Food Antioxidants and Aging: Theory, Current Evidence and Perspectives
Abstract
:Introduction
1. Aging Theories
1.1. Genetic Factors Associated with Aging
1.1.1. Program Theory
1.1.2. Error Theory
1.2. Non-Genetic Factors Associated with Aging
1.2.1. Wear-and-Tear Theory
1.2.2. Cross-Linking Theory
1.2.3. Autoimmune Theory
1.2.4. Glycation Theory
1.2.5. Oxidative Damage Theory
1.2.6. Other Biological Aging-Related Theories
1.3. Sociology of Aging
1.3.1. Disengagement Theory
1.3.2. Activity Theory
1.3.3. Life-Course Theory
1.3.4. Continuity Theory
1.4. Aging and Senescence
2. Food and Aging
2.1. Potential Foods for Anti-Aging
2.2. Mediterranean Diet
2.3. Other Traditional Foods
2.4. Individual Foodstuffs
2.4.1. Fruits and Vegetables
2.4.2. Nuts
2.4.3. Beverages
2.5. Age and Food Intake
2.6. Potential Foods That May Accelerate Aging
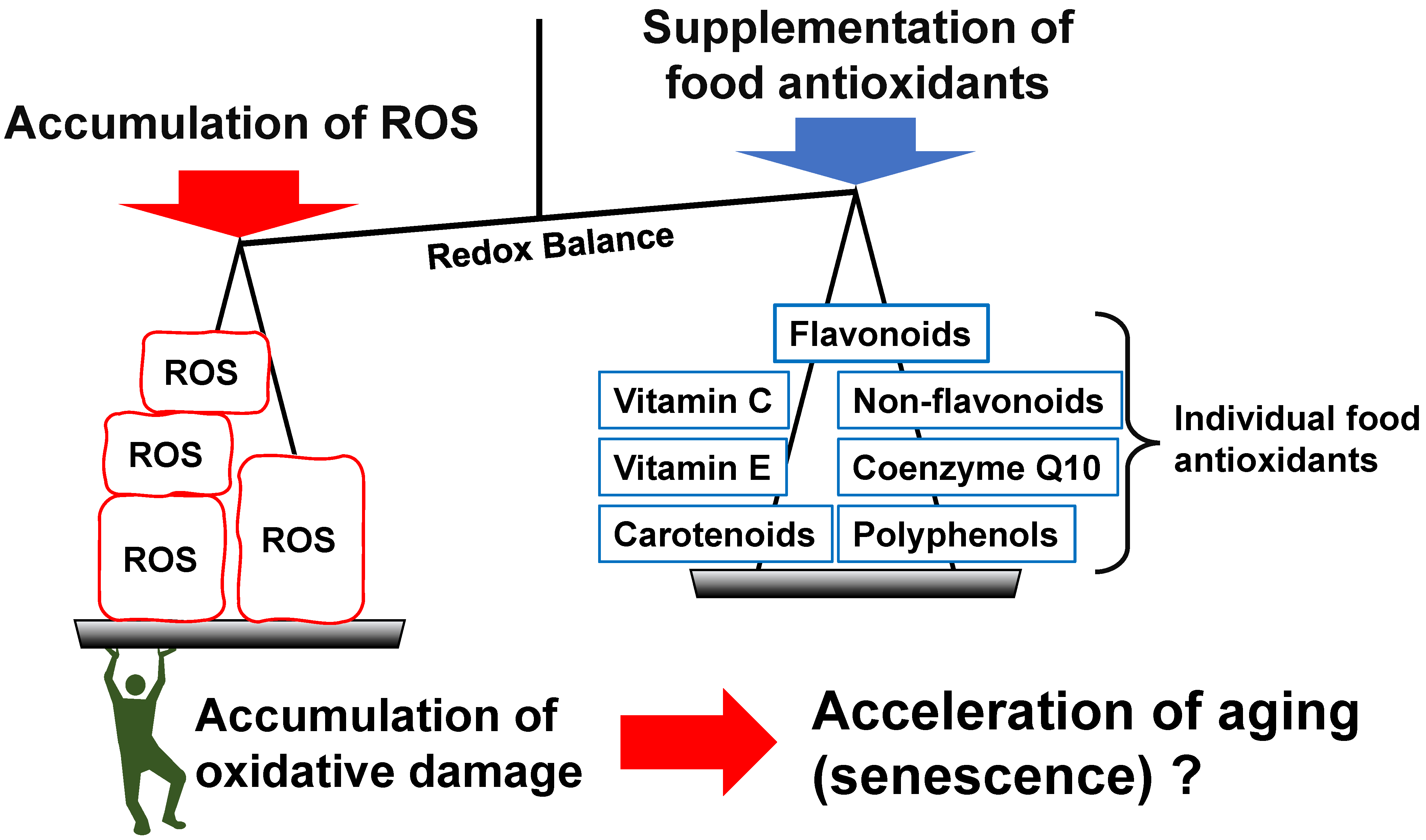
3. Individual Food Antioxidants and Aging
3.1. Vitamins
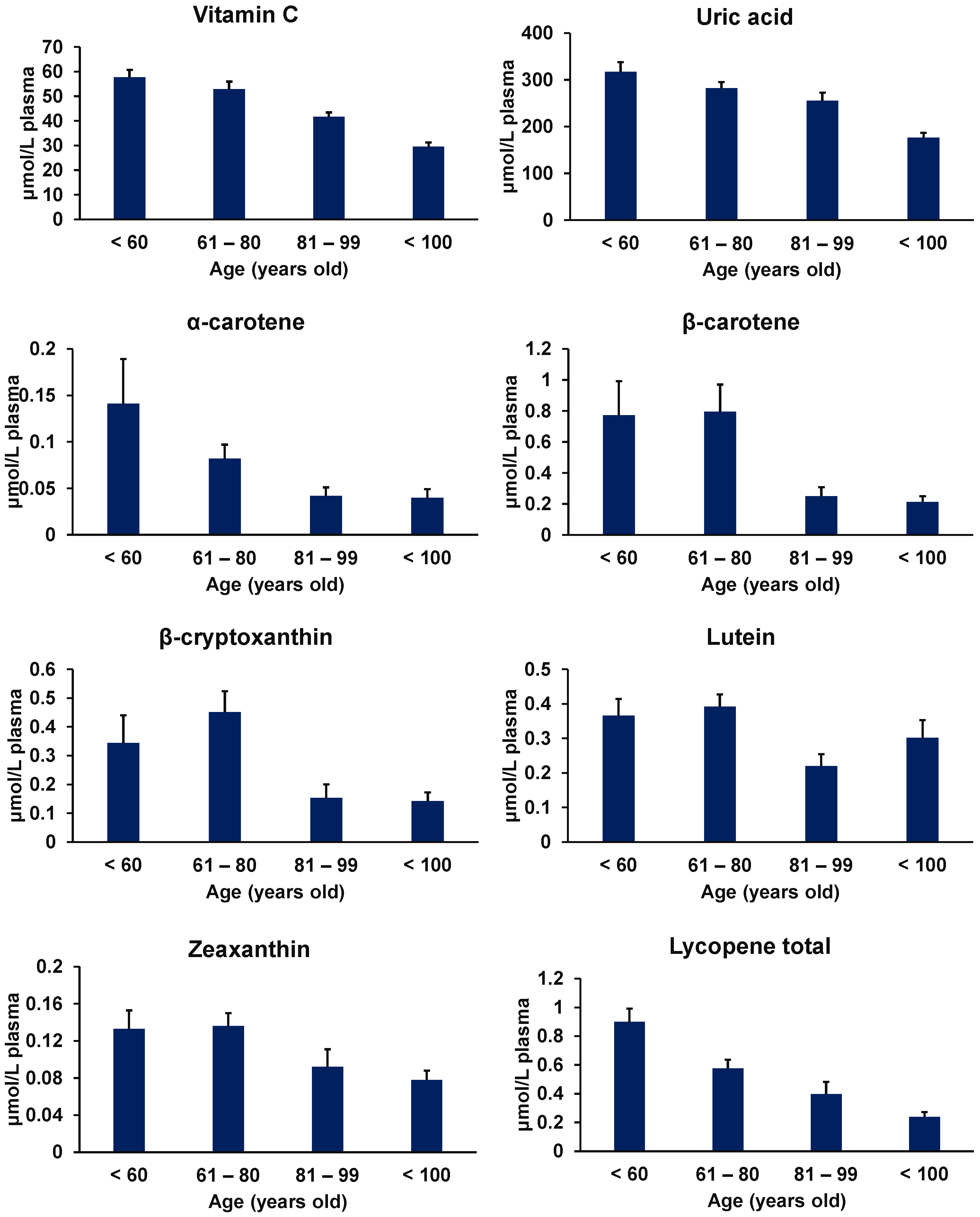
3.1.1. Vitamin C
3.1.2. Vitamin E
3.1.3. Carotenoids
3.2. Polyphenols
3.2.1. Flavonoids
3.2.2. Non-Flavonoids
3.3. Coenzyme Q10
4. Limitations of the Current Investigations
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colloca, G.; Di Capua, B.; Bellieni, A.; Fusco, D.; Ciciarello, F.; Tagliaferri, L.; Valentini, V.; Balducci, L. Biological and functional biomarkers of aging: Definition, characteristics, and how they can impact everyday cancer treatment. Curr. Oncol. Rep. 2020, 22, 115. [Google Scholar] [CrossRef]
- Gladyshev, T.V.; Gladyshev, V.N. A disease or not a disease? Aging as a pathology. Trends. Mol. Med. 2016, 22, 995–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Vina, J.; Borras, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Vidacek, N.S.; Nanic, L.; Ravlic, S.; Sopta, M.; Geric, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferron, S.R.; Marques-Torrejon, M.A.; Mira, H.; Flores, I.; Taylor, K.; Blasco, M.A.; Farinas, I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 2009, 29, 14394–14407. [Google Scholar] [CrossRef]
- Derevyanko, A.; Whittemore, K.; Schneider, R.P.; Jiménez, V.; Bosch, F.; Blasco, M.A. Gene therapy with the TRF1 telomere gene rescues decreased TRF1 levels with aging and prolongs mouse health span. Aging Cell 2017, 16, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, B.B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA 1963, 49, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troen, B.R. The biology of aging. Mt. Sinai J. Med. 2003, 70, 3–22. [Google Scholar] [PubMed]
- Edelmann, P.; Gallant, J. On the translational error theory of aging. Proc. Natl. Acad. Sci. USA 1977, 74, 3396–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weismann, A. Essays Upon Heredity and Kindred Biological Problems; Poulton, E.B., Selmar, S., Arthur, S.E., Eds.; Clarendon Press: Oxford, UK, 1889. [Google Scholar]
- Hanson, R.W.; Hakimi, P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie 2008, 90, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorksten, J. The Crosslinkage Theory of Aging. Finska Kemists. Medd. 1971, 2, 23–38. [Google Scholar] [CrossRef]
- Monnier, V.M.; Mustata, G.T.; Biemel, K.L.; Reihl, O.; Lederer, M.O.; Dai, Y.; Sell, D.R. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: An update on “a puzzle nearing resolution”. Ann. N. Y. Acad. Sci. 2005, 1043, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Bjorksten, J. Theoretical Aspects of Aging; Rockstein, M., Ed.; Academic Press: New York, NY, USA, 1974; p. 43. [Google Scholar]
- Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Toubi, E.; Porat, B.-S.; Shoenfeld, Y. Autoimmunity in the elderly: Insights from basic science and clinics-a mini-review. Gerontology 2017, 63, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B cell dysfunction associated with aging and autoimmune diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- Shanley, D.P.; Aw, D.; Manley, N.R.; Palmer, D.B. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009, 30, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Stroehla, B.C. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2003, 2, 119–125. [Google Scholar] [CrossRef]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycationLa réaction de Maillard dans le corps humain. Découvertes majeures et facteurs qui affectent la glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Simm, A. Protein glycation during aging and in cardiovascular disease. J. Proteom. 2013, 92, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Sohal, R.S. DNA oxidative damage and life expectancy in houseflies. Proc. Natl. Acad. Sci. USA 1994, 91, 12332–12335. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 161–196. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Kulinsky, V.I. Biochemical aspects of inflammation. Biochemistry 2007, 72, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Witkowski, J.M. Human Inflammaging. Gerontology 2019, 65, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020, 64, 101142. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Awad, P.; Campisi, J.; Desprez, P.Y. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012, 5, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Colchero, F.; Aburto, J.M.; Archie, E.A.; Boesch, C.; Breuer, T.; Campos, F.A.; Collins, A.; Conde, D.A.; Cords, M.; Crockford, C.; et al. The long lives of primates and the ‘invariant rate of ageing’ hypothesis. Nat. Commun. 2021, 12, 3666. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.H. Successful Aging. Gerontologist 1961, 1, 8–13. [Google Scholar]
- Cumming, E.; Henry, W. Growing Old: The Process of Disengagement. Basic Books, New York, 1961. (Reprint: Arno, New York, 1979, ISBN 0405 118147). Ageing Soc. 1991, 11, 217–220. [Google Scholar]
- Atchley, R.C. Activity theory. In The Encyclopedia of Aging, 2nd ed.; Maddox, G.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 9–12. [Google Scholar]
- Elder, G.H., Jr. Life Course and Human Development. In Handbook of Child Psychology; Damon, W., Ed.; Wiley: New York, NY, USA, 1998; pp. 939–991. [Google Scholar]
- Cheval, B.; Sieber, S.; Guessous, I.; Orsholits, D.; Courvoisier, D.S.; Kliegel, M.; Stringhini, S.; Swinnen, S.P.; Burton-Jeangros, C.; Cullati, S.; et al. Effect of early-and adult-life socioeconomic circumstances on physical inactivity. Med. Sci. Sports Exerc. 2018, 50, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Selvamani, Y.; Arokiasamy, P. Association of life course socioeconomic status and adult height with cognitive functioning of older adults in India and China. BMC Geriatr. 2021, 21, 354. [Google Scholar] [CrossRef]
- Atchley, R.C. Continuity Theory. In The Encyclopedia of Aging, 2nd ed.; Maddox, G.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 227–230. [Google Scholar]
- Dodig, S.; Cepelak, I.; Pavic, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Bohr, V.A.; de Cabo, R. Nutrition and aging. Mech. Ageing Dev. 2010, 131, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T.; Partridge, L. Horizons in the evolution of aging. BMC Biol. 2018, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [PubMed] [Green Version]
- Levitan, E.B.; Lewis, C.E.; Tinker, L.F.; Eaton, C.B.; Ahmed, A.; Manson, J.E.; Snetselaar, L.G.; Martin, L.W.; Trevisan, M.; Howard, B.V.; et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women’s Health Initiative. Circ. Heart Fail. 2013, 6, 1116–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, A.; Egeberg, R.; Halkjær, J.; Christensen, J.; Overvad, K.; Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J. Nutr. 2011, 141, 639–644. [Google Scholar] [CrossRef]
- Roswall, N.; Sandin, S.; Lof, M.; Skeie, G.; Olsen, A.; Adami, H.O.; Weiderpass, E. Adherence to the Healthy Nordic Food Index and total and cause-specific mortality among Swedish women. Eur. J. Epidemiol. 2015, 30, 509–517. [Google Scholar] [CrossRef]
- Abe, S.; Zhang, S.; Tomata, Y.; Tsuduki, T.; Sugawara, Y.; Tsuji, I. Japanese diet and survival time: The Ohsaki Cohort 1994 study. Clin. Nutr. 2020, 39, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, E.; Nakamura, K.; Ukawa, S.; Wakai, K.; Date, C.; Iso, H.; Tamakoshi, A. The Japanese food score and risk of all-cause, CVD and cancer mortality: The Japan Collaborative Cohort Study. Br. J. Nutr. 2018, 120, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Leenders, M.; Sluijs, I.; Ros, M.M.; Boshuizen, H.C.; Siersema, P.D.; Ferrari, P.; Weikert, C.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.C.; et al. Fruit and vegetable consumption and mortality: European prospective investigation into cancer and nutrition. Am. J. Epidemiol. 2013, 178, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Stefler, D.; Pikhart, H.; Kubinova, R.; Pajak, A.; Stepaniak, U.; Malyutina, S.; Simonova, G.; Peasey, A.; Marmot, M.G.; Bobak, M. Fruit and vegetable consumption and mortality in Eastern Europe: Longitudinal results from the Health, Alcohol and Psychosocial Factors in Eastern Europe study. Eur. J. Prev. Cardiol. 2016, 23, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shu, X.O.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; Ruospo, P.; Palmer, S.C.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Campbell, K.L.; Carrero, J.-J.; Stenvinkel, P.; et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 250–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleby, P.N.; Crowe, F.L.; Bradbury, K.E.; Travis, R.C.; Key, T.J. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am. J. Clin. Nutr. 2016, 103, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Kim, E.; Buring, J.E.; Lee, I.M.; Gaziano, J.M.; Djousse, L. Nut consumption, risk of cardiovascular mortality, and potential mediating mechanisms: The Women’s Health Study. J. Clin. Lipidol. 2021, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, M.; Kenfield, S.A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Giovannucci, E.L.; Bao, Y. Nut consumption and prostate cancer risk and mortality. Br. J. Cancer 2016, 115, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccio, M.; Castelnuovo, A.D.; Curtis, D.A.; Costanzo, S.; Bracone, F.; Persichillo, M.; Donati, M.B.; Gaetano, G.; Iacoviello, L. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: Prospective results from the Moli-sani study. Br. J. Nutr. 2015, 114, 804–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, M.; Wada, K.; Koda, S.; Uji, T.; Nakashima, Y.; Onuma, S.; Oba, S.; Nagata, C. Associations of total nut and peanut intakes with all-cause and cause-specific mortality in a Japanese community: The Takayama study. Br. J. Nutr. 2022, 127, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Sharafkhah, M.; Poustchi, H.; Hashemian, M.; Dawsey, S.M.; Freedman, N.D.; Boffetta, P.; Abnet, C.C.; Etemadi, A.; Pourshams, A.; et al. Nut consumption and total and cause-specific mortality: Results from the Golestan Cohort Study. Int. J. Epidemiol. 2017, 46, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferre, M.; Bullo, M.; Martinez-Gonzalez, M.A.; Ros, E.; Corella, D.; Estruch, R.; Fito, M.; Aros, F.; Warnberg, J.; Fiol, M.; et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Gu, K.; Wang, K.; Cai, H.; Zheng, W.; Bao, P.; Shu, X.-O. Nut consumption in association with overall mortality and recurrence/disease-specific mortality among long-term breast cancer survivors. Int. J. Cancer 2022, 150, 572–579. [Google Scholar] [CrossRef]
- Abe, S.K.; Saito, E.; Sawada, N.; Tsugane, S.; Ito, H.; Lin, Y.; Tamakoshi, A.; Sado, J.; Kitamura, Y.; Sugawara, Y.; et al. Green tea consumption and mortality in Japanese men and women: A pooled analysis of eight population-based cohort studies in Japan. Eur. J. Epidemiol. 2019, 34, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Nakamura, Y. Green tea suppresses brain aging. Molecules 2021, 26, 4897. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Liu, Y.; Li, Y.; Song, W.; Yu, J.; Li, H.; Wang, W. Association between longevity and element levels in food and drinking water of typical Chinese longevity area. J. Nutr. Health Aging 2016, 20, 897–903. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Ouyang, Y.; Liu, J.; Zhao, G.; Bao, W.; Yan, M. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. BMC Med. 2014, 12, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.M.; Martinez-Gonzalez, M.A.; Gea, A.; Grosso, G.; Martín-Moreno, J.M.; Lopez-Garcia, E.; Martin-Calvo, N.; Toledo, E. Coffee consumption and total mortality in a Mediterranean prospective cohort. Am. J. Clin. Nutr. 2018, 108, 1113–1120. [Google Scholar] [CrossRef]
- Shadyab, A.H.; Manson, J.E.; Luo, J.; Haring, B.; Saquib, N.; Snetselaar, L.G.; Chen, J.C.; Groessl, E.J.; Wassertheil-Smoller, S.; Sun, Y.; et al. Associations of coffee and tea consumption with survival to age 90 years among older women. J. Am. Geriatr. Soc. 2020, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Shu, X.O.; Gao, Y.T.; Li, H.; Zhang, X.; Gao, J.; Cai, H.; Yang, G.; Xiang, Y.B.; Zheng, W. Red meat and poultry intakes and risk of total and cause-specific mortality: Results from cohort studies of Chinese adults in Shanghai. PLoS ONE 2013, 8, e56963. [Google Scholar]
- Lv, Y.; Kraus, V.B.; Gao, X.; Yin, Z.; Zhou, J.; Mao, C.; Duan, J.; Zeng, Y.; Brasher, M.S.; Shi, W.; et al. Higher dietary diversity scores and protein-rich food consumption were associated with lower risk of all-cause mortality in the oldest old. Clin. Nutr. 2020, 39, 2246–2254. [Google Scholar] [CrossRef]
- Stefler, D.; Brett, D.; Sarkadi-Nagy, E.; Kopczynska, E.; Detchev, S.; Bati, A.; Scrob, M.; Koenker, D.; Aleksov, B.; Douarin, E.; et al. Traditional Eastern European diet and mortality: Prospective evidence from the HAPIEE study. Eur. J. Nutr. 2021, 60, 1091–1100. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Alles, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; De Curtis, A.; Persichillo, M.; Sofi, F.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am. J. Clin. Nutr. 2021, 113, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Sandoval-Insausti, H.; Lopez-Garcia, E.; Graciani, A.; Ordovas, J.M.; Banegas, J.R.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Consumption of ultra-processed foods and mortality: A national prospective cohort in Spain. Mayo Clin. Proc. 2019, 94, 2178–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: Prospective cohort study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Vaona, A.; Demurtas, J.; Nicetto, D.; Crepaldi, G.; Schofield, P.; Koyanagi, A.; et al. Fried potato consumption is associated with elevated mortality: An 8-y longitudinal cohort study. Am. J. Clin. Nutr. 2017, 106, 162–167. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S. What is the scientific definition of the Japanese diet from the viewpoint of nutrition and health? Nutr. Rev. 2020, 78, 18–26. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourd, E. Ultra-processed foods might increase cancer risk. Lancet Oncol. 2018, 19, e186. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.D.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Sante prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [Green Version]
- da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef]
- Jalel, A.; Soumaya, G.S.; Hamdaoui, M.H. Vitiligo treatment with vitamins, minerals and polyphenol supplementation. Indian J. Dermatol. 2009, 54, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, I.H.; Kim, C.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef]
- Najjar, F.M.; Taghavi, F.; Ghadari, R.; Sheibani, N.; Moosavi-Movahedi, A.A. Destructive effect of non-enzymatic glycation on catalase and remediation via curcumin. Arch. Biochem. Biophys. 2017, 630, 81–90. [Google Scholar] [CrossRef]
- Matsumaru, D.; Motohashi, H. The KEAP1-NRF2 system in healthy aging and longevity. Antioxidants 2021, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Tapia, P.C. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med. Hypotheses 2006, 66, 832–843. [Google Scholar] [PubMed]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araujo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, L.; Chen, X.; Ding, C.; Hao, M.; Peng, X.; Zhang, Y.; Zhu, H.; Liu, W. Anti-aging effect of phlorizin on D-galactose–induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 2022, 163, 111769. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wen, Z.; Lei, L.; Li, F.; Zhao, J.; Zhi, Q.; Li, F.; Yin, R.; Ming, J. Coreopsis tinctoria flowers extract ameliorates D-galactose induced aging in mice via regulation of Sirt1-Nrf2 signaling pathway. J. Funct. Foods. 2019, 60, 103464. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.-J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Zhang, A.; Zhao, N.; Zhang, J.; Zhao, D.; Yu, Z.; Xu, N.; Yin, Y.; Luan, X.; et al. Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Sci. Nutr. 2020, 8, 3872–3881. [Google Scholar] [CrossRef]
- Maleki, M.; Khelghati, N.; Alemi, F.; Bazdar, M.; Asemi, Z.; Majidinia, M.; Sadeghpoor, A.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Targhazeh, N.; et al. Stabilization of telomere by the antioxidant property of polyphenols: Anti-aging potential. Life Sci. 2020, 259, 118341. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Miyazawa, T. Effects of dietary food components on cognitive functions in older adults. Nutrients 2021, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon dos Santos, J.; Schaan de Quadros, A.; Weschenfelder, C.; Bueno Garofallo, S.; Marcadenti, A. Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Dictionary by Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/vitamin (accessed on 27 January 2022).
- Cambridge Dictionary. Available online: https://dictionary.cambridge.org/dictionary/english/vitamin (accessed on 27 January 2022).
- Meyers, D.G.; Maloley, P.A.; Weeks, D. Safety of Antioxidant Vitamins. Arch. Intern. Med. 1996, 156, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Higuchi, O.; Matsumoto, A.; Miyazawa, T. Determination of intracellular ascorbic acid using tandem mass spectrometry. Analyst 2022, 147, 2640–2643. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Matsumoto, A.; Miyahara, Y. Determination of cellular vitamin C dynamics by HPLC-DAD. Analyst 2019, 144, 3483–3487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.N.; Hayhoe, R.P.G.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Lower dietary and circulating vitamin C in middle-and older-aged men and women are associated with lower estimated skeletal muscle mass. J. Nutr. 2020, 150, 2789–2798. [Google Scholar] [CrossRef]
- Qu, Y.N.; Zhang, L.; Wang, T.; Zhang, H.Y.; Yang, Z.J.; Yuan, F.F.; Wang, Y.; Li, S.W.; Jiang, X.X.; Xie, X.H. Vitamin C Treatment rescues prelamin A-induced premature senescence of subchondral bone mesenchymal stem cells. Stem Cells Int. 2020, 2020, 3150716. [Google Scholar] [CrossRef]
- Aumailley, L.; Warren, A.; Garand, C.; Dubois, M.J.; Paquet, E.R.; Le Couteur, D.G.; Marette, A.; Cogger, V.C.; Lebel, M. Vitamin C modulates the metabolic and cytokine profiles, alleviates hepatic endoplasmic reticulum stress, and increases the life span of Gulo−/− mice. Aging 2016, 8, 458–483. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Bernardo, A.; Walker, J.M.; Kennard, J.A.; Kim, G.Y.; Kessler, E.S.; Harrison, F.E. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally-aging mice. ACS Chem. Neurosci. 2015, 6, 570–581. [Google Scholar] [CrossRef] [Green Version]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox. Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Lai, G.Y.; Weinstein, S.J.; Taylor, P.R.; McGlynn, K.A.; Virtamo, J.; Gail, M.H.; Albanes, D.; Freedman, N.D. Effects of alpha-tocopherol and beta-carotene supplementation on liver cancer incidence and chronic liver disease mortality in the ATBC study. Br. J. Cancer 2014, 111, 2220–2223. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Weinstein, S.J.; Yu, K.; Mannisto, S.; Albanes, D. Relationship between serum alpha-tocopherol and overall and cause-specific mortality: A 30-year prospective cohort analysis. Circ. Res. 2019, 125, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef]
- Guan, J.Z.; Guan, W.P.; Maeda, T.; Makino, N. Effect of vitamin E administration on the elevated oxygen stress and the telomeric and subtelomeric status in Alzheimer’s disease. Gerontology 2012, 58, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Alpha-and gamma-tocopherol and telomere length in 5768 US men and women: A NHANES study. Nutrients 2017, 9, 601. [Google Scholar] [CrossRef] [Green Version]
- Hanson, C.; Lyden, E.; Furtado, J.; Campos, H.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin. Nutr. 2016, 35, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Boccardi, V.; Arosio, B.; Cari, L.; Bastiani, P.; Scamosci, M.; Casati, M.; Ferri, E.; Bertagnoli, L.; Ciccone, S.; Rossi, P.D.; et al. Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 2020, 59, 119–126. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Mannisto, S.; Albanes, D. Serum beta carotene and overall and cause-specific mortality: A prospective cohort study. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Association between leukocyte telomere length and serum carotenoid in US adults. Eur. J. Nutr. 2017, 56, 1045–1052. [Google Scholar] [CrossRef]
- Yazaki, K.; Yoshikoshi, C.; Oshiro, S.; Yanase, S. Supplemental cellular protection by a carotenoid extends lifespan via Ins/IGF-1 signaling in Caenorhabditis elegans. Oxid. Med. Cell Longev. 2011, 2011, 596240. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Lebda, M.A.; Noreldin, A.E.; Atta, M.S.; Elewa, Y.H.A.; Elfeky, M.; Mousa, S.A. Quercetin attenuates pancreatic and renal D-galactose-induced aging-related oxidative alterations in rats. Int. J. Mol. Sci. 2020, 21, 4348. [Google Scholar] [CrossRef]
- Wang, H.; Jo, Y.-J.; Oh, J.S.; Kim, N.-H. Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 2017, 8, 38631–38641. [Google Scholar] [CrossRef]
- Geng, L.; Liu, Z.; Zhang, W.; Li, W.; Wu, Z.; Wang, W.; Ren, R.; Su, Y.; Wang, P.; Sun, L.; et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of aging-induced oxidative stress and activation of autophagy by bilberry anthocyanin supplementation via the AMPK-mTOR signaling pathway in aged female rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.; Gonzalez-Pardo, H.; Garrido, P.; Conejo, N.M.; Llaneza, P.; Diaz, F.; Rey, C.G.D.; Gonzalez, C. Acute effects of 17 β-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age 2010, 32, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ren, X.; Lio, C.; Sun, W.; Lai, K.; Liu, Y.; Zhang, Z.; Liang, J.; Zhou, H.; Liu, L.; et al. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol. Res. 2018, 130, 110–122. [Google Scholar] [CrossRef]
- Gines, C.; Cuesta, S.; Kireev, R.; Garcia, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A.F. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesus, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegri, C.; Sanfeliu, C.; Camins, A.; Pallas, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Cruz, A.R.; Ramirez, Y.; Ayala, R.; Ochoa-Velasco, C.; Brambila, E.; Avila-Sosa, R.; Perez-Fernandez, S.; Morales-Medina, J.C.; Aguilar-Alonso, P. Effect of chronic administration of resveratrol on cognitive performance during aging process in rats. Oxid. Med. Cell Longev. 2017, 2017, 8510761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, C.A.; Santos, M.A.; Araujo, G.R.; Lara, R.C.; Franco, F.N.; Chaves, M.M. Resveratrol: Change of SIRT 1 and AMPK signaling pattern during the aging process. Exp. Gerontol. 2021, 146, 111226. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Bartali, B.; Urpi-Sarda, M.; Zamora-Ros, R.; Sun, K.; Cherubini, A.; Bandinelli, S.; Andres-Lacueva, C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern. Med. 2014, 174, 1077–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliemann, L.; Nyberg, M.; Hellsten, Y. Effects of exercise training and resveratrol on vascular health in aging. Free Radic. Biol. Med. 2016, 98, 165–176. [Google Scholar] [CrossRef]
- Shen, L.R.; Xiao, F.; Yuan, P.; Chen, Y.; Gao, Q.K.; Parnell, L.D.; Meydani, M.; Ordovas, J.M.; Li, D.; Lai, C.Q. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age 2013, 35, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, T.; Nakagawa, K.; Kim, S.H.; Thomas, M.J.; Paul, L.; Zingg, J.-M.; Dolnikowski, G.G.; Roberts, S.B.; Kimura, F.; Miyazawa, T.; et al. Curcumin and piperine supplementation of obese mice under caloric restriction modulates body fat and interleukin-1β. Nutr. Metab. 2018, 15, 12. [Google Scholar] [CrossRef]
- Shailaja, M.; Gowda, K.M.D.; Vishakh, K.; Kumari, N.S. Anti-aging role of curcumin by modulating the inflammatory markers in albino wistar rats. J. Natl. Med. Assoc. 2017, 109, 9–13. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Hasani, A.S.; Hamid, N.; Amin, A.M.; Fatemeh, E.; Allahbakhshian, F.M.; Ghaffari, N.M. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod. Fertil. Dev. 2020, 32, 292–303. [Google Scholar]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.R.; Franks, S.; Sumien, N.; Thangthaeng, N.; Filipetto, F.; Forster, M. Curcumin mimics the neurocognitive and anti-inflammatory effects of caloric restriction in a mouse model of midlife obesity. PLoS ONE 2015, 10, e0140431. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shuvo, A.A.; Bepari, A.K.; Hasan, A.M.; Shill, M.C.; Hossain, M.; Uddin, M.; Islam, M.R.; Bakshi, M.K.; Hasan, J.; et al. Curcumin improves D-galactose and normal-aging associated memory impairment in mice: In vivo and in silico-based studies. PLoS ONE 2022, 17, e0270123. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Bowley, B.; Shultz, P.; Calderazzo, S.; Shobin, E.; Killiany, R.J.; Rosene, D.L.; Moss, M.B. Chronic curcumin treatment improves spatial working memory but not recognition memory in middle-aged rhesus monkeys. Geroscience 2017, 39, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Camargo, A.; Delgado-Casado, N.; Cruz-Teno, C.; Santos-Gonzalez, M.; Rodriguez-Cantalejo, F.; Castano, J.P.; et al. Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age 2012, 34, 389–403. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, B.; Yu, S.; Zhang, C.; Wang, B.; Wang, Y.; Wang, J.; Yuan, Z.; Zhang, L.; Pan, J. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid. Med. Cell Longev. 2015, 2015, 867293. [Google Scholar] [CrossRef]
- Huo, J.; Xu, Z.; Hosoe, K.; Kubo, H.; Miyahara, H.; Dai, J.; Mori, M.; Sawashita, J.; Higuchi, K. Coenzyme Q10 prevents senescence and dysfunction caused by oxidative stress in vascular endothelial cells. Oxid. Med. Cell Longev. 2018, 2018, 3181759. [Google Scholar] [CrossRef]
- Miyazawa, T.; Hiratsuka, Y.; Toda, M.; Hatakeyama, N.; Ozawa, H.; Abe, C.; Cheng, T.Y.; Matsushima, Y.; Miyawaki, Y.; Ashida, K.; et al. Artificial intelligence in food science and nutrition: A narrative review. Nutr. Rev. 2022, nuac033, epub ahead of print. [Google Scholar] [CrossRef]
- Mezgec, S.; Seljak, B.K. NutriNet: A deep learning food and drink image recognition system for dietary assessment. Nutrients 2017, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Lemay, D.G.; Baldiviez, L.M.; Chin, E.L.; Spearman, S.S.; Cervantes, E.; Woodhouse, L.R.; Keim, N.L.; Stephensen, C.B.; Laugero, K.D. Technician-scored stool consistency spans the full range of the bristol scale in a healthy US population and differs by diet and chronic stress load. J. Nutr. 2021, 151, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A critical review of the use of surfactant-coated nanoparticles in nanomedicine and food nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Plagg, B.; Zerbe, S. How does the environment affect human ageing? An interdisciplinary review. J. Gerontol. Geriatr. 2020, 61, 53–67. [Google Scholar] [CrossRef]
- Trzeciak, A.R.; Barnes, J.; Ejiogu, N.; Foster, K.; Brant, L.J.; Zonderman, A.B.; Evans, M.K. Age, sex, and race influence single-strand break repair capacity in a human population. Free Radic. Biol. Med. 2008, 45, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M.; Macian, F. Autophagy and the immune function in aging. Curr. Opin. Immunol. 2014, 29, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Rando, T.A.; Chang, H.Y. Aging, rejuvenation, and epigenetic reprogramming: Resetting the aging clock. Cell 2012, 148, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Perez, V.I.; Van Remmen, H.; Bokov, A.; Epstein, C.J.; Vijg, J.; Richardson, A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009, 8, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Perez-Estrada, J.R.; Hernandez-Garcia, D.; Leyva-Castro, F.; Ramos-Leon, J.; Cuevas-Benítez, O.; Diaz-Munoz, M.; Castro-Obregon, S.; Ramirez-Solis, R.; Garcia, C.; Covarrubias, L. Reduced lifespan of mice lacking catalase correlates with altered lipid metabolism without oxidative damage or premature aging. Free Radic. Biol. Med. 2019, 135, 102–115. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Superoxide dismutase is dispensable for normal animal lifespan. Proc. Natl. Acad. Sci. USA 2012, 109, 5785–5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Target | Diet | Country | Populations Analyzed/Total | Age | Follow-Up Time | Reducing Mortality/Increasing Life Span | Ref. |
|---|---|---|---|---|---|---|---|
| Mortality | Mediterranean diet | Greece | 22,043/28,572 (M, F) | 20–86 | 3.7 years | Yes | [47] |
| Mortality | Mediterranean diet | US | 3215/(F) (with heart failure) | 50–79 | 4.6 years | Yes | [48] |
| Mortality | Traditional Nordic foods | Denmark | 2383/27,178 (M) 1743/29,875 (F) | 50–64 | 12 years | Yes (especially in middle-aged men) | [49] |
| Mortality | Nordic foods | Sweden | 44,961/49,259 (F) | 29–49 | 21.3 years | Yes (especially by cancer, non-cancer, non-cardiovascular, non-injury/suicide) | [50] |
| Longevity | Japanese diet | Japan | 14,764/32,126 (M, F) | 40–79 | 20 years | Yes | [51] |
| Mortality | Japanese diet | Japan | 23,162 (M) and 34,232 (F) /110,585 (M, F) | 40–79 | 18.9 years (M) 19.4 years (F) | Yes (especially in females) | [52] |
| Mortalityrisk of CVD | fruits, vegetables | US | 9608/14,407 (M, F) | 25–74 | 19 years | Yes | [53] |
| Mortality | fresh fruits, vegetables | Europe (DNK, FRA, DEU, GRC, ITA, NLD, NOR, ESP, SWE, GBR) | 129,882 (M) and 321,269 (F)/ 521,448 | 25–70 | 13 years | Yes (especially by CVD) | [54] |
| Mortality | 21 fruits, 24 vegetables | POL, RUS, CZE | 19,333/28,945 (M, F) | Middle age | 7.1 years | Yes (especially by CVD in smokers/hypertension) | [55] |
| Mortality | 8 fruits, 33 vegetables | China | 73,360/74,942 (F) 61,436/61,500 (M) | 40–70 (F) 40–74 (M) | 10.2 years (F) 4.6 (M) | Yes (by CVD), no (by cancer) | [56] |
| Mortality | fruits, vegetables | Europe (FRA, DU, ITA, HUN, POL, PRT, ROU, ESP, SWE, TUR) and ARG | 8078/9757 (M, F) (with hemodialysis) | mean 63 | 2.7 years | Yes (except by CVD) | [57] |
| Mortality | Meat, fish, dairy products, eggs, and vegetables | UK | 11,140 (M, F) (Vegetarians and meat-eaters) | mean 38.7 (V), mean 39.3 (NV) | 12 years | No | [58] |
| 65,411 (M, F) (Meat/fish eaters, vegetarians and vegans) | 20–97 | 15 years | |||||
| Mortality | Nuts (peanuts and tree nuts) | U.S. | 39,167/39,876 (F) | ≥45 | 19 years | Yes (except by CVD) | [59] |
| Mortality | Nuts (peanuts and others) | U.S. | 47,299/51,529 (M) (PCa patients) | 40–75 | 26 years | No (except in men diagnosed with non-metastatic PCa) | [60] |
| Mortality | Nuts (walnuts, hazelnuts, almonds and peanuts) | Italy | 19,386/24,325 (M, F) | ≥35 | 4.3 years | Yes | [61] |
| Mortality | Nuts (peanut butter, nut bread and rice cooked with chestnuts) | Japan | 13,355 (M) 15,724 (F) /36,990 | ≥35 | 17 years | Yes | [62] |
| Mortality | Nuts (peanuts, tree nuts and overall nuts consumption) | Iran | 20,855 (M) 28,257 (F) /50,045 | 40–87 | 7 years | Yes | [63] |
| Mortalityrisk of type-2 diabetesrisk of CVD(meta- analysis) | Nuts | US | 134,486 (F) | 55–69 | 11 years | Yes | [64] |
| China | 64,227 (F) | 40–70 | 4.6 years | ||||
| US | 20,224 (M) | 41–87 | 21.1 years | ||||
| US | 1,164,248 (F) | 30–55 | 22 years | ||||
| US | 1,599,667 (F) | 20–45 | 18 years | ||||
| US | 31,208 (M, F) | >25 | 6 years | ||||
| US | 21,454 (M) | 40–84 | 17 years | ||||
| US | 31,778 (F) | 55–69 | 15 years | ||||
| US | 6309 (F) | 52.8 ± 8.5 | 22 years | ||||
| US | 34,492 (F) | 30–55 | 26 years | ||||
| US | 21,078 (M) | 41–87 | 21.1 years | ||||
| US | 43,150 (M) 84,010 (F) | 40–75 (M) 30–55 (F) | 26 years (M) 22 years (F) | ||||
| US | 87,025 (F) | 50–79 | 4 years | ||||
| US | NS (M, F) | >25 | – | ||||
| US | NS (M, F) | >85 | 12 years | ||||
| UK | 10,802 (M, F) | 16–79 | 13.3 years | ||||
| Netherlands | NS (M, F) | 55–69 | 24 years | ||||
| US | 3,038,853 (M, F) | 30–55 (NHS) 40–75 (HPFS) | 30 years (NHS) 24 years (HPFS) | ||||
| Spain | 31,077 (M, F) | 55–80 | 4.8 years | ||||
| Mortality | Mediterranean diet, (especially nuts) | Spain | 7216/7447 (M, F) | 55–80 (M) 60–80 (F) | 4.8 years | Yes | [65] |
| Mortality | Nuts (peanuts, walnuts and other nuts) | China | 3449/5042 (F) | 20–75 | 8.27 years | Yes | [66] |
| Mortality | Green tea | Japan | 22,597 (M) 25,382 (F) | 40–59 | 20.25 years | Yes (especially by HD and CVD) | [67] |
| Japan | 27,941 (M) 31,415 (F) | 40–69 | 17.3 years | ||||
| Japan | 28,258 (M) 39,446 (F) | 40–79 | 16.1 years | ||||
| Japan | 16,874 (M) 18,077 (F) | 40–64 | 20.52 years | ||||
| Japan | 12,152 (M) 13,194 (F) | 40–79 | 11.66 years | ||||
| Japan | 16,749 (M) 18,185 (F) | ≥40 | 10.98 years | ||||
| Japan | 8663 (M) 9951 (F) | 40–103 | 11.67 years | ||||
| Japan | 11,516 (M) 12,981 (F) | 40–97 | 12.56 years | ||||
| Mortality | Green tea | Japan | 42,836/68,722 (M) 48,078/71,698 (F) | 40–69 | 18.7 years | Yes | [68] |
| Tea | China | 51,668/65,212 (M, F) | ≥65 | 3.5–3.8 years | |||
| Longevity | Elements in food and water | China | NA | ≥65, ≥90 | - | Yes | [69] |
| MortalityCVD mortality | Calcium | China | 3139 (M, F) | ≥65 | 9.1 years | Yes (except high dose > 900 mg calcium/day) | [70] |
| China | 61,414 (M) 73,232 (F) | 40–74 (M) 40–70 (F) | 5.5 years (M) 11 years (F) | ||||
| Canada | 9033 (M, F) | ≥25 | 10 years | ||||
| Sweden | 61,433 (F) | 39–74 | 19 years | ||||
| US | 388,229 (M, F) | 50–71 | 12 years | ||||
| US | 18,714 (M, F) | ≥17 | 18 years | ||||
| Germany | 23,980 (M, F) | 35–64 | 11 years | ||||
| US | 38,772 (F) | 55–69 | 11 years | ||||
| Sweden | 23,366 (M) | 45–79 | 10 years | ||||
| Japan | 21,068 (M) 32,319 (F) | 40–59 | 9.6 years | ||||
| Netherlands | 1340 (M) 1265 (F) | 40–65 | 28 years | ||||
| Mortality | Coffee | Spain | 19,888/22,320 (M, F) | Middle age | 10 years | Yes (≥54 years) | [71] |
| Longevity | Coffee | US | 27,480/93,676 (F) (postmenopausal) | 65–81 | 25 years | No | [72] |
| Morality | Meat intake | China | 61,128/61,483 (M) 73,162/74,941 (F) | 40–74 (M) 40–70 (F) | 5.5 years (M) 11.2 years (F) | No (increased mortality in men) | [73] |
| Mortality | Dietary diversity (meat, fish and seafood, eggs, beans, fruits, salty vegetables, tea, garlic, and fresh vegetables) | China | 28,790/43,487 | ≥80 | 3.4 years | Yes (especially by consumption of protein-rich food) | [74] |
| Mortality | Eastern European diet | Eastern Europe (RUS, POL and CZE) | 18,852/29,845 (M, F) | 45–70 | 8–15 years | No (rather than increased) | [75] |
| Mortality | Ultra-processed food | France | 44,551/158,361 (M, F) | ≥45 | 7.1 years | No (rather than increased) | [76] |
| Mortality | Ultra-processed food | Italy | 22,475/24,325 (M, F) | ≥35 | 8.2 years | No (rather than increased) | [77] |
| Mortality | Ultra-processed food | Spain | 11,898/12,948 | ≥18 | 7.7 years | No (rather than increased) | [78] |
| Mortality | Fried food | US | 106,966/373,092 (F) (postmenopausal) | 50–79 | 17.9 years | No (rather than increased by CVD) | [79] |
| Mortality | Potato | North America | 4400/4796 (M, F) | 45–79 | 8 years | No (rather than increased) | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazawa, T.; Abe, C.; Burdeos, G.C.; Matsumoto, A.; Toda, M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals 2022, 2, 181-204. https://doi.org/10.3390/nutraceuticals2030014
Miyazawa T, Abe C, Burdeos GC, Matsumoto A, Toda M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals. 2022; 2(3):181-204. https://doi.org/10.3390/nutraceuticals2030014
Chicago/Turabian StyleMiyazawa, Taiki, Chizumi Abe, Gregor Carpentero Burdeos, Akira Matsumoto, and Masako Toda. 2022. "Food Antioxidants and Aging: Theory, Current Evidence and Perspectives" Nutraceuticals 2, no. 3: 181-204. https://doi.org/10.3390/nutraceuticals2030014
APA StyleMiyazawa, T., Abe, C., Burdeos, G. C., Matsumoto, A., & Toda, M. (2022). Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals, 2(3), 181-204. https://doi.org/10.3390/nutraceuticals2030014