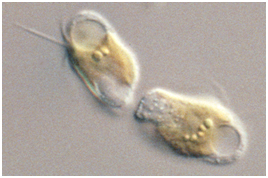
Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta)
Abstract
:1. Introduction
2. Prymnesins
2.1. Isolation and Characterization of Prymnesins
2.2. Putative Synthesis of the Polyketides, Prym1 and Prym2
3. Modes of Prymnesin Toxicity
4. Variables Affecting the Presence and Toxicity of Prymnesins
4.1. Nutrient Availability
4.2. Salinity
4.3. Light
4.4. Temperature
4.5. pH
4.6. Co-factors
4.7. Biotic Factors
5. Biological Relevance of Prymnesins
6. Detection of Prymnesins
7. Conclusions and Future Prospects
Acknowledgements
- Samples Availability: Not available
References and Notes
- Ulitzur, S; Shilo, M. Mode of action of Prymnesium parvum ichthyotoxin. J Protozool 1966, 13, 332–336. [Google Scholar]
- Moestrup, Ø. Economic aspects: Blooms, nuisance species and toxins. In The Haptophyte Algae, The Systematics Association; Green, JC, Leadbeater, BSC, Eds.; Oxford University Press: New York, NY, USA, 1994; Special Volume 51, pp. 265–285. [Google Scholar]
- Edvardsen, B; Paasche, E. Bloom Dynamics and Physiology of Prymnesium and Chrysochromulina. In Physiological Ecology of Harmful Algal Blooms: NATO ASI Series; Anderson, DM, Cembella, AD, Hallegraeff, GM, Eds.; Springer-Verlag: Berlin, Germany, 1998; Volume G41, pp. 193–208. [Google Scholar]
- Houdan, A; Bonnard, A; Fresnel, J; Fouchard, S; Billard, C; Probert, I. Toxicity of coastal coccolithophores (Prymnesiophyceae, Haptophyta). J Plankton Res 2004, 26, 875–883. [Google Scholar]
- Larsen, A; Eikrem, W; Paasche, E. Growth and toxicity in Prymnesium patelliferum (Prymnesiophyceae) isolated from Norwegian waters. Can J Bot 1993, 71, 1357–1362. [Google Scholar]
- Chang, FH; Ryan, KG. Prymnesium calathiferum sp. nov. (Prymnesiophyceae), a new species isolated from Northland, New Zealand. Phycologia 1985, 24, 191–198. [Google Scholar]
- Fresnel, J; Probert, I; Billard, C. Prymnesium faveolatum sp. nov. (Prymnesiophyceae), a new toxic species from the Mediterranean Sea. Vie Milieu 2001, 51, 89–97. [Google Scholar]
- Conrad, W. Notes protistologiques. XXI. Sur les Chrysomonadines à trois fouets. Aperçu synoptique. Bull Mus R Hist Nat Belg 1941, 17, 1–16. [Google Scholar]
- Kell, V; Noack, B. Mass mortality caused by Prymnesium saltans Massart in the Kleiner Jasmunder Bodden (Rugen), April 1990. J. Appl. Ichthyol 1991, 7, 187–192. [Google Scholar]
- Reich, K; Aschner, M. Mass development and control of the phytoflagellate Prymnesium in fish ponds in Palestine. Palestine J Bot 1947, 4, 14–23. [Google Scholar]
- Hallegraeff, GM. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar]
- Larsen, A. Prymnesium parvum and P. patelliferum (Haptophyta) – one species. Phycologia 1999, 38, 541–543. [Google Scholar]
- Meldahl, AS; Fonnum, F. Effect of toxin of Prymnesium patelliferum on neurotransmitter transport mechanisms: development of a sensitive test method. J Toxicol Environ Health 1993, 38, 57–67. [Google Scholar]
- Manton, I; Leedale, GF. Observations on the fine structure of Prymnesium parvum Carter. Arch Mikrobiol 1963, 45, 285–303. [Google Scholar]
- Green, JC; Hibberd, DJ; Pienaar, RN. The taxonomy of Prymnesium (Prymnesiophyceae) including a description of a new cosmopolitan species, P. patellifera sp. nov., and further observations on P. parvum N. Carter. Brit Phycol J 1982, 17, 363–382. [Google Scholar]
- Green, JC; Hori, T. Flagella and flagellar roots. In The Haptophyte Algae, The Systematics Association; Green, JC, Leadbeater, BSC, Eds.; Oxford University Press: New York, USA, 1994; Special Volume 51, pp. 47–71. [Google Scholar]
- Bjørnland, T; Liaaen-Jensen, S. Distribution patterns of carotenoids in relation to chromophyte phylogeny and systematics. In The Chromophyte Algae: Problems and Perspectives; Green, JC, Leadbeater, BSC, Diver, WI, Eds.; Clarendon Press: Oxford, UK, 1989; Systematics Association Special Volume , 38, pp. 37–60. [Google Scholar]
- Jeffrey, SW; Wright, SW. Photosynthetic pigments in the Haptophyta. In The Haptophyte Algae, The Systematics Association; Green, JC, Leadbeater, BSC, Eds.; Oxford University Press: New York, NY, USA, 1994; Special Volume 51, pp. 111–132. [Google Scholar]
- Yoon, HS; Hackett, JD; Pinto, G; Bhattacharya, D. The single ancient origin of chromist plastids. P Nat Acad Sci USA 2002, 99, 15507–15512. [Google Scholar]
- Parke, M; Manton, I; Clarke, B. Studies on marine flagellates: three new species of Chrysochromulina. J Mar Biol Assoc UK 1955, 34, 579–609. [Google Scholar]
- Inouye, I; Kawachi, M. The haptonema. In The Haptophyte Algae, The Systematics Association; Green, JC, Leadbeater, BSC, Eds.; Oxford University Press: New York NY, USA, 1994; Special Volume 51, pp. 73–90. [Google Scholar]
- McLaughlin, JJA. Euryhaline chrysomonads: Nutrition and toxigenesis in Prymnesium parvum, with notes on Isochrysis galbana and Monochrysis lutheri. J Protozool 1958, 5, 75–81. [Google Scholar]
- Liebert, F; Deerns, WM. Onderzoek naar de oorzak van een Vischsterfte in den Polder Workumer Nieuwland, nabij Workum. Verhandungen en Rapporten uitgegeven door Rijkinstituten voor Visscherijonderzoek 1920, 1, 81–93. [Google Scholar]
- Otterstrøm, CV; Steemann-Nielsen, E. Two cases of extensive mortality in fishes caused by the flagellate Prymnesium parvum Carter. Rept Danish Biol Stat 1940, 44, 1–24. [Google Scholar]
- Strodtmann, S. Über die vermeintliche Schädlichkeit der Wasserblüte. Forschungsber Biol Stn Plön 1898, 6, 206–212. [Google Scholar]
- Watson, S. Literature review of the microalga Prymnesium parvum and its associated toxicity. Prepared for the Texas Parks and Wildlife Department. 2001. Available online: http://www.tpwd.state.tx.us/landwater/water/environconcerns/hab/ga/literature/ Accessed December 2009.
- Dickson, DMJ; Kirst, GO. Osmotic adjustment in marine eukaryotic algae: The role of inorganic ions, quaternary ammonium, tertiary sulphonium and carbohydrate solutes. II. Prasinophytes and haptophytes. New Phytol 1987, 106, 657–666. [Google Scholar]
- Guo, MX; Harrison, PJ; Taylor, FJR. Fish kills related to Prymnesium parvum N. Carter (Haptophyta) in the People’s Republic of China. J Appl Phycol 1996, 8, 111–117. [Google Scholar]
- Nicholls, KH. Haptophyte Algae. In Freshwater Algae of North America; Wehr, D, Smith, RG, Eds.; Academic Press: Boston, MA, USA, 2003; pp. 511–521. [Google Scholar]
- Shilo, M. Toxins of Chrysophyceae. In Microbial Toxins; Kadis, S, Ciegler, A, Ajl, SJ, Eds.; Academic Press: New York, NY, USA, 1971; pp. 7–14. [Google Scholar]
- Yariv, J; Hestrin, S. Toxicity of the extracellular phase of Prymnesium parvum cultures. J Gen Microbiol 1961, 24, 165–175. [Google Scholar]
- Paster, Z. Pharmacology and mode of action of prymnesin. In Marine Pharmacognosy: Action of Marine Biotoxins at the Cellular Level; Martin, DF, Padilla, GM, Eds.; Academic Press: New York, NY, USA, 1973; pp. 241–263. [Google Scholar]
- Shilo, M. The toxic principles of Prymnesium parvum. In The Water Environment: Algal Toxins and Health; Carmichael, WW, Ed.; Plenum Press: New York, NY, USA, 1981; pp. 37–47. [Google Scholar]
- Igarashi, T; Satake, M; Yasumoto, T. Prymnesin-2: A potent ichthyotoxic and hemolytic glycoside isolated from the red alga Prymnesium parvum. J Am Chem Soc 1996, 118, 479–480. [Google Scholar]
- Igarashi, T; Satake, M; Yasumoto, T. Structures and partial stereochemical assignments for prymnesin-1 and prymnesin-2: Potent ichthyotoxic and hemolytic glycosides isolated from the red alga Prymnesium parvum. J Am Chem Soc 1999, 121, 8499–8511. [Google Scholar]
- Sasaki, M; Shida, T; Tachibana, K. Synthesis and stereochemical confirmation of the HI/JK ring system of prymnesins, potent hemolytic and ichthyotoxic glycoside toxins isolated from the red tide alga. Tetrahedron Lett 2001, 42, 5725–5728. [Google Scholar]
- Sasaki, M; Ebine, M; Takagi, H; Takakura, H; Shida, T; Satake, M; Oshima, Y; Igarashi, T; Yasumotao, T. Synthesis of the CDE/FG ring models of prymnesins: reassignment of the relative configuration of the E/F ring juncture. Org Lett 2004, 6, 1501–1504. [Google Scholar]
- Ulitzur, S; Shilo, M. Effect of Prymnesium parvum toxin, cetyltrimethyl-ammonium bromide and sodium dodecyl sulphate on bacteria. J Gen Microbiol 1970, 62, 363–370. [Google Scholar]
- Paster, Z. Purification and properties of prymnesin, the toxin formed by Prymnesium parvum (Chrysomonadinae). PhD Thesis, Hebrew University, Jerusalem, Israel, 1968. [Google Scholar]
- Larsen, A; Bryant, S. Growth rate and toxicity of Prymnesium parvum and Prymnesium patelliferum (Haptophyta) in response to changes in salinity, light and temperature. Sarsia 1998, 83, 409–418. [Google Scholar]
- La Claire, JW. II. Analysis of expressed sequence tags from the harmful alga, Prymnesium parvum (Prymnesiophyceae, Haptophyta). Mar Biotech 2006, 8, 534–546. [Google Scholar]
- Baker, JW; Grover, JP; Brooks, BW; Urena-Boeck, F; Roelke, DL; Errera, RM; Kiesling, RL. Growth and toxicity of Prymnesium parvum (Haptophyta) as a function of salinity, light, and temperature. J Phycol 2007, 43, 219–227. [Google Scholar]
- Roelke, DL; Errera, E; Kiesling, R; Brooks, BW; Grover, JP; Schwierzke, L; Urena-Brock, F; Baker, J; Pinckney, JL. Effects of nutrient enrichment on Prymnesium parvum population dynamics and toxicity: Results from field experiments, Lake Possum Kingdom, USA. Aquat Microb Ecol 2007, 46, 125–140. [Google Scholar]
- Carter, N. New or interesting algae from brackish water. Arch Protistenkd 1937, 90, 1–68. [Google Scholar]
- Reich, K; Bergmann, F; Kidron, M. Studies on the homogeneity of prymnesin, the toxin isolated from Prymnesium parvum Carter. Toxicon 1965, 3, 33–39. [Google Scholar]
- Shilo, M; Rosenberger, M. Studies on the toxic principles formed by the chrysomonad Prymnesium parvum Carter. Ann NY Acad Sci 1960, 90, 866–876. [Google Scholar]
- Yariv, J. Toxicity of Prymnesium cultures. In PhD Thesis; Hebrew University: Jerusalem, Israel, 1958. [Google Scholar]
- Ulitzur, S; Shilo, M. Procedure for purification and separation of Prymnesium parvum toxins. Biochim Biophys Acta 1970, 201, 350–363. [Google Scholar]
- Kozakai, H; Oshima, Y; Yasumoto, T. Isolation and structural elucidation of hemolysin from the phytoflagellate Prymnesium parvum.
- Moran, A; Ilani, A. The effect of prymnesin on the electric conductivity of thin lipid membranes. J Membr Biol 1974, 16, 237–256. [Google Scholar]
- Dafni, Z; Shilo, M. The cytotoxic principle of the phytoflagellate Prymnesium parvum. J Cell Biol 1966, 28, 461–471. [Google Scholar]
- Meldahl, AS; Kvernstuen, J; Grasbakken, GJ; Edvardsen, B; Fonnum, F. Toxic Activity of Prymnesium spp. and Chrysochromulina spp. tested by different test methods. Harmful Marine Algal Blooms: Proceedings of the Sixth International Conference on Toxic Marine Phytoplankton, Nantes, France, October 1993, Lassus, P, Arzul, G, Erard-Le Denn, E, Gentien, P, Marcaillou-Le Baut, C, Eds.; Springer-Verlag: New York, NY, USA, 1995; pp. 315–320. [Google Scholar]
- Lindholm, T; Ohman, P; Kurki-Helasmo, K; Kincaid, B; Meriluoto, J. Toxic algae and fish mortality in a brackish-water lake in Aland, SW Finland. Hydrobiologia 1999, 397, 109–120. [Google Scholar]
- Kvernstuen, J. Factors of importance for the stability of a toxic actively extract from the phytoflagellate Prymnesium patelliferum Green, Hibberd & Pienaar. MSc Thesis, University of Oslo, Oslo, Norway, 1993. [Google Scholar]
- Usami, Y. Recent synthesis studies leading to structural revisions of marine natural products. Mar Drugs 2009, 7, 314–330. [Google Scholar]
- Glendenning, L; Igarashi, T; Yasumoto, T. Examination of the conformational properties of the prymnesin system: A computational chemistry approach. Bull Chem Soc Jpn 1996, 69, 2253–2263. [Google Scholar]
- Cane, DE; Walsh, CT. The parallel and convergent universes of polyketide synthases and non-ribosomal peptide synthases. Chem Biol 1999, 6, R319–325. [Google Scholar]
- Mann, J. Secondary metabolites derived from acetate: Fatty acids and polyketides. In Secondary Metabolism, 2nd Ed; Atkins, PW, Holker, JSE, Holliday, AK, Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 25–94. [Google Scholar]
- Baerson, SR; Rimando, AM. A Plethora of Polyketides: Structures, Biological Activities, and Enzymes. In Polyketides: Biosynthesis, Biological Activity, and Genetic Engineering; ACS Symposium Series 955; Rimando, AM, Baerson, SR, Eds.; American Chemical Society: Washington, D.C., USA, 2005; ACS Symposium Series 955; pp. 2–14. [Google Scholar]
- Hopwood, DA; Khosla, C. Genes for polyketide secondary metabolic pathways in microorganisms and plants. Symposium on Secondary Metabolites: Their Function and Evolution, Ciba Foundation: London, UK; 18–20 February 1992, Chadwick, DJ, Whelan, J, Eds.; John Wiley and Sons Ltd: London, UK, 1992; Volume 151, pp. 88–112. [Google Scholar]
- Shimizu, Y. Microalgal metabolites: a new perspective. Annu Rev Microbiol 1996, 50, 431–465. [Google Scholar]
- Murata, M; Yasumoto, T. The structure elucidation and biological activities of high molecular weight algal toxins: Maitotoxin, prymnesins and zooxanthellatoxins. Nat Prod Rep 2000, 17, 293–314. [Google Scholar]
- Doucette, GJ. Interactions between bacteria and harmful algae: A review. Nat Toxins 1995, 3, 65–74. [Google Scholar]
- Perez, R; Liu, L; Lopez, J; An, T; Rein, KS. Diverse bacterial PKS sequences derived from okadaic acid-producing dinoflagellates. Mar Drugs 2008, 6, 164–179. [Google Scholar]
- John, U; Beszteri, B; Derelle, E; Van de Peer, Y; Read, B; Moreau, H; Cembella, A. Novel insights into evolution of protistan polyketide synthases through phylogenomic analyses. Protist 2008, 159, 21–30. [Google Scholar]
- Tsantrizos, YS; Yang, XS. Macrolide and polyether polyketides: Biosynthesis and molecular diversity. In Recent advances in phytochemistry: Evolution of Metabolic Pathways; Romeo, JT, Ibrahim, R, Varin, L, De Luca, V, Eds.; Elsevier Science: Oxford, UK, 2000; Volume 34, pp. 91–108. [Google Scholar]
- Guschina, IA; Harwood, JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 2006, 45, 160–186. [Google Scholar]
- Vogt, T. Glycosyltransferases involved in plant secondary metabolism. In Recent Advances in Phytochemistry: Evolution of Metabolic Pathways; Romeo, JT, Ibrahim, R, Varin, L, De Luca, V, Eds.; Elsevier Science: Oxford, UK, 2000; Volume 34, pp. 317–347. [Google Scholar]
- Vaistij, FE; Lim, EK; Edwards, R; Bowles, DJ. Glycosylation of secondary metabolites and xenobiotics. In Plant-derived Natural Products: Synthesis, Function, and Application; Osbourn, AE, Lanzotti, V, Eds.; Springer: New York, NY, USA, 2009; Volume 1, pp. 209–228. [Google Scholar]
- Ulitzur, S. The amphiphatic nature of Prymnesium parvum hemolysin. Biochim Biophys Acta 1973, 298, 673–679. [Google Scholar]
- Imai, M; Inoue, K. The mechanism on the action of Prymnesium toxin on membranes. Biochim Biophys Acta 1974, 352, 344–348. [Google Scholar]
- Moran, A; Bamberg, E; Froelich, O. Unit response and membrane composition dependence of conductance changes produced by Prymnesium toxin. Isr J Med Sci 1975, 11, 853–853. [Google Scholar]
- Parnas, I; Abbott, BC. Physiological activity of the ichthyotoxin from Prymnesium parvum. Toxicon 1965, 3, 133–145. [Google Scholar]
- Shilo, M. Formation and mode of action of algal toxins. Bacteriol Rev 1967, 31, 180–193. [Google Scholar]
- Meldahl, AS; Aas, P; Fonnum, F. Extract of the marine alga Prymnesium patelliferum induces release of acetylcholine from cholinergic nerves in the rat bronchial smooth tissue. Acta Physiol Scand 1996, 156, 99–107. [Google Scholar]
- Parnas, I; Bergmann, F; Reich, K. Pharmacological effects of ichthyotoxin of Prymnesium parvum. Bull Res Counc Isr 1963, E10, 225. [Google Scholar]
- Meldahl, AS; Fonnum, F. Effect of toxin of Prymnesium patelliferum on neurotransmitter transport mechanisms: Development of a sensitive test method. J Toxicol Environ Health 1993, 38, 57–67. [Google Scholar]
- Meldahl, AS; Fonnum, F. The effects of a purified toxic extract of Prymnesium patelliferum on transport of ions through the plasma membrane of synaptosomes. Toxicon 1995, 33, 1071–1086. [Google Scholar]
- Mariussen, E; Nelson, GN; Fonnum, F. A toxic extract of the marine phytoflagellate Prymnesium parvum induces calcium-dependent release of glutamate from rat brain synaptosomes. J Toxicol Environ Heal A 2005, 68, 67–79. [Google Scholar]
- Padilla, GM; Martin, DF. Interactions of prymnesin with erythrocyte membranes. In Marine Pharmacognosy: Action of Marine Biotoxins at the Cellular Level; Martin, DF, Padilla, GM, Eds.; Academic Press: New York, NY, USA, 1973; pp. 265–295. [Google Scholar]
- Binford, JS, Jr; Martin, DF; Padilla, GM. Hemolysis induced by Prymnesium parvum toxin calorimetric studies.
- Meldahl, AS; Edvardsen, B; Fonnum, F. Toxicity of four potentially ichthyotoxic marine phytoflagellates determined by four different test methods. J Toxicol Environ Health 1994, 42, 289–301. [Google Scholar]
- Shilo, M; Aschner, M. Factors governing the toxicity of cultures containing phytoflagellate Prymnesium parvum Carter. J Gen Microbiol 1953, 8, 333–343. [Google Scholar]
- Rahat, M; Jahn, TL. Growth of Prymnesium parvum in the dark: Note on ichthyotoxin formation. J Protozool 1965, 12, 246–250. [Google Scholar]
- Dafni, Z; Ulitzur, S; Shilo, M. Influence of light and phosphate on toxin production and growth of Prymnesium parvum. J Gen Microbiol 1972, 70, 199–207. [Google Scholar]
- Demain, AL. Microbial secondary metabolism: A new theoretical frontier for academia a new opportunity for industry. Symposium on Secondary Metabolites: Their Function and Evolution, London, UK, 18–20 February 1992, Chadwick, DJ, Whelan, J, Eds.; John Wiley and Sons Ltd: London, UK, 1992; Volume 151, pp. 3–23. [Google Scholar]
- Plumley, FG. Marine algal toxins: Biochemistry, genetics, and molecular biology. Limnol Oceanogr 1997, 42, 1252–1264. [Google Scholar]
- Ulitzur, S; Shilo, M. A sensitive assay system for determination of the ichthyotoxicity of Prymnesium parvum. J Gen Microbiol 1964, 36, 161–169. [Google Scholar]
- Rijn, J; Shilo, M. Environmental factors in fish culture systems. In Fish Culture in Warm Water Systems; Shilo, M, Sarig, S, Eds.; CRC Press Inc: Boca Raton, FL, USA, 1989; pp. 163–177. [Google Scholar]
- Simonsen, S; Moestrup, Ø. Toxicity tests in eight species of Chrysochromulina (Haptophyta). Can J Bot 1997, 75, 129–136. [Google Scholar]
- Collins, M. Algal toxins. Microbiol Rev 1978, 42, 725–746. [Google Scholar]
- Holdway, PA; Watson, RA; Moss, B. Aspects of ecology of Prymnesium parvum (Haptophyta) and water chemistry in Norfolk Broads, England. Freshwater Biol 1978, 8, 295–311. [Google Scholar]
- Sabour, B; Loudiki, LM; Oudra, B; Oubraim, S; Fawzi, B; Fadlaoui, S; Chlaida, M; Vasconcelos, V. Blooms of Prymnesium parvum associated with fish mortalities in a hyper eutrophic brackish lake in Morocco. In Harmful Algae: An IOC Newsletter on Toxic Algae and Algal Blooms; Wyatt, T, Ed.; IOC: Vigo, Spain, 2000; 21. [Google Scholar]
- Legrand, C; Johansson, N; Johnsen, G; Borsheim, KY; Granéli, E. Phagotrophy and toxicity variation in the mixotrophic Prymnesium patelliferum (Haptophyceae). Limnol Oceanogr 2001, 46, 1208–1214. [Google Scholar]
- Johansson, N; Granéli, E. Influence of different nutrient condition on cell density, chemical composition and toxicity of Prymnesium parvum (Haptophyta) in semi-continuous cultures. J Exp Mar Biol Ecol 1999, 239, 243–258. [Google Scholar]
- Hagström, JA; Granéli, E. Removal of Prymnesium parvum (Haptophyceae) cells under different nutrient conditions by clay. Harmful Algae 2004, 4, 249–260. [Google Scholar]
- Padilla, GM. Growth and toxigenesis of the chrysomonad Prymnesium as a function of salinity. J Protozool 1970, 17, 456–462. [Google Scholar]
- Nygaard, K; Tobiesen, A. Bacterivory in algae - a survival strategy during nutrient limitation. Limnol Oceanogr 1993, 38, 273–279. [Google Scholar]
- Martin-Cerceda, M; Novarino, G; Young, JR. Grazing by Prymnesium parvum on small planktonic diatoms. Aquat Microb Ecol 2003, 33, 191–199. [Google Scholar]
- Rahat, M; Reich, K. The B12 vitamins and methionine in the metabolism of Prymnesium parvum. J Gen Microbiol 1963, 31, 203–209. [Google Scholar]
- Carvalho, WF; Granéli, E. Contribution of phagotrophy versus autotrophy to Prymnesium parvum growth under nitrogen and phosphorus sufficiency and deficiency. Harmful Algae 2010, 9, 105–115. [Google Scholar]
- Sundh, I. Characterization of phytoplankton extracellular products (PDOC) and their subsequent uptake by heterotrophic organisms in a mesotrophic forest lake. J Plankton Res 1989, 11, 463–486. [Google Scholar]
- Brand, LE. The salinity tolerance of forty-six marine phytoplankton isolates. Estuar Coast Shelf Sci 1984, 18, 543–556. [Google Scholar]
- Baker, JW; Grover, JP; Ramachandrannair, R; Black, C; Valenti, TW; Brooks, BW; Roelke, DL. Growth at the edge of the niche: An experimental study of the harmful alga Prymnesium parvum. Limnol Oceanogr 2009, 54, 1679–1687. [Google Scholar]
- Reich, K; Rotberg, J. Some factors influencing the production of toxin poisonous to fish in bacteria-free cultures of Prymnesium. Bull Res Counc Isr 1958, 7B, 208. [Google Scholar]
- Reich, K; Parnas, I. Effect of illumination on ichthyotoxin in an axenic culture of Prymnesium parvum Carter. J Protozool 1962, 9, 38–40. [Google Scholar]
- Parnas, I; Spiegelstein, M. Photoinactivation of ichthyotoxin of Prymnesium parvum in cultures and fish ponds. Fish Fish Breed Isr 1963, 1, 13–17. [Google Scholar]
- Rahat, M; Kushnir, M. Toxin synthesis in dark and light grown cultures of Prymnesium parvum. In Toxins from Plant and Animal Origin; de Vries, A, Kochva, E, Eds.; Gordon and Breach: New York, NY, USA, 1971; Volume 1, pp. 191–202. [Google Scholar]
- Parnas, I; Reich, K; Bergmann, F. Photoinactivation of ichthyotoxin from axenic cultures of Prymnesium parvum Carter. Appl Microbiol 1962, 10, 237–239. [Google Scholar]
- Almeida-Paz, FA; Gates, PJ; Fowler, S; Gallimore, A; Harvey, B; Lopes, NP; Stark, J; Staunton, CBW; Klinowski, J; Spencer, JB. Sodium monensin dihydrate. Acta Crystallogr 2003, E59, m1050–m1052. [Google Scholar]
- Berman, I. Formation of toxic principles in concentrated cell suspensions of Prymnesium parvum. MSc, 1960, Thesis, Hebrew Univ., Jerusalem, Israel.
- Vining, LC. Role of secondary metabolites from microbes. Symposium on Secondary Metabolites: Their Function and Evolution, Ciba Foundation, London, UK, 18–20 February 1992, Chadwick, DJ, Whelan, J, Eds.; John Wiley and Sons Ltd: London, UK, 1992; Volume 151, pp. 184–198. [Google Scholar]
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem Rec 2001, 1, 228–242. [Google Scholar]
- Skovgaard, A; Hansen, PJ. Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnol Oceanogr 2003, 48, 1161–1166. [Google Scholar]
- Koski, M; Rosenberg, M; Viitasalo, M; Tanskanen, S; Sjölund, U. Is Prymnesium patelliferum toxic for copepods? – grazing, egg production, and egestion of the calanoid copepod Eurytemora affinis in mixtures of “good” and “bad” food. J Mar Sci 1999, 56(supplement), 131–139. [Google Scholar]
- Granéli, E; Johansson, N. Effects of the toxic haptophyte Prymnesium parvum on the survival and feeding of a ciliate: the influence of different nutrient conditions. Mar Ecol-Prog Ser 2003, 254, 49–56. [Google Scholar]
- Tillmann, U. Kill and eat your predator: A winning strategy of the planktonic flagellate Prymnesium parvum. Aquat Microb Ecol 2003, 32, 73–84. [Google Scholar]
- Nejstgaard, JC; Solberg, PT. Repression of copepod feeding and fecundity by the toxic haptophyte Prymnesium patelliferum. Sarsia 1996, 81, 339–344. [Google Scholar]
- Sopanen, S; Koski, M; Uronen, P; Kuuppo, P; Lehtinen, S; Legrand, C; Tamminen, T. Prymnesium parvum exotoxins affect the grazing and viability of the calanoid copepod Eurytemora affinis. Mar Ecol-Prog Ser 2008, 361, 191–202. [Google Scholar]
- Tillmann, U. Phagotrophy by a plastidic haptophyte Prymnesium patelliferum. Aquat Microb Ecol 1998, 14, 155–160. [Google Scholar]
- Skovgaard, A; Legrand, C; Hansen, PJ; Granéli, E. Effects of nutrient limitation on food uptake in the toxic haptophyte Prymnesium parvum. Aquat Microb Ecol 2003, 31, 259–265. [Google Scholar]
- Arlstad, S. The allelopathic effect of Prymnesium parvum, Prymnesium patelliferum, and Chrysochromulina polylepis. In M.Sc. Thesis; 1991; University of Copenhagen: Demark. [Google Scholar]
- Granéli, E; Weberg, M; Salomon, PS. Harmful algal blooms of allelopathic microalgal species: The role of eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar]
- Legrand, C; Rengefors, K; Fistarol, GO; Granéli, E. Allelopathy in phytoplankton - biochemical, ecological and evolutionary aspects. Phycologia 2003, 42, 406–419. [Google Scholar]
- Fistarol, GO; Legrand, C; Granéli, E. Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar Ecol-Prog Ser 2003, 255, 115–125. [Google Scholar]
- Willis, RJ. The historical bases of the concept of allelopathy. J Hist Biol 1985, 18, 71–102. [Google Scholar]
- Eschbach, E; Scharsack, JP; Uwe, J; Medlin, LK. Improved erythrocyte lysis assay in microtiter plates for sensitive detection and efficient measurement of haemolytic compounds from ichthyotoxic algae. J Appl Toxicol 2001, 21, 512–519. [Google Scholar]
- Igarashi, T; Aritake, S; Yasumoto, T. Biological activities of prymnesin-2 isolated from a red tide alga Prymnesium parvum. Nat Toxins 1998, 6, 35–41. [Google Scholar]
- Simon, N; Brenner, J; Edvardsen, B; Medlin, LK. The identification of Chrysochromulina and Prymnesium species (Haptophyta, Prymnesiophyceae) using fluorescent or chemiluminescent oligonucleotide probes: A means for improving studies on toxic algae. Eur J Phycol 1997, 32, 393–401. [Google Scholar]
- Simon, N; Campbell, L; Ornolfsdottir, E; Groben, R; Guillou, L; Lange, M; Medlin, LK. Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole-cell hybridization. J Eukaryot Microbiol 2000, 47, 76–84. [Google Scholar]
- Lange, M; Guillou, L; Vaulot, D; Simon, N; Amann, RI; Ludwig, W; Medlin, LK. Identification of the Class Prymnesiophyceae and the genus Phaeocystis with ribosomal RNA-targeted nucleic acid probes detected by flow cytometry. J Phycol 1996, 32, 858–868. [Google Scholar]
- Töbe, K; Eller, G; Medlin, LK. Automated detection and enumeration for toxic algae by solid-phase cytometry and the introduction of a new probe for Prymnesium parvum (Haptophyta: Prymnesiophyceae). J Plank Res 2006, 28, 643–657. [Google Scholar]
- West, NJ; Bacchieri, R; Hansen, G; Tomas, C; Lebaron, P; Moreau, H. Rapid quantification of the toxic alga Prymnesium parvum in natural samples by use of a specific monoclonal antibody and solid-phase cytometry. Appl Environ Microb 2006, 72, 860–886. [Google Scholar]
- Manning, SR. Multiplex polymerase chain reaction (PCR) method for the rapid and sensitive species-specific detection of the harmful alga, Prymnesium parvum Carter (Haptophyta). MA Thesis, The University of Texas, Austin, USA, 2006. [Google Scholar]
- Galluzzi, L; Bertozzini, E; Penna, A; Perini, F; Pigalarga, A; Granéli, E; Magnani, M. Detection and quantification of Prymnesium parvum (Haptophyceae) by real-time PCR. Lett Appl Microbiol 2008, 46, 261–266. [Google Scholar]
- Manning, SR; La Claire, JW. II. Multiplex PCR methods for the species-specific detection and quantification of Prymnesium parvum (Haptophyta). J Appl Phycol 2010, in press. [Google Scholar]
- Barkoh, A; Smith, D; Schlechte, JW. An effective minimum concentration of un-ionized ammonia nitrogen for controlling Prymnesium parvum. N Am J Aquacult 2003, 65, 220–225. [Google Scholar]
- Hickel, B. Fish mortality in a carp pond associated with mass development of toxic phytoflagellate Prymnesium parvum Carter (Haptophyceae). Archiv Fischereiwiss 1976, 27, 143–148. [Google Scholar]






| Species | Source |
|---|---|
| Prymnesium parvum | [2,3] |
| P. parvum f. patelliferum | [5] |
| P. calathiferum | [6] |
| P. faveolatum | [7] |
| P. saltans | [7–9*] |
| P. zebrinum | [7] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manning, S.R.; La Claire, J.W., II. Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta). Mar. Drugs 2010, 8, 678-704. https://doi.org/10.3390/md8030678
Manning SR, La Claire JW II. Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta). Marine Drugs. 2010; 8(3):678-704. https://doi.org/10.3390/md8030678
Chicago/Turabian StyleManning, Schonna R., and John W. La Claire, II. 2010. "Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta)" Marine Drugs 8, no. 3: 678-704. https://doi.org/10.3390/md8030678
APA StyleManning, S. R., & La Claire, J. W., II. (2010). Prymnesins: Toxic Metabolites of the Golden Alga, Prymnesium parvum Carter (Haptophyta). Marine Drugs, 8(3), 678-704. https://doi.org/10.3390/md8030678