Recent Advances in Research on Inhibitory Effects of Seaweed Extracts Against Parasites
Abstract
1. Introduction
2. Retrieval Strategy and Results
2.1. Methods
2.1.1. Study Design and Eligibility Criteria
2.1.2. Search Strategy
2.1.3. Research Selection and Data Extraction
2.2. Results
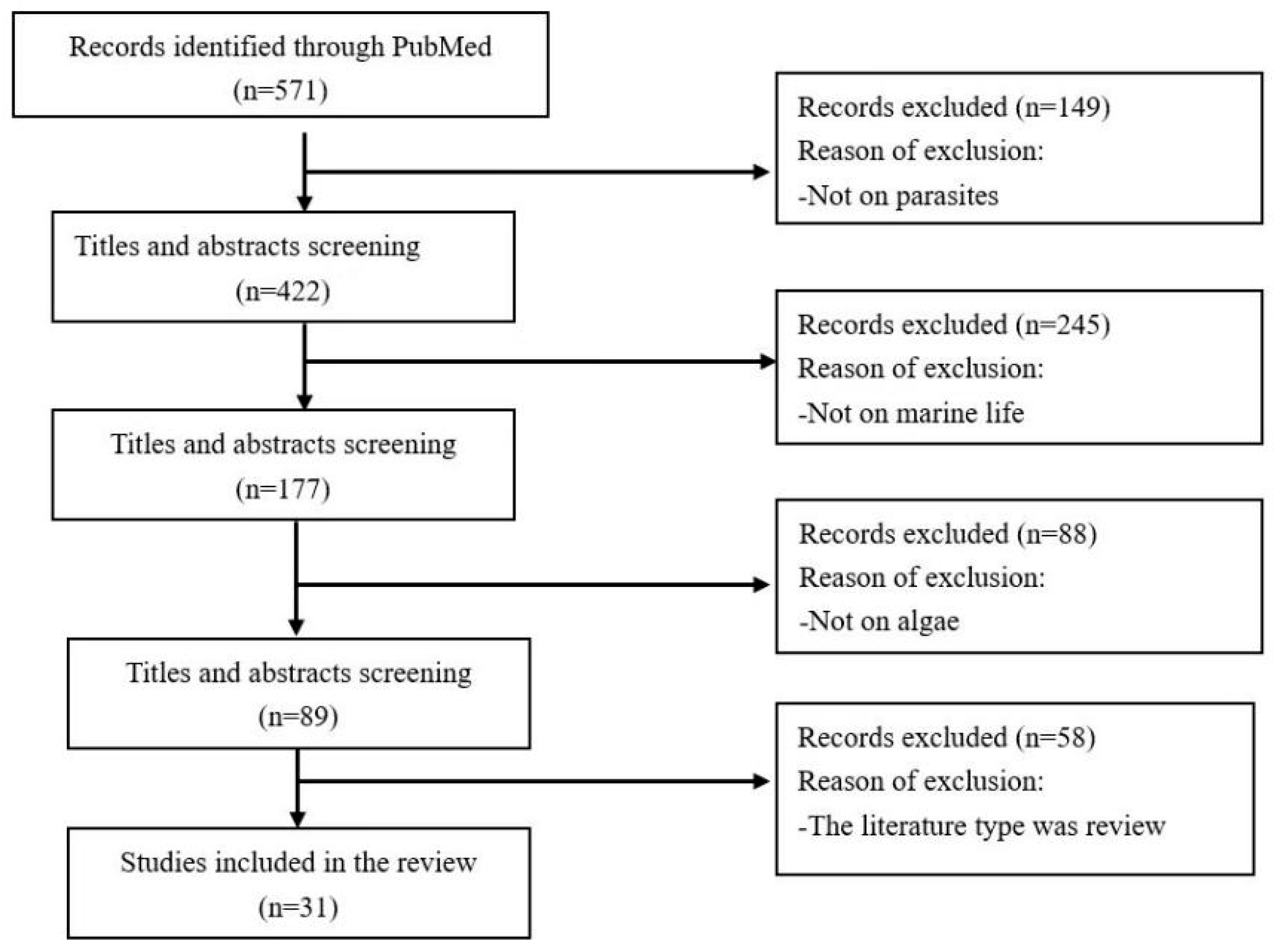
2.2.1. Study Selection
2.2.2. Characteristics Included in the Study
3. Red Algae
3.1. Terpenoids Derived from Red Algae
3.2. Other Components Derived from Red Algae
4. Brown Algae
4.1. Terpenoids Derived from Brown Algae
4.2. Extract Mixture from Brown Algae
5. Green Algae
6. Parasitic Diseases
6.1. Development of Seaweed Extract for Disease Delivery
6.2. The Treatment of Parasitic Diseases with Seaweed Extract
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L. donovani | Leishmania donovani |
| L. amazonensis | Leishmania amazonensis |
| M. infantum | Leishmania infantum |
| L. cruzi | Leishmania cruzi |
| P. falciparum | Plasmodium falciparum |
| S. mansoni | Schistosoma mansoni |
| T. cruzi | Trypanosoma cruzi |
| T. b. rhodesiense | Trypanosoma brucei rhodesiense |
| EC50 | half-maximal effective concentration |
| IC50 | half-maximal inhibitory concentration |
| CC50 | cytotoxic concentration 50 |
| SI | selectivity index |
References
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef] [PubMed]
- Talman, A.M.; Clain, J.; Duval, R.; Ménard, R.; Ariey, F. Artemisinin Bioactivity and Resistance in Malaria Parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.M.; Cruz, I.; Picado, A.; Van Bocxlaer, K.; Croft, S.L. Leishmaniasis immunopathology-impact on design and use of vaccines, diagnostics and drugs. Semin. Immunopathol. 2020, 42, 247–264. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Kedzierski, L.; Andrews, P.C. Do bismuth complexes hold promise as antileishmanial drugs? Future Med. Chem. 2018, 10, 1721–1733. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2025, 405, 937–950. [Google Scholar] [CrossRef]
- Kasozi, K.I.; MacLeod, E.T.; Ntulume, I.; Welburn, S.C. An Update on African Trypanocide Pharmaceutics and Resistance. Front. Vet. Sci. 2022, 9, 828111. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef]
- Silva, M.; Avni, D.; Varela, J.; Barreira, L. The Ocean’s Pharmacy: Health Discoveries in Marine Algae. Molecules 2024, 29, 1900. [Google Scholar] [CrossRef]
- Afolayan, A.F.; Mann, M.G.; Lategan, C.A.; Smith, P.J.; Bolton, J.J.; Beukes, D.R. Antiplasmodial halogenated monoterpenes from the marine red alga Plocamium cornutum. Phytochemistry 2009, 70, 597–600. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.R.; Haddadi, A. Biogenic trypanocidal sesquiterpenes: Lead compounds to design future trypanocidal drugs—A mini review. DARU J. Pharm. Sci. 2013, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Filho, B.P.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Arberas-Jiménez, I.; Nocchi, N.; Chao-Pellicer, J.; Sifaoui, I.; Soares, A.R.; Díaz-Marrero, A.R.; Fernández, J.J.; Piñero, J.E.; Lorenzo-Morales, J. Chamigrane-Type Sesquiterpenes from Laurencia dendroidea as Lead Compounds against Naegleria fowleri. Mar. Drugs 2023, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.L.d.S.; Pacienza-Lima, W.; Rossi-Bergmann, B.; Gestinari, L.M.d.S.; Fujii, M.T.; de Paula, J.C.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the brazilian red Alga Laurencia dendroidea. Planta Medica 2010, 77, 733–735. [Google Scholar] [CrossRef]
- dos Santos, G.S.; Miyasato, P.A.; Stein, E.M.; Colepicolo, P.; Wright, A.D.; Pereira, C.A.d.B.; Falkenberg, M.; Nakano, E. Algal-Derived Halogenated Sesquiterpenes from Laurencia dendroidea as Lead Compounds in Schistosomiasis Environmental Control. Mar. Drugs 2022, 20, 111. [Google Scholar] [CrossRef]
- Topcu, G.; Aydogmus, Z.; Imre, S.; Gören, A.C.; Pezzuto, J.M.; Clement, J.A.; Kingston, D.G. Brominated sesquiterpenes from the red alga Laurencia obtusa. J. Nat. Prod. 2003, 66, 1505–1508. [Google Scholar] [CrossRef]
- Etahiri, S.; Bultel-Poncé, V.; Caux, C.; Guyot, M. New Bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; López-Arencibia, A.; Bethencout-Estrella, C.J.; Cen-Pacheco, F.; Sifaoui, I.; Creus, A.H.; Duque-Ramírez, M.C.; Souto, M.L.; Daranas, A.H.; Lorenzo-Morales, J.; et al. Antiprotozoal activities of marine polyether triterpenoids. Bioorg. Chem. 2019, 92, 103276. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Díaz-Marrero, A.R.; Cen-Pacheco, F.; Sifaoui, I.; Reyes-Batlle, M.; Souto, M.L.; Daranas, A.H.; Piñero, J.E.; Fernández, J.J. Evaluation of Oxasqualenoids from the Red Alga Laurencia viridis against Acanthamoeba. Mar. Drugs 2019, 17, 420. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Lima, J.C.R.; Lechuga, G.C.; Ramos, C.J.B.; Pereira, M.C.d.S.; Calvet, C.M.; Bourguignon, S.C. Natural products from marine red and brown algae against Trypanosoma cruzi. Rev. Bras. Farm. 2019, 29, 735–738. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Genovese, G.; Bruno, F.; Castelli, G.; Piazza, M.; Migliazzo, A.; Minicante, S.A.; Manghisi, A.; Morabito, M. Effectiveness of red alga Asparagopsis taxiformis extracts against Leishmania infantum. Open Life Sci. 2015, 10. [Google Scholar] [CrossRef]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In vitro amoebicidal and antioxidant activities of some Tunisian seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef]
- Gallé, J.B.; Attioua, B.; Kaiser, M.; Rusig, A.M.; Lobstein, A.; Vonthron-Sénécheau, C. Eleganolone, a diterpene from the French marine alga Bifurcaria bifurcata inhibits growth of the human pathogens Trypanosoma brucei and Plasmodium falciparum. Mar. Drugs 2013, 11, 599–610. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a New Antiprotozoal Acyclic Diterpene from the Brown Alga Bifurcaria bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef]
- Chiboub, O.; Sifaoui, I.; Lorenzo-Morales, J.; Abderrabba, M.; Mejri, M.; Fernández, J.J.; Piñero, J.E.; Díaz-Marrero, A.R. Spiralyde A, an Antikinetoplastid Dolabellane from the Brown Alga Dictyota spiralis. Mar. Drugs 2019, 17, 192. [Google Scholar] [CrossRef]
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; Paixão, I.C.N.d.P.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Neglected Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef]
- Santos, A.O.d.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis as an-tileishmanial agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef]
- Miclon, M.; Courtot, É.; Guégnard, F.; Lenhof, O.; Boudesocque-Delaye, L.; Matard-Mann, M.; Collén, P.N.; Castagnone-Sereno, P.; Neveu, C. The Brown Alga Bifurcaria bifurcata Presents an Anthelmintic Activity on All Developmental Stages of the Parasitic Nematode Heligmosomoides polygyrus bakeri. Pathogens 2023, 12, 540. [Google Scholar] [CrossRef]
- Geisshirt, H.A.; Bonde, C.S.; Marcussen, C.; Mejer, H.; Williams, A.R. Development of In Vitro Assays with the Canine Hookworm Uncinaria stenocephala and Assessment of Natural Plant Products for Anti-Parasitic Activity. Pathogens 2023, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, S.S.; Hassan, F.A.M.; Hifney, A.F.; Salem, S.H. Combating the causative agent of amoebic keratitis, Acanthamoeba castellanii, using Padina pavonica alcoholic extract: Toxicokinetic and molecular docking approaches. Sci. Rep. 2024, 14, 13610. [Google Scholar] [CrossRef] [PubMed]
- Ghania, A.; Nabila, B.B.; Larbi, B.; Elisabeth, M.; Philippe, G.; Mariem, B.; Khadidja, K.K.; Wacila, B.R.; Fawzia, A.B. Antimicrobial and antiparasitic activities of three algae from the northwest coast of Algeria. Nat. Prod. Res. 2019, 33, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Aliança, A.S.d.S.; dos Anjos, K.F.L.; Reis, T.N.D.V.; Higino, T.M.M.; Brelaz-De-Castro, M.C.A.; Bianco, É.M.; De Figueiredo, R.C.B.Q. The in vitro biological activity of the brazilian brown seaweed Dictyota mertensii against Leishmania amazonensis. Molecules 2014, 19, 14052–14065. [Google Scholar] [CrossRef]
- Lira, M.-L.F.; Lopes, R.; Gomes, A.P.; Barcellos, G.; Verícimo, M.; Osako, K.; Ortiz-Ramirez, F.A.; Ramos, C.J.B.; Cavalcanti, D.N.; Teixeira, V.L.; et al. Anti-leishmanial activity of Brazilian green, brown, and red algae. J. Appl. Phycol. 2015, 28, 591–598. [Google Scholar] [CrossRef]
- Vonthron-Sénécheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.-M. Antiprotozoal activities of organic extracts from french marine seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef]
- Ainane, T.; Abourriche, A.; Bennamara, A.; Talbi, M.; Lemrani, M. Activité anti-leishmanienne des extraits d’une algue brune Bifurcaria bifurcata de la côte atlantique du Maroc. Phytotherapie 2018, 16, 68–73. [Google Scholar] [CrossRef]
- Ainane, T.; Abourriche, A.; Bennamara, A.; Talbi, M.; Lemrani, M. Anti-leishmanial activity of extracts from a brown seaweed Bifurcaria bifurcata the Atlantic coast of Morocco. Phytothérapie 2015, 1–6. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Zahran, E.M.; Soltane, R.; Alasiri, A.; Saber, H.; Ngwa, C.J.; Pradel, G.; Alsenani, F.; Sayed, A.M.; Abdelmohsen, U.R. New Halogenated Compounds from Halimeda macroloba Seaweed with Potential Inhibitory Activity against Malaria. Molecules 2022, 27, 5617. [Google Scholar] [CrossRef]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of some british green algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008, 120, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z. The antitumor activity of a red alga polysaccharide complexes carrying 5-fluorouracil. Int. J. Biol. Macromol. 2014, 69, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Sathuvan, M.; Thangam, R.; Gajendiran, M.; Vivek, R.; Balasubramanian, S.; Nagaraj, S.; Gunasekaran, P.; Madhan, B.; Rengasamy, R. κ-Carrageenan: An effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr. Polym. 2017, 160, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, J.; Deng, W.; Cao, X.; Wang, Y.; Zhou, J.; Xu, W.; Du, P.; Wang, Q.; Yu, J.; et al. Direct reprogramming of mouse fibroblasts into neural cells via Porphyra yezoensis polysaccharide based high efficient gene co-delivery. J. Nanobiotechnol. 2017, 15, 82. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Zhao, X.; Tang, W.; Wang, F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci. Rep. 2019, 9, 6754. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Pan, Y.; Qu, W.; Hao, H.; Wang, X.; Liu, Z.; Xie, S. Nanoparticles for antiparasitic drug delivery. Drug Deliv. 2019, 26, 1206–1221. [Google Scholar] [CrossRef]
- Veeragoni, D.; Deshpande, S.S.; Singh, V.; Misra, S.; Mutheneni, S.R. In vitro and in vivo antimalarial activity of green synthesized silver nanoparticles using Sargassum tenerrimum—A marine seaweed. Acta Trop. 2023, 245, 106982. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; El-Din, N.G.E.-D.S.; El Maghraby, D.M.; Ibrahim, D.S.S.; Abdel-Megeed, A.; Abdelsalam, N.R. Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci. Rep. 2022, 12, 3841. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.; Yang, X.; Yang, D.; Zhang, T.; Tian, L.; Dao, J.; Feng, Z.; Hu, W. Recent Advances in Research on Inhibitory Effects of Seaweed Extracts Against Parasites. Mar. Drugs 2025, 23, 171. https://doi.org/10.3390/md23040171
Cheng W, Yang X, Yang D, Zhang T, Tian L, Dao J, Feng Z, Hu W. Recent Advances in Research on Inhibitory Effects of Seaweed Extracts Against Parasites. Marine Drugs. 2025; 23(4):171. https://doi.org/10.3390/md23040171
Chicago/Turabian StyleCheng, Wenbing, Xiangyang Yang, Dengfeng Yang, Ting Zhang, Liguang Tian, Jiahao Dao, Zheng Feng, and Wei Hu. 2025. "Recent Advances in Research on Inhibitory Effects of Seaweed Extracts Against Parasites" Marine Drugs 23, no. 4: 171. https://doi.org/10.3390/md23040171
APA StyleCheng, W., Yang, X., Yang, D., Zhang, T., Tian, L., Dao, J., Feng, Z., & Hu, W. (2025). Recent Advances in Research on Inhibitory Effects of Seaweed Extracts Against Parasites. Marine Drugs, 23(4), 171. https://doi.org/10.3390/md23040171








