Anti-Phytopathogenic Activities of Macro-Algae Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity
2.2. In Vitro Antifungal Activity
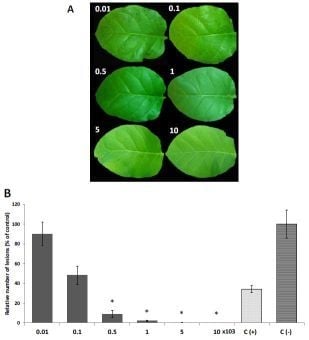
2.3. In Vivo Antifungal Activity
3. Experimental Section
3.1. Algal Materials
3.2. Preparation of Aqueous and Ethanolic Extracts
3.3. Plant Material
3.4. In Vitro Antibacterial Activity Assays
3.5. In Vitro Fungicide Activity Assays
3.6. Virus Bioassay
3.7. In Vivo Assays in Tomato Leaves with Botrytis Cinerea
3.8. Statistics
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Spoel, S; Dong, X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 2008, 3, 348–351. [Google Scholar]
- Dodds, PN; Rathjen, JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nature 2010, 11, 539–548. [Google Scholar]
- Delattre, C; Michaud, B; Courtois, B; Courtois, J. Oligosaccharides engineering from plants and algae applications in biotechnology and therapeutics. Minerva Biotechnol 2005, 17, 107–117. [Google Scholar]
- Chandía, NP; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int J Biol Macromol 2008, 42, 235–240. [Google Scholar]
- Gonzalez del Val, A; Platas, G; Basilio, A; Cabello, A; Garrochategui, J; Suay, I; Vicente, F; Portillo, E; Jimenez del Rio, M; Reina, G; Pelaez, F. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int Microbiol 2001, 4, 35–40. [Google Scholar]
- Newman, D; Cragg, G; Snader, K. Natural products as source of new drugs over the period 1981–2002. J. Nat. Prod 2003, 66, 1022–1037. [Google Scholar]
- Damonte, E; Matulewicz, M; Cerezo, A. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 2004, 11, 2399–2419. [Google Scholar]
- Mayer, AM; Rodríguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim Biophys Acta 2009, 1790, 283–308. [Google Scholar]
- Caccamese, S; Azzolina, R; Furnari, G; Comaxi, M; Grasso, S. Antimicrobial and antiviral activities of extracts from Mediterranean algae. Bot Mar 1980, 23, 285–288. [Google Scholar]
- Manimala, K; Rengasamy, R. Effect of bioactive compounds of seaweeds on the phytopathogen. Xanthomonas oryzae Phycos 1993, 32, 77–83. [Google Scholar]
- Sultana, V; Ehteshamul-Haque, S; Ara, J; Athar, M. Comparative efficacy of brown, green and red seaweeds in the control of root infecting fungi and okra. Int J Environ Sci Technol 2005, 2, 129–132. [Google Scholar]
- Kulic, M. The potential for using Cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur J Plant Pathol 1995, 101, 35–59. [Google Scholar]
- Rizvi, M; Shameel, M. Biological activity and elementology of benthic algae from Karachi coast. Pak J Bot 2003, 35, 717–729. [Google Scholar]
- Ara, J; Sultana, V; Qasim, R; Ehteshamul-Haque, S; Ahmad, V. Biological activity of Spatoglossum asperum: A brown alga. Phytother Res 2005, 19, 618–623. [Google Scholar]
- Kumar, CS; Sarada, D; Rengasamy, R. Seaweed extracts control the leaf spot disease of the medicinal plant Gymnema sylvestre Indian. J Sci Technol 2008, 3, 1–5. [Google Scholar]
- Paulert, R; Talamini, V; Cassolato, J; Duarte, M; Noseda, M; Smania, A; Stadnik, M. Effects of sulfated polysaccharides and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J Plant Dis Prot 2009, 116, 263–270. [Google Scholar]
- Aruna, P; Mansuya, P; Sridhar, S; Suresh, J; Babu, S. Pharmacognostical and antifungal activity of selected seaweeds from Gulf of Mannar region. Recent Res Sci Technol 2010, 2, 115–119. [Google Scholar]
- Arunkumar, K; Sivakumar, S; Rengasamy, R. Review of bioactive potential in seaweeds (Marine macroalgae): A special emphasis on bioactivity of seaweeds against plant pathogens. Asian J Plant Sci 2010, 9, 227–240. [Google Scholar]
- Dang, J; Jones, J. Plant pathogens and integrated defence response to infection. Nature 2001, 411, 926–933. [Google Scholar]
- Gachomo, E; Shonukan, O; Kotchoni, O. The molecular initiation and subsequent acquisition of disease resistance in plants. Afr J Biotechnol 2003, 2, 26–32. [Google Scholar]
- Berger, S; Sinha, A; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J Exp Bot 2007, 58, 4019–4026. [Google Scholar]
- Cul, J; Bahrami, A; Pringle, E; Hernandez-Guzman, G; Bender, C; Pierce, N; Ausubel, F. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 2005, 102, 1791–1796. [Google Scholar]
- Kvitko, B; Collmer, A. Construction of Pseudomonas syringae pv. Tomato DC3000 mutant and polymutant strains. Methods Mol Biol 2011, 712, 109–128. [Google Scholar]
- Chatterjee, A; Cui, Y; Chatterjee, A. RsmC of Erwinia carotovora subsp. carotovora negatively controls motility extracelular protein production, and virulence by binding FlhD and modulating transcriptional activity of the master regulator, FlhDC. J Bacteriol 2009, 191, 4582–4593. [Google Scholar]
- Choquer, M; Fournier, E; Kunz, C. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol Lett 2007, 277, 1–10. [Google Scholar]
- Horta, M; Sousa, N; Coelho, A; Neves, D; Cravador, A. In vitro and in vivo quantification of elicitin expression in Phytophthora cinnamomi. Physiol Mol Plant Pathol 2008, 73, 48–57. [Google Scholar]
- Kuda, T; Ikemori, T. Minerals, Polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 2009, 112, 575–581. [Google Scholar]
- Stern, JL; Hagerman, AE; Steinberg, PD; Winter, FC; Estes, JA. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J Chem Ecol 1996, 22, 1273–1293. [Google Scholar]
- Pavia, H; Toth, GB. Influence of light and nitrogen on phlorotannin content of the Brown seaweeds Ascophyllum nodosum andFucus vesiculosus. Hydrobiologia 2000, 440, 299–305. [Google Scholar]
- Markom, M; Hasan, M; Daud, WR; Singh, H; Jahim, JM. Extraction of hydrolysable tannins from Phyllanthus niruri L.: Effects of solvent and extraction methods. Sep Purif Technol 2007, 52, 487–496. [Google Scholar]
- Cole, MD. Key Antifungal, antibacterial and anti-insect assays—a critical review. Biochem Syst Ecol 1994, 22, 837–856. [Google Scholar]
- Zampini, I; Vattuone, M; Isla, M. Antibacterial activity of Zuccagnia punctata Cav. ethanolic extracts. J Ethnopharmacol 2005, 102, 450–456. [Google Scholar]
- Cos, P; Vlietinck, AJ; Vanden Berghe, D; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “Proof-of-Concept”. J Ethnopharmacol 2006, 106, 290–302. [Google Scholar]
- McManus, PS; Stockwell, V; Sundin, G; Jones, A. Antibiotic use in plant agriculture. Ann Rev Phytopathol 2002, 40, 443–465. [Google Scholar]
- Riggoti, S; Gindro, K; Richter, H; Viret, O. Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: Fr. in strawberry (Fragaria × ananassa Duch.) using PCR. FEMS Microbiol Lett 2002, 209, 169–174. [Google Scholar]
- Bekker, T; Labuschagne, N; Kaiser, C. Effects of soluble silicon against Phytophthora cinnamomi root rot of avocado (Persea americana Mill.) nursey plants. S Afr Avocado Grow Assoc Yearb 2005, 28, 60–64. [Google Scholar]
- Horta, M; Sousa, N; Coelho, A; Neves, D; Cravador, A. In vitro and in vivo quantification of elicitin expression in Phytophthora cinammomi. Physiol Mol Plant Pathol 2008, 73, 48–57. [Google Scholar]
- Salzman, R; Tikhonova, I; Bordelon, B; Hasegawa, P; Bressan, R. Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiol 1998, 117, 465–472. [Google Scholar]
- Kirubakaran, SI; Begum, SM; Ulaganathan, K; Sakthivel, N. Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol Biochem 2008, 46, 918–927. [Google Scholar]
- Zhu, G; Huang, F; Feng, L; Qin, B; Yang, Y; Hen, Y; Lu, X. Sensitivities of Phytophthora infestans to metalaxyl, cymoxanil, and dimethomorph. Agric Sci China 2008, 7, 831–840. [Google Scholar]
- Meepagala, M; Sturtz, G; Wedge, G. Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. Vardracunculus. J Agric Food Chem 2002, 50, 6989–6992. [Google Scholar]
- Alizadeh, H; Sharifi-Tehrani, A; Hedjaroude, G. Evaluation of the effects of chemical versus biological control on Botrytis cinerea agent of gray mould disease of strawberry. Commun Agric Appl Biol Sci 2007, 724, 795–800. [Google Scholar]
- Enyedi, A; Yalpani, N; Silverman, P; Raskin, I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA 1992, 89, 2480–2484. [Google Scholar]
- Lerch, B. On the inhibition of plant virus multiplication by Ribavirin. Antivir Res 1987, 7, 257–270. [Google Scholar]
- Li, W; Xia, Y; Fan, Z; Qu, F; Wu, Q; Peng, L. Bitriazolyl acyclonucleosides with antiviral activity against tobacco mosaic virus. Tetrahedron Lett 2008, 49, 2804–2809. [Google Scholar]
- Zuo, X; Mi, N; Fan, Z; Zheng, Q; Zhang, H; Wang, H; Yang, Z. Synthesis of 4-methyl-1,2,3-thiadiazole derivatives via Ugi reaction and their Biological Activities. J Agric Food Chem 2010, 58, 2755–2762. [Google Scholar]
- The R project for statistical computing, 2010. Available online: http://www.r-project.org (accessed on 25 November 2010).
- Audenaert, K; Geert, B; Meyer, D; Hofte, M. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 2002, 128, 491–501. [Google Scholar]
- Allmendinger, A; Spavieri, J; Kaiser, M; Casey, R; Hingley-Wilson, S; Lalvani, A; Guiry, M; Blunden, G; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother Res 2010, 24, 1099–1103. [Google Scholar]
- Lima-Filho, J; Carvalho, A; Freitas, S; Melo, V. Antibacterial activity of extracts of six macroalgae from the northeastern Brazilian coast. Braz J Microbiol 2002, 33, 311–313. [Google Scholar]
- Bansemir, A; Just, N; Michalik, M; Lindequist, U; Lalk, M. Extracts and sesquiterpene derivatives from the red alga Laurencia chondriodes with antibacterial activity against fish and human pathogenic bacteria. Chem Biodiv 2004, 1, 463–467. [Google Scholar]
- Kolanjinathan, K; Ganesh, P; Govindarajan, M. Antibacterial activity of ethanol extracts of seaweeds against fish bacterial pathogens. Eur Rev Med Pharmacol Sci 2009, 13, 173–177. [Google Scholar]
- Hellio, C; De la Broise, D; Dufosse, L; Gal, L; Bourgougnon, N. Inhibition of marine bacteria by extracts of macroalgae: potential use for environmentally friendly antifouling paints. Mar Environ Res 2001, 52, 231–247. [Google Scholar]
- Selvin, J; Huxley, A; Lipton, A. Immunomodulatory potential of marine secondary metabolites against bacterial diseases of shrimp. Aquaculture 2004, 230, 241–248. [Google Scholar]
- Chakraborty, K; Lipton, A; Paulraj, R; Chakraborty, R. Guaiane sesquiterpenes from seaweed Ulva fasciata Delile and their antibacterial properties. Eur J Med Chem 2010, 45, 2237–2244. [Google Scholar]
- Marechal, JP; Culioli, G; Hellio, C; Thomas-Guyon, H; Callow, M; Clare, A; Ortalo-Magne, A. Seasonal variation in antofouling activity of crude extracts of the brown alga Bifurcaria bifurcate (Cystoseiraceae) against cyprids of Bolanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplannktis. J Exp Mar Biol Ecol 2004, 313, 47–62. [Google Scholar]
- Wiesemeier, T; Hay, M; Pohnert, G. The potential role of wound-activated volatile release in the chemical defence of the brown alga Dictyota dichotoma: Blend recognition by marine herbivores. Aquat Sci 2007, 69, 403–412. [Google Scholar]
- Stout, EP; Hasemeyer, A; Lane, AL; Davenport, T; Engel, S; Hay, ME; Fairchild, CR; Prudhomme, J; Le Roch, K; Aalbersberg, W; Kubanek, J. Antibacterial neurymenolides from the Fijian red alga Neurymenia fraxinifolia. Org Lett 2009, 11, 225–228. [Google Scholar]
- Lane, A; Mular, L; Drenkard, EJ; Shearer, TL; Engel, S; Fredericq, S; Fairchild, CR; Prudhomme, J; Le Roch, K; Hay, ME; Aalbersberg, W; Kubanek, J. Ecological leads for natural product discovery: Novel sesquiterpene hydroquinones from the red macroalga Peyssonnelia sp. Tetrahedron 2010, 66, 455–461. [Google Scholar]
- Raghavendra, V; Lokesh, S; Prakash, H. Dravya, a product of seaweed extracts (Sargassum wightii), induces resistance in cotton against Xanthomonas campestris pv. malvacearum. Phytopathology 2007, 35, 442–449. [Google Scholar]
- Abourriche, A; Charrouf, M; Berrada, M; Bennamara, A; Chaib, N; Francisco, C. Antimicrobial activities and cytotoxity of the brown alga Cystoseira tamariscifolia. Fitoterapia 1999, 70, 611–614. [Google Scholar]
- Bhosale, S; Jagtap, T; Naik, C. Antifungal activity of some marine organisms from India against food spoilage Aspergillus strains. Mycopathologia 1999, 147, 33–138. [Google Scholar]
- Matsuhiro, B; Conte, A; Damonte, E; Kolender, A; Matulewicz, M; Mejias, E; Pujol, C; Zuniga, E. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr Res 2005, 340, 2392–2402. [Google Scholar]
- Pujol, C; Scolaro, L; Cianca, M; Matulewicz, M; Cerezo, A; Damonte, E. Antiviral activity of a carrageenan from Gigartina skottsbergii against intraperitoneal murine herpes simples virus infection. Planta Med 2006, 72, 121–125. [Google Scholar]
- Wang, H; Ooi, E; Ang, P. Antiviral activities of extracts from Hong Kong seaweeds. J Zhejiang Univ Sci B 2008, 9, 969–976. [Google Scholar]
- Rioux, LE; Turgeon, S; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharia longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar]
- Cassolato, J; Noseda, M; Pujol, C; Pellizzari, F; Damonte, E; Duarte, M. Chemical structure and antiviral activity from the green seaweed Gayralia oxysperma. Carbohydr Res 2008, 343, 3085–3095. [Google Scholar]








| Name | Abbreviation | Type | Location | Season 1 Summer | Season 2 Autumn | Season 3 Spring | Season 4 Spring–Summer |
|---|---|---|---|---|---|---|---|
| Macrocystis pyrifera | MP | Brown | Punta Arenas | Jan 2009 | Apr 2009 | Sept 2009 | Nov 2009 |
| Macrocystis integrifolia | MI | Brown | Puerto Aldea | Jan 2009 | May 2009 | Sept 2009 | Nov 2009 |
| Lessonia nigrescens | LN | Brown | Puerto Aldea | Jan 2009 | May 2009 | Sept 2009 | Dec 2009 |
| Lessonia trabeculata | LT | Brown | Puerto Aldea | Jan 2009 | May 2009 | Sept 2009 | Nov 2009 |
| Durvillaea antarctica | DA | Brown | Matanzas | Feb 2009 | May 2009 | Sept 2009 | Dec 2009 |
| Gracilaria chilensis | GC | Red | Quetalmapu | Dec 2008 | Apr 2009 | Sept 2009 | Dec 2009 |
| Porphyra columbina | PC | Red | Punta Arenas | Jan 2009 | Apr 2009 | Sept 2009 | Dec 2009 |
| Gigartina skottsbergii | GS | Red | Punta Arenas | Jan 2009 | Apr 2009 | Sept 2009 | Dec 2009 |
| Ulva costata | UC | Green | Coliumo | Jan 2009 | Apr 2009 | Sept 2009 | Nov 2009 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-Phytopathogenic Activities of Macro-Algae Extracts. Mar. Drugs 2011, 9, 739-756. https://doi.org/10.3390/md9050739
Jiménez E, Dorta F, Medina C, Ramírez A, Ramírez I, Peña-Cortés H. Anti-Phytopathogenic Activities of Macro-Algae Extracts. Marine Drugs. 2011; 9(5):739-756. https://doi.org/10.3390/md9050739
Chicago/Turabian StyleJiménez, Edra, Fernando Dorta, Cristian Medina, Alberto Ramírez, Ingrid Ramírez, and Hugo Peña-Cortés. 2011. "Anti-Phytopathogenic Activities of Macro-Algae Extracts" Marine Drugs 9, no. 5: 739-756. https://doi.org/10.3390/md9050739
APA StyleJiménez, E., Dorta, F., Medina, C., Ramírez, A., Ramírez, I., & Peña-Cortés, H. (2011). Anti-Phytopathogenic Activities of Macro-Algae Extracts. Marine Drugs, 9(5), 739-756. https://doi.org/10.3390/md9050739