014; p. 345. [Google Scholar]
- Samaddar, S.; Koneri, R. Polyphenols of marine red macroalga Symphyocladia latiuscula ameliorate diabetic peripheral neuropathy in experimental animals. Heliyon 2019, 5, e01781. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Kumar, D.; Saravanan, C.; Badgujar, P.C.; Pharande, A.; Pareek, S.; Fawole, O.A. Polyphenols of Edible Macroalgae: Estimation of In Vitro Bio-Accessibility and Cytotoxicity, Quantification by LC-MS/MS and Potential Utilization as an Antimicrobial and Functional Food Ingredient. Antioxidants 2022, 11, 993. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and Analysis of Mycosporine-Like Amino Acids in Marine Algae. Methods Mol. Biol. 2015, 1308, 119–129. [Google Scholar]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar] [PubMed]
- Guo, X.; Luo, J.; Qi, J.; Zhao, X.; An, P.; Luo, Y.; Wang, G. The Role and Mechanism of Polysaccharides in Anti-Aging. Nutrients 2022, 14, 5330. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Miguel, S.P.; D’Angelo, C.; Ribeiro, M.P.; Simões, R.; Coutinho, P. Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Mar. Drugs 2023, 21, 589. [Google Scholar] [CrossRef]
- Li, S.; Guo, W.; Zhang, M.; Zeng, M.; Wu, H. Microalgae polysaccharides exert antioxidant and anti-inflammatory protective effects on human intestinal epithelial cells in vitro and dextran sodium sulfate-induced mouse colitis in vivo. Int. J. Biol. Macromol. 2024, 254, 127811. [Google Scholar] [CrossRef]
- Severo, I.A.; Dias, R.R.; do Nascimento, T.C.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Microalgae-derived polysaccharides: Potential building blocks for biomedical applications. World J. Microbiol. Biotechnol. 2022, 38, 150. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Rosic, N.N. Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl. Microbiol. Biotechnol. 2012, 94, 29–37. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence Regarding the UV Sunscreen Role of a Mycosporine-Like Compound in the Cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Figueroa, F.L. Mycosporine-Like Amino Acids from Marine Resource. Mar. Drugs 2021, 19, 18. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Woodhouse, J.N.; Liew, H.T.; Sehnal, L.; Pickford, R.; Wong, H.L.; Burns, B.P.; Neilan, B.A. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ. Microbiol. 2019, 21, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B Biol. 2014, 130, 287–292. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution. Photochem. Photobiol. Sci. 2007, 6, 669–674. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef]
- Lopez Figueroa, F.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of Mycosporine-like Amino Acids in Asparagopsis armata Grown in Tanks with Fishpond Effluents of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2008, 39, 692–699. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Effects of UV Radiation on Photosynthesis, Antioxidant Capacity and the Accumulation of Bioactive Compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; West, J.; Wiencke, C. Ultraviolet sunscreen compounds in epiphytic red algae from mangroves. Hydrobiologia 2000, 432, 159–171. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, Isolation and Characterization of Mycosporine-like Amino Acids from Four Species of Red Macroalgae. Mar. Drugs 2021, 19, 615. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.-S.; Seo, H.H.; Song, M.Y.; Kulkarni, A.; Choi, Y.-H.; Kim, K.W.; Moh, S.H. Antiaging Effects of Algae-Derived Mycosporine-Like Amino Acids (MAAs) on Skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; p. 1. [Google Scholar]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory Effects of the Mycosporine-Like Amino Acids Shinorine and Porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef]
- Rosic, N.; Climstein, M.; Boyle, G.M.; Thanh Nguyen, D.; Feng, Y. Exploring Mycosporine-like Amino Acid UV-Absorbing Natural Products for a New Generation of Environmentally Friendly Sunscreens. Mar. Drugs 2023, 21, 253. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Caetano, P.A.; do Nascimento, T.C.; Fernandes, A.S.; Nass, P.P.; Vieira, K.R.; Maróstica Junior, M.R.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae-based polysaccharides: Insights on production, applications, analysis, and future challenges. Biocatal. Agric. Biotechnol. 2022, 45, 102491. [Google Scholar] [CrossRef]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Lin, J.; Jiao, G.; Kermanshahi-Pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y.-J. Antioxidant and anti-photoaging effects of a fucoidan isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar. Biol. 2002, 141, 619–627. [Google Scholar]
- Choi, S.Y.; Lee, S.Y.; Kim, H.G.; Jeong, J.C.; Batara, D.C.; Kim, S.H.; Cho, J.Y. Shinorine and porphyra-334 isolated from laver (Porphyra dentata) inhibit adipogenesis in 3T3-L1 cells. Food Sci. Biotechnol. 2022, 31, 617–625. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Sommaruga, R.; Garcia-Pichel, F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Arch. Hydrobiol. 1999, 144, 255–269. [Google Scholar] [CrossRef]
- Görünmek, M.; Ballık, B.; Çakmak, Z.E.; Çakmak, T. Mycosporine-like amino acids in microalgae and cyanobacteria: Biosynthesis, diversity, and applications in biotechnology. Algal Res. 2024, 80, 103507. [Google Scholar] [CrossRef]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P.; Jeyakumar, R.B.; Bajhaiya, A.K. Microalgae as a Source of Mycosporine-like Amino Acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Hashimoto, A.; Yamaba, M.; Wada, N.; Yoshida, T.; Inoue-Sakamoto, K.; Nishiuchi, T.; Matsugo, S. Four chemotypes of the terrestrial cyanobacterium Nostoc commune characterized by differences in the mycosporine-like amino acids. Phycol. Res. 2019, 67, 3–11. [Google Scholar] [CrossRef]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.P. Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem. Photobiol. 2008, 84, 1500–1505. [Google Scholar] [CrossRef]
- Rosic, N. Molecular Mechanisms of Stress Tolerance in Cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Rastogi, R.P., Ed.; Springer: Singapore, 2021; p. 131. [Google Scholar]
- Shick, J.M.; Dunlap, W.C.; Pearse, J.S.; Pearse, V.B. Mycosporine-like Amino Acid Content in Four Species of Sea Anemones in the Genus Anthopleura Reflects Phylogenetic but Not Environmental or Symbiotic Relationships. Biol. Bull. 2002, 203, 315–330. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Cohen, G.; Jakus, J.; Portillo, M.; Gvirtz, R.; Ogen-Shtern, N.; Silberstein, E.; Ayzenberg, T.; Rozenblat, S. In vitro, ex vivo, and clinical evaluation of anti-aging gel containing EPA and CBD. J. Cosmet. Dermatol. 2023, 22, 3047–3057. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cheng, W.; Yang, G. A novel antioxidant activity index (AAU) for natural products using the DPPH assay. Food Chem. 2011, 125, 1430–1435. [Google Scholar] [CrossRef]
- Storey, A.; McArdle, F.; Friedmann, P.S.; Jackson, M.J.; Rhodes, L.E. Eicosapentaenoic Acid and Docosahexaenoic Acid Reduce UVB- and TNF-α-induced IL-8 Secretion in Keratinocytes and UVB-induced IL-8 in Fibroblasts. J. Investig. Dermatol. 2005, 124, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Pilkington, S.M.; Murphy, S.A.; Carratore, F.D.; Sunarwidhi, A.L.; Kiezel-Tsugunova, M.; Urquhart, P.; Watson, R.E.B.; Breitling, R.; Rhodes, L.E.; et al. Dynamics of the human skin mediator lipidome in response to dietary ω-3 fatty acid supplementation. FASEB J. 2019, 33, 13014–13027. [Google Scholar] [CrossRef]
- Xie, C.-L.; Liu, Q.; Xia, J.-M.; Gao, Y.; Yang, Q.; Shao, Z.-Z.; Liu, G.; Yang, X.-W. Anti-Allergic Compounds from the Deep-Sea-Derived Actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs 2017, 15, 71. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Graves, P.R.; Haystead, T.A. Molecular biologist’s guide to proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine natural products from microalgae: An-omics overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- He, S.-J.; Li, J.; Zhou, J.-C.; Yang, Z.-Y.; Liu, X.; Ge, Y.-W. Chemical proteomics accelerates the target discovery of natural products. Biochem. Pharmacol. 2024, 230, 116609. [Google Scholar] [CrossRef]
- Patwary, Z.P.; Zhao, M.; Wang, T.; Paul, N.A.; Cummins, S.F. A Proteomic Analysis for the Red Seaweed Asparagopsis taxiformis. Biology 2023, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Tucholski, T.; Eken, C.; Knott, S.; Zhu, Y.; Jin, S.; Ge, Y. High-Throughput Proteomics Enabled by a Photocleavable Surfactant. Angew. Chem. Int. Ed. Engl. 2020, 59, 8406–8410. [Google Scholar] [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef]
- Schmollinger, S.; Mühlhaus, T.; Boyle, N.R.; Blaby, I.K.; Casero, D.; Mettler, T.; Moseley, J.L.; Kropat, J.; Sommer, F.; Strenkert, D.; et al. Nitrogen-Sparing Mechanisms in Chlamydomonas Affect the Transcriptome, the Proteome, and Photosynthetic Metabolism. Plant Cell 2014, 26, 1410–1435. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhu, S.; Wang, Z.; Qin, L.; Alam, M.A.; Xie, J.; Yuan, Z. Proteome response of Dunaliella parva induced by nitrogen limitation. Algal Res. 2017, 23, 196–202. [Google Scholar] [CrossRef]
- Mühlroth, A.; Li, K.; Røkke, G.; Winge, P.; Olsen, Y.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Pathways of Lipid Metabolism in Marine Algae, Co-Expression Network, Bottlenecks and Candidate Genes for Enhanced Production of EPA and DHA in Species of Chromista. Mar. Drugs 2013, 11, 4662. [Google Scholar] [CrossRef]
- Andrade, L.M.; Tito, C.A.; Mascarenhas, C.; Lima, F.A.; Dias, M.; Andrade, C.J.; Mendes, M.A.; Nascimento, C.A.O. Chlorella vulgaris phycoremediation at low Cu(+2) contents: Proteomic profiling of microalgal metabolism related to fatty acids and CO(2) fixation. Chemosphere 2021, 284, 131272. [Google Scholar] [CrossRef]
- Plouviez, M.; Dubreucq, E. Key Proteomics Tools for Fundamental and Applied Microalgal Research. Proteomes 2024, 12, 13. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosic, N. Unveiling the Anti-Aging Potential of Marine Natural Bioproducts. Mar. Drugs 2025, 23, 165. https://doi.org/10.3390/md23040165
Rosic N. Unveiling the Anti-Aging Potential of Marine Natural Bioproducts. Marine Drugs. 2025; 23(4):165. https://doi.org/10.3390/md23040165
Chicago/Turabian StyleRosic, Nedeljka. 2025. "Unveiling the Anti-Aging Potential of Marine Natural Bioproducts" Marine Drugs 23, no. 4: 165. https://doi.org/10.3390/md23040165
APA StyleRosic, N. (2025). Unveiling the Anti-Aging Potential of Marine Natural Bioproducts. Marine Drugs, 23(4), 165. https://doi.org/10.3390/md23040165







