Long-Term Pterostilbene Supplementation of a High-Fat Diet Increases Adiponectin Expression in the Subcutaneous White Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Treatments
2.3. RNA and Protein Extraction
2.4. Quantitative Real Time PCR (RT-qPCR)
2.5. Pt Quantification in the Adipose Tissue
2.6. Western Blot
2.7. Statistical Analyses
3. Results
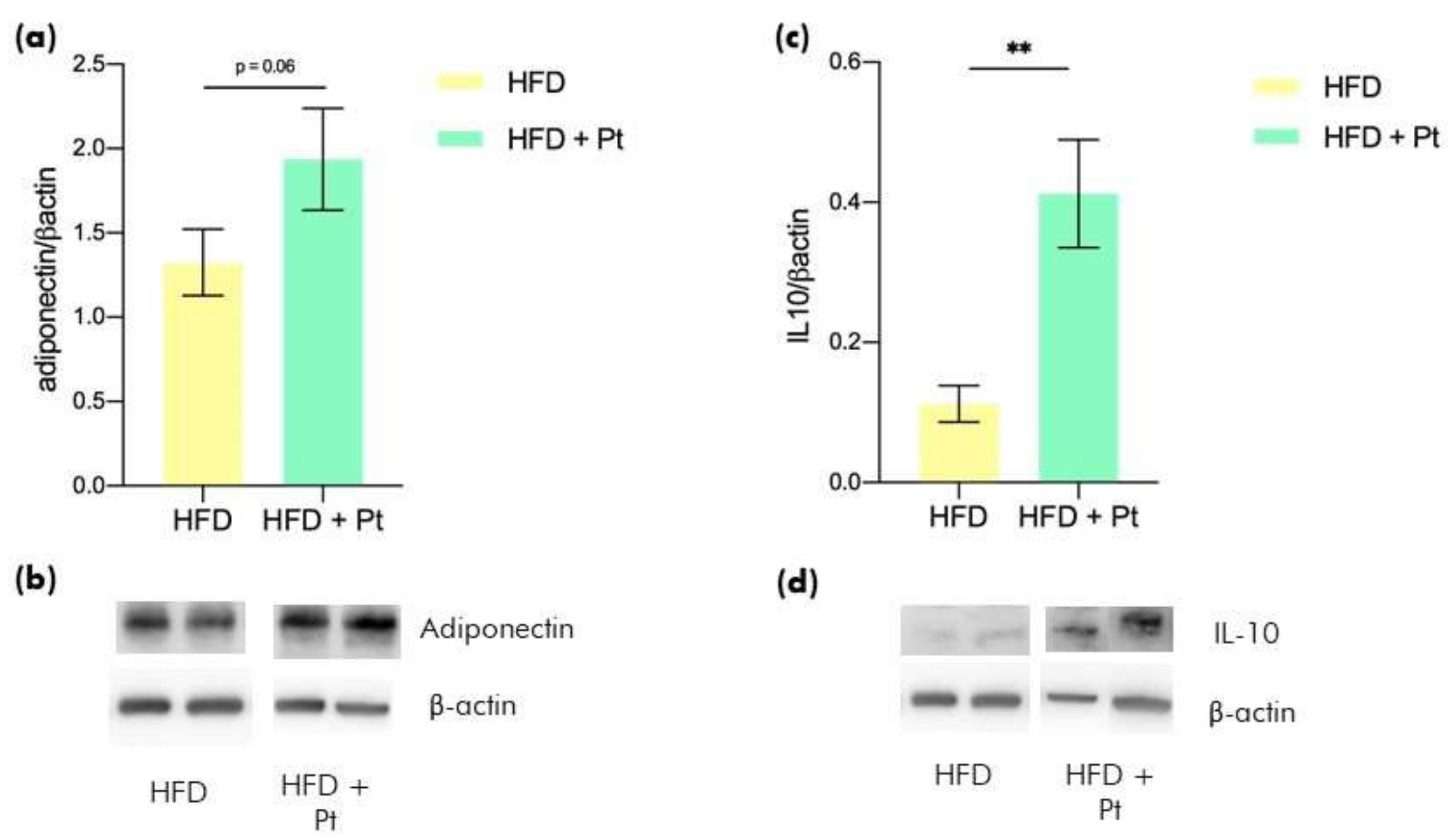
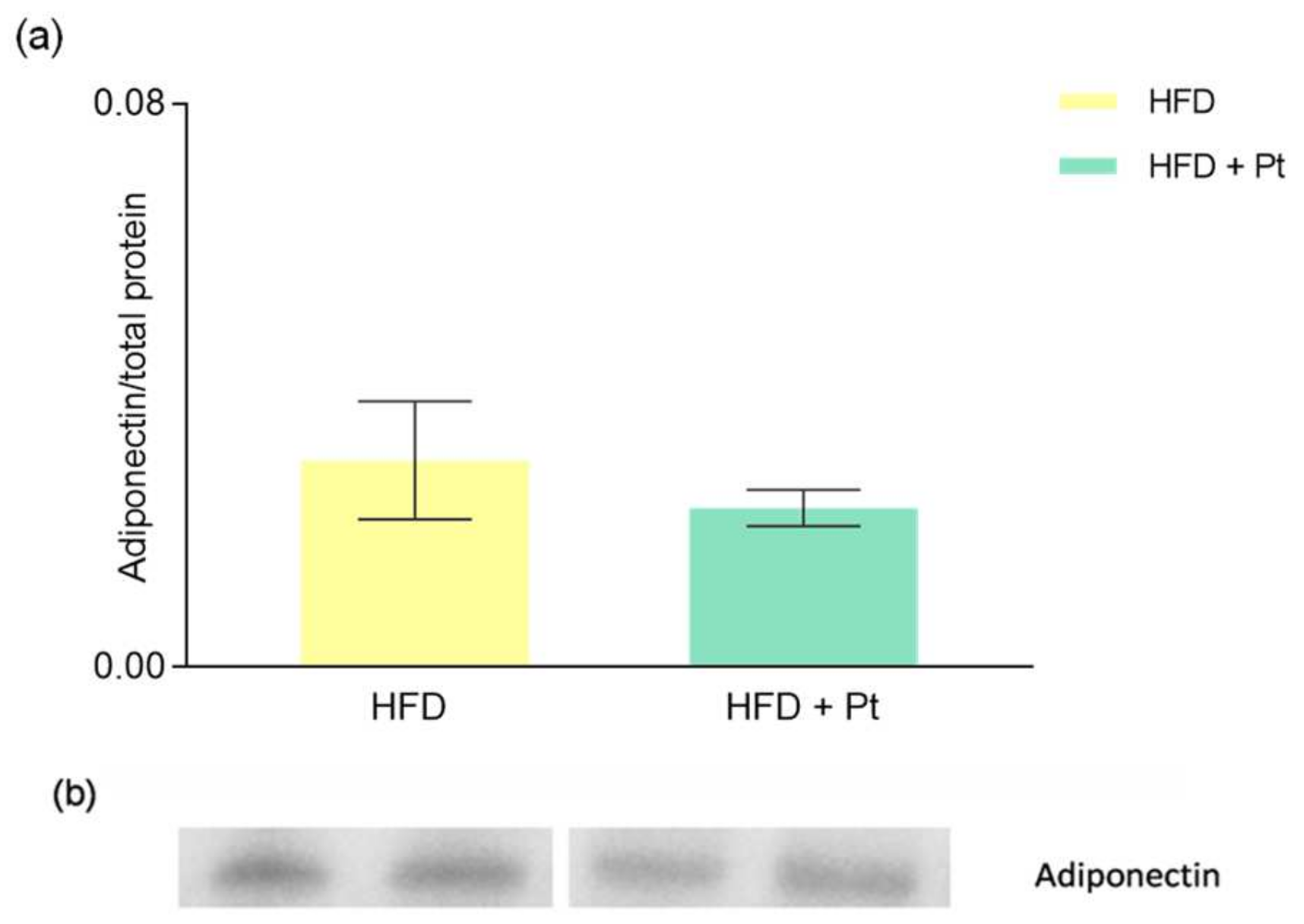
3.1. Effects of Pt on Adiponectin
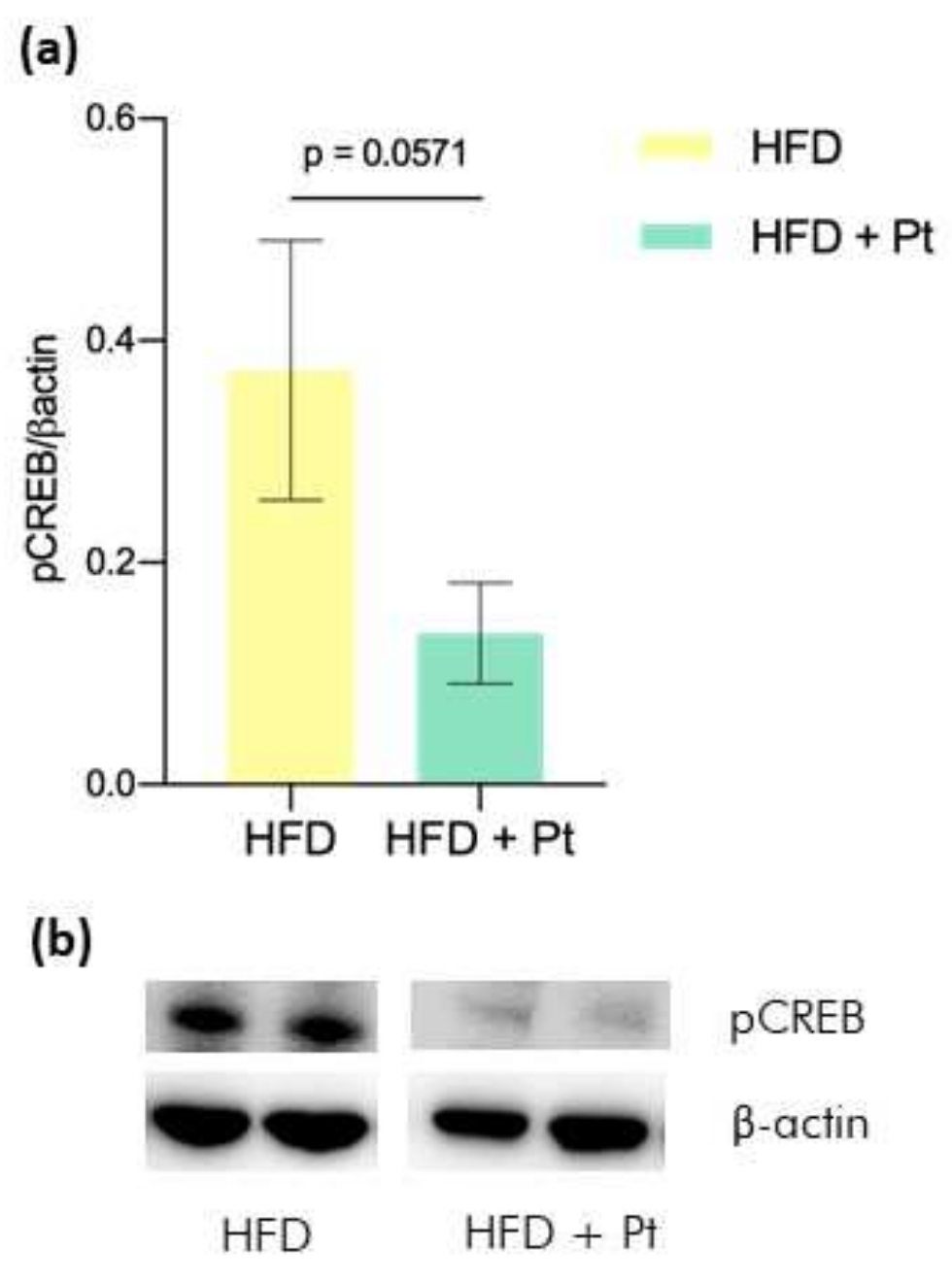
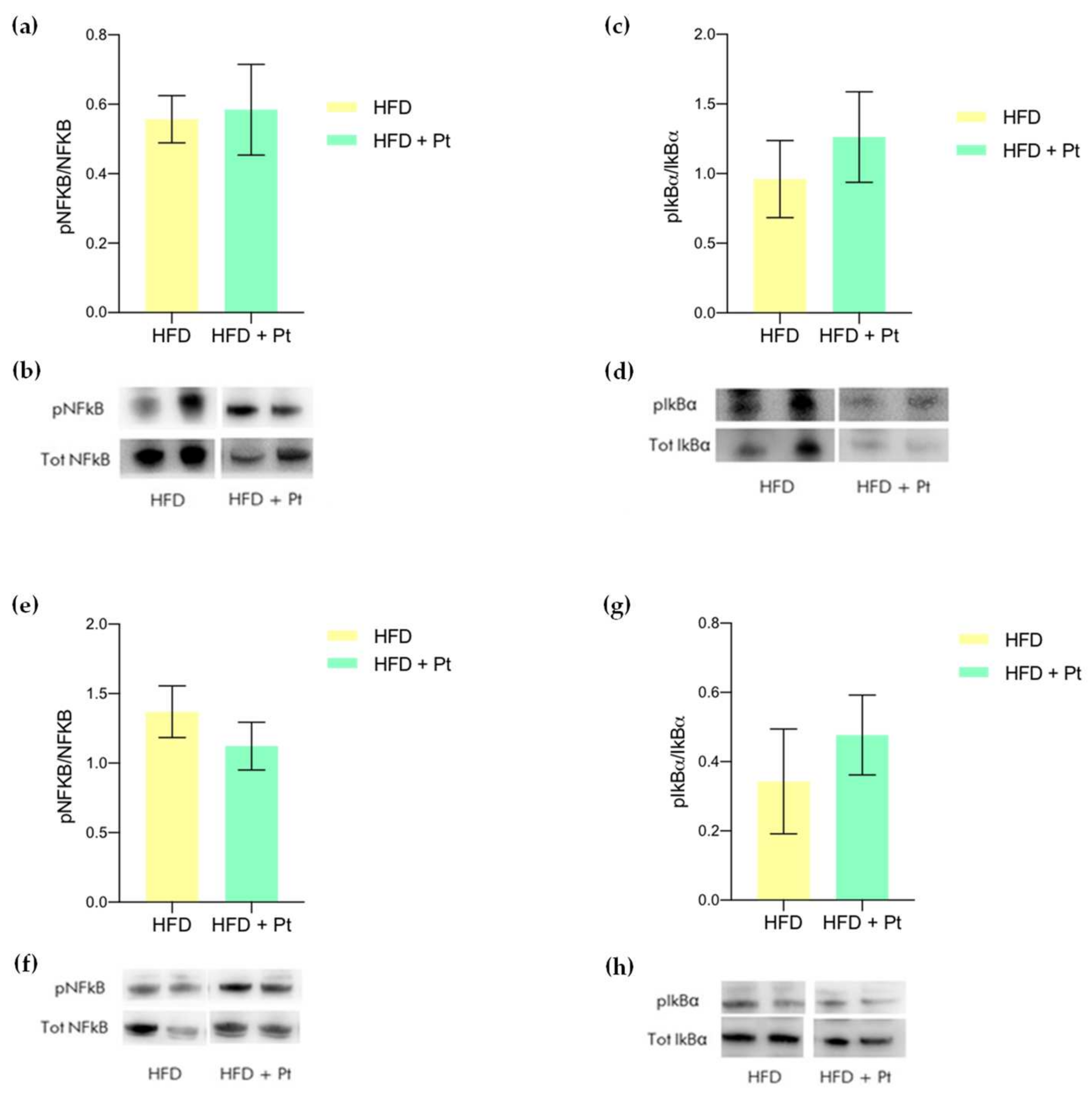
3.2. Effects on CREB
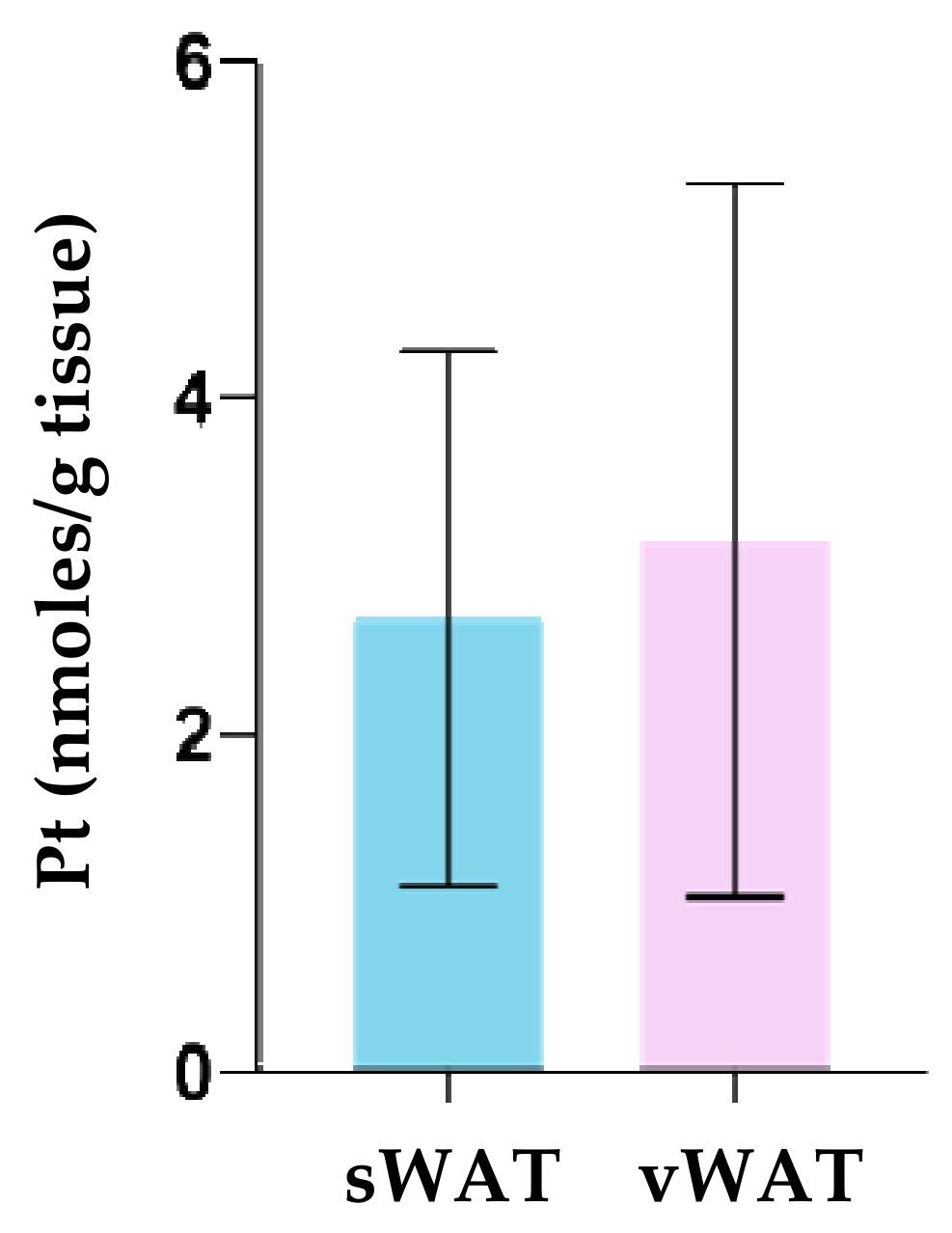
3.3. Pt Quantification in WAT Depots
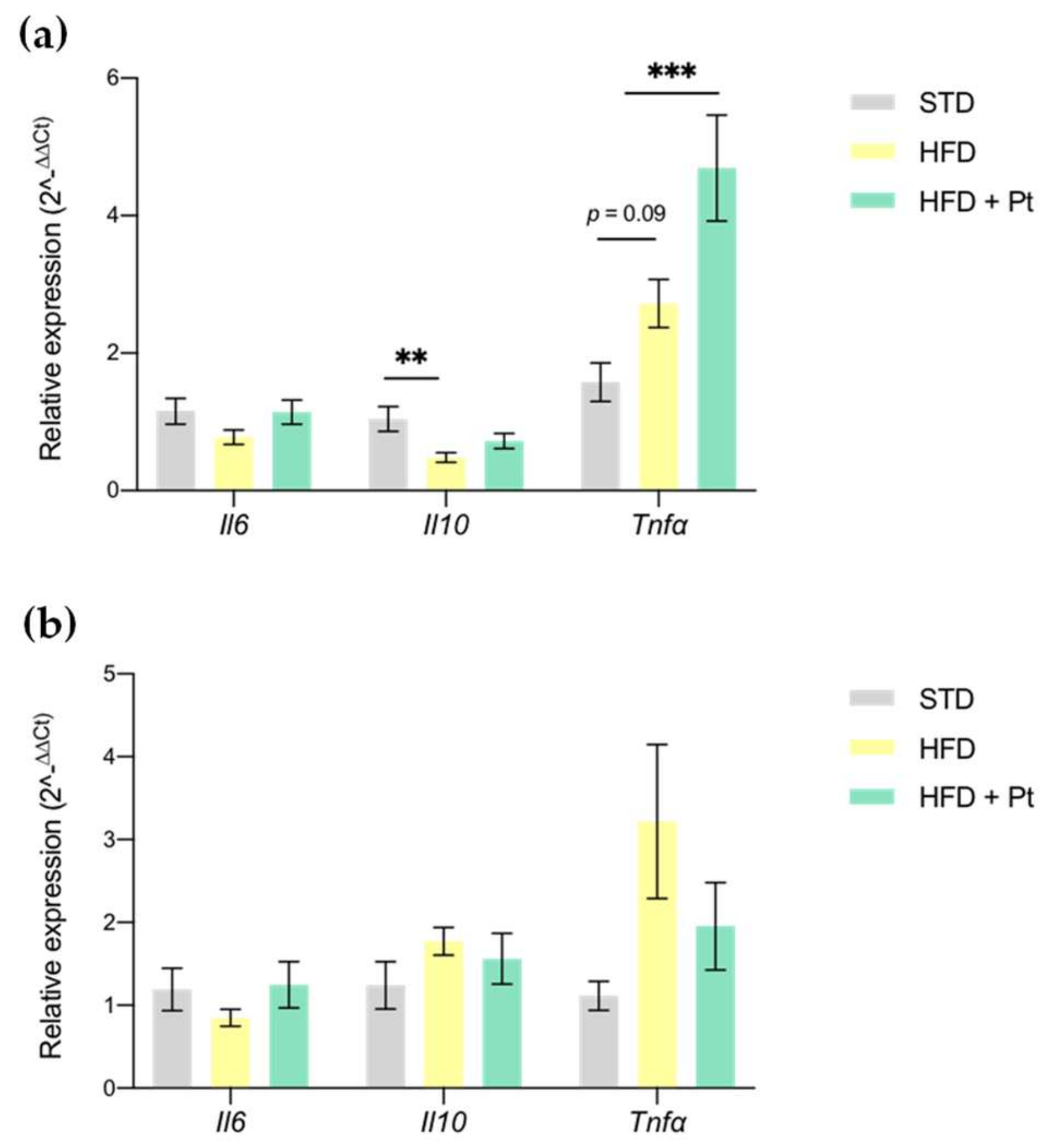
3.4. Effects on Adipose Tissue Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, Y.C.; Ho, C.T.; Pan, M.H. Recent Advances in Health Benefits of Stilbenoids. J. Agric. Food Chem. 2021, 69, 10036–10057. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef] [PubMed]
- Milton-Laskíbar, I.; Gómez-Zorita, S.; Arias, N.; Romo-Miguel, N.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Effects of resveratrol and its derivative pterostilbene on brown adipose tissue thermogenic activation and on white adipose tissue browning process. J. Physiol. Biochem. 2020, 76, 269–278. [Google Scholar] [CrossRef]
- La Spina, M.; Galletta, E.; Azzolini, M.; Gomez Zorita, S.; Parrasia, S.; Salvalaio, M.; Salmaso, A.; Biasutto, L. Browning Effects of a Chronic Pterostilbene Supplementation in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2019, 20, 5377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Aguirre, L.; Crujeiras, A.B.; Irles, E.; Segues, N.; Bujanda, L.; Portillo, M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants 2020, 9, 1042. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Milton-Laskibar, I.; Macarulla, M.T.; Biasutto, L.; Fernández-Quintela, A.; Miranda, J.; Lasa, A.; Segues, N.; Bujanda, L.; Portillo, M.P. Pterostilbene modifies triglyceride metabolism in hepatic steatosis induced by high-fat high-fructose feeding: A comparison with its analog resveratrol. Food Funct. 2021, 12, 3266–3279. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Milton-Laskíbar, I.; Aguirre, L.; Fernández-Quintela, A.; Xiao, J.; Portillo, M.P. Effects of Pterostilbene on Diabetes, Liver Steatosis and Serum Lipids. Curr. Med. Chem. 2021, 28, 238–252. [Google Scholar] [CrossRef]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef]
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef]
- Wang, C.H.; Wei, Y.H. Therapeutic Perspectives of Thermogenic Adipocytes in Obesity and Related Complications. Int. J. Mol. Sci. 2021, 22, 7177. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Calcaterra, V.; Di Profio, E.; Fiore, G.; Rey, F.; Magenes, V.C.; Todisco, C.F.; Carelli, S.; Zuccotti, G.V. Brown Adipose Tissue: New Challenges for Prevention of Childhood Obesity. A Narrative Review. Nutrients 2021, 13, 1450. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Pope, M.; Bloor, I.; Law, J.; Alagal, R.; Budge, H. Adipose tissue growth and development: The modulating role of ambient temperature. J. Endocrinol. 2021, 248, R19–R28. [Google Scholar] [CrossRef] [PubMed]
- Motta, V.F.; Bargut, T.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning is activated in the subcutaneous white adipose tissue of mice metabolically challenged with a high-fructose diet submitted to high-intensity interval training. J. Nutr. Biochem. 2019, 70, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Volke, L.; Krause, K. Effect of Thyroid Hormones on Adipose Tissue Flexibility. Eur. Thyroid J. 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Sentis, S.C.; Oelkrug, R.; Mittag, J. Thyroid hormones in the regulation of brown adipose tissue thermogenesis. Endocr. Connect. 2021, 10, R106–R115. [Google Scholar] [CrossRef]
- Tabuchi, C.; Sul, H.S. Signaling Pathways Regulating Thermogenesis. Front. Endocrinol. 2021, 12, 595020. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Xiang, J.; Zhou, J.; Cao, H.; Che, Q.; Bai, Y.; Guo, J.; Su, Z. Non-shivering Thermogenesis Signalling Regulation and Potential Therapeutic Applications of Brown Adipose Tissue. Int. J. Biol. Sci. 2021, 17, 2853–2870. [Google Scholar] [CrossRef]
- Lu, K.Y.; Primus Dass, K.T.; Tsai, S.F.; Chuang, H.M.; Lin, S.Z.; Liu, S.P.; Harn, H.J. Clinical Application Potential of Small Molecules that Induce Brown Adipose Tissue Thermogenesis by Improving Fat Metabolism. Cell Transplant. 2020, 29, 1–13. [Google Scholar] [CrossRef]
- Hui, X.; Gu, P.; Zhang, J.; Nie, T.; Pan, Y.; Wu, D.; Feng, T.; Zhong, C.; Wang, Y.; Lam, K.S.; et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015, 22, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Lee, J.H.; Wang, H.; Bongmba, O.Y.N.; Wu, C.S.; Pradhan, G.; Sun, Z.; Chew, L.; Bajaj, M.; Chan, L.; et al. Adiponectin is required for maintaining normal body temperature in a cold environment. BMC Physiol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.D.C.; Pazos, P.; Lima, L.; Diéguez, C. Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors. Int. J. Mol. Sci. 2018, 19, 2569. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Odegaard, J.I.; Mukundan, L.; Qiu, Y.; Molofsky, A.B.; Nussbaum, J.C.; Yun, K.; Locksley, R.M.; Chawla, A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015, 160, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-kappaB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Pani, S.; Sethy, C.; Banerjee, A.; Das, S.; Patnaik, S.; Kundu, C.N. Mild cold induced thermogenesis: Are BAT and skeletal muscle synergistic partners? Biosci. Rep. 2017, 37, BSR20171087. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Lian, S.; Li, S.Z.; Guo, J.R.; Wang, J.F.; Wang, D.; Zhang, L.P.; Yang, H.M. GABAB receptor mediate hippocampal neuroinflammation in adolescent male and female mice after cold expose. Brain Res. Bull. 2018, 142, 163–175. [Google Scholar] [CrossRef]
- Wernstedt, I.; Edgley, A.; Berndtsson, A.; Fäldt, J.; Bergström, G.; Wallenius, V.; Jansson, J.O. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R551–R557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egecioglu, E.; Anesten, F.; Schéle, E.; Palsdottir, V. Interleukin-6 is important for regulation of core body temperature during long-term cold exposure in mice. Biomed. Rep. 2018, 9, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, R.P.; Peixoto, M.S.; de Oliveira, K.A.; Ferreira, A.C.F.; Coelho-de-Souza, A.N.; Carvalho, D.P.; de Oliveira, A.C.; Fortunato, R.S. Sex differences in subcutaneous adipose tissue redox homeostasis and inflammation markers in control and high-fat diet fed rats. Appl. Physiol. Nutr. Metab. 2019, 44, 720–726. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mattarei, A.; Rossa, A.; Bombardelli, V.; Azzolini, M.; La Spina, M.; Paradisi, C.; Zoratti, M.; Biasutto, L. Novel lipid-mimetic prodrugs delivering active compounds to adipose tissue. Eur. J. Med. Chem. 2017, 135, 77–88. [Google Scholar] [CrossRef]
- Azzolini, M.; La Spina, M.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014, 58, 2122–2132. [Google Scholar] [CrossRef]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T. Adiponectin/AdipoR Research and Its Implications for Lifestyle-Related Diseases. Front. Cardiovasc. Med. 2019, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Saberi, M.; Zmuda, E.; Wang, Y.; Altarejos, J.; Zhang, X.; Dentin, R.; Hedrick, S.; Bandyopadhyay, G.; Hai, T.; et al. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 2009, 9, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Fernandez-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Pallebage-Gamarallage, M.M.; Lam, V.; Giles, C.; Mamo, J.C. Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood-brain barrier function in wild-type mice. J. Neuroinflamm. 2013, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, C.D.; Bellentani, F.F.; Fernandes, G.S.; Perobelli, J.E.; Favareto, A.P.; Nascimento, A.F.; Cicogna, A.C.; Kempinas, W.D. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod. Biol. Endocrinol. 2011, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Tauer, J.T.; Boraschi-Diaz, I.; Al Rifai, O.; Rauch, F.; Ferron, M.; Komarova, S.V. Male but not female mice with severe osteogenesis imperfecta are partially protected from high-fat diet-induced obesity. Mol. Genet. Metab. 2021, 133, 211–221. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernandez, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Clayton, J.A. Studying both sexes: A guiding principle for biomedicine. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Luong, Q.; Huang, J.; Lee, K.Y. Deciphering White Adipose Tissue Heterogeneity. Biology 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sackmann-Sala, L.; Berryman, D.E.; Munn, R.D.; Lubbers, E.R.; Kopchick, J.J. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity 2012, 20, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caesar, R.; Manieri, M.; Kelder, T.; Boekschoten, M.; Evelo, C.; Muller, M.; Kooistra, T.; Cinti, S.; Kleemann, R.; Drevon, C.A. A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences. PloS ONE 2010, 5, e11525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Kikuchi, R.; Tigges, U.; Yoshida, S.; Denzel, M.S.; Ranscht, B.; Walsh, K. T-cadherin is essential for adiponectin-mediated revascularization. J. Biol. Chem. 2013, 288, 24886–24897. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, H.; Kishida, K.; Sekimoto, R.; Komura, N.; Kihara, S.; Funahashi, T.; Shimomura, I. Accumulation of adiponectin in inflamed adipose tissues of obese mice. Metab. Clin. Exp. 2014, 63, 542–553. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef] [Green Version]
- Benoit, B.; Laugerette, F.; Plaisancié, P.; Géloën, A.; Bodennec, J.; Estienne, M.; Pineau, G.; Bernalier-Donadille, A.; Vidal, H.; Michalski, M.C. Increasing fat content from 20 to 45 wt% in a complex diet induces lower endotoxemia in parallel with an increased number of intestinal goblet cells in mice. Nutr. Res. 2015, 35, 346–356. [Google Scholar] [CrossRef]
- Benoit, B.; Plaisancié, P.; Awada, M.; Géloën, A.; Estienne, M.; Capel, F.; Malpuech-Brugère, C.; Debard, C.; Pesenti, S.; Morio, B.; et al. High-fat diet action on adiposity, inflammation, and insulin sensitivity depends on the control low-fat diet. Nutr. Res. 2013, 33, 952–960. [Google Scholar] [CrossRef]

| Gene | Transcript Accession Number (Ensembl) | Primer |
|---|---|---|
| Adiponectin (Adipoq) | ENSMUST00000023593.6 | F: TGTTCCTCTTAATCCTGCCCA R: CCAACCTGCACAAGTTCCCTT |
| Cd206 (Mrc1) | ENSMUST00000028045.4 | F: TGTGGTGAGCTGAAAGGTGA R: CAGGTGTGGGCTCAGGTAGT |
| Gapdh | ENSMUST00000073605.15 | F: TGTGTCCGTCGTGGATCTGA R: TTGCTGTTGAAGTCGCAGGAG |
| Il6 | ENSMUST00000026845.12 | F: CCAGTTGCCTTCTTGGGACT R: GGTCTGTTGGGAGTGGTATCC |
| Il10 | ENSMUST00000016673.6 | F: GCTCTTACTGACTGGCATGAG R: CGCAGCTCTAGGAGCATGTG |
| Tnfα | ENSMUST00000025263.15 | F: GTGCCTATGTCTCAGCCTCT R: CTGATGAGAGGGAGGCCATT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrasia, S.; Galletta, E.; La Spina, M.; Magrini, A.; Azzolini, M.; Salvalaio, M.; Biasutto, L. Long-Term Pterostilbene Supplementation of a High-Fat Diet Increases Adiponectin Expression in the Subcutaneous White Adipose Tissue. Nutraceuticals 2022, 2, 102-115. https://doi.org/10.3390/nutraceuticals2020008
Parrasia S, Galletta E, La Spina M, Magrini A, Azzolini M, Salvalaio M, Biasutto L. Long-Term Pterostilbene Supplementation of a High-Fat Diet Increases Adiponectin Expression in the Subcutaneous White Adipose Tissue. Nutraceuticals. 2022; 2(2):102-115. https://doi.org/10.3390/nutraceuticals2020008
Chicago/Turabian StyleParrasia, Sofia, Eva Galletta, Martina La Spina, Arianna Magrini, Michele Azzolini, Marika Salvalaio, and Lucia Biasutto. 2022. "Long-Term Pterostilbene Supplementation of a High-Fat Diet Increases Adiponectin Expression in the Subcutaneous White Adipose Tissue" Nutraceuticals 2, no. 2: 102-115. https://doi.org/10.3390/nutraceuticals2020008
APA StyleParrasia, S., Galletta, E., La Spina, M., Magrini, A., Azzolini, M., Salvalaio, M., & Biasutto, L. (2022). Long-Term Pterostilbene Supplementation of a High-Fat Diet Increases Adiponectin Expression in the Subcutaneous White Adipose Tissue. Nutraceuticals, 2(2), 102-115. https://doi.org/10.3390/nutraceuticals2020008






