Abstract
Several lines of evidence indicate that plant-derived antioxidant compounds can be used as anticancer agents to support conventional pharmacological therapy. In this context, heme-oxygenase-1 (HO-1) modulation has been proven to represent a valid approach for reducing cancer cells’ proliferation through the activation of apoptosis and ferroptosis. This study focused on three little studied HO-1 inducers (paeonol, rosolic acid and dimethoxy resveratrol) in order to evaluate their efficacy as antiproliferative compounds on breast cancer cells (MCF-7 and MDA-MB 231). Cell viability data showed an interesting selectivity of dimethoxy resveratrol (DMR) for MDA-MB 231 cells. The ineffectiveness of Ferrostatin-1 and Trolox treatment led to the exclusion of ferroptosis involvement; meanwhile, cell viability reduction was associated with caspase 3/7 activation and apoptosis. Taken together, our results suggest a potential role of DMR as an adjuvant in conventional chemotherapy for breast cancer treatment.
Keywords:
pterostilbene; dimethoxy resveratrol; rosolic acid; aurin; paeonol; heme oxygenase; cancer; nutraceuticals 1. Introduction
Several bioactive compounds derived from plants have the ability to induce heme oxygenase 1 (HO-1), which represents a cytoprotective system under physiological conditions; however, several lines of evidence have suggested its potential role in cancer.
Data reported that HO-1 modulation can represent a valid therapeutic approach for cancer treatment depending on basal cellular levels of the enzyme. Indeed, HO-1 inhibition has been widely used as an effective therapeutic approach for cancer treatment [1,2,3]. However, more and more data point to a different approach for the pharmacological modulation of HO-1 in cancer treatment, displaying an antiproliferative effect following protein induction [4,5].
Among plant-derived HO inducers, we selected three less investigated compounds: paeonol [6], rosolic acid [7] and pterostilbene, also known as dimethoxy resveratrol (DMR) [8] (Figure 1).

Figure 1.
Chemical structures of selected plant-derived natural compounds.
These three can be potentially considered valid nutraceuticals; indeed, formulations of paeonol and pterostilbene already exist as over-the-counter (OTC) products and drugs.
Paeonia suffruticosa Andr. has been reported to contain many polyphenols, such as gallic acid, paeoniflorin, paeonidamin and paeonol [9]. Different paeonol formulations have been reported and approved by the State Food and Drug Administration (SFDA) of China; in particular, injection was recorded the earliest in the Chinese pharmacopoeia 1977 edition [10]. Sun et al. demonstrated that paeonol was able to prevent UVB-induced photoaging through the activation of nuclear factor erythroid 2–related factor 2 (Nrf2)-HO-1 axis in vitro and in vivo [11]. Additionally, paeonol was observed as potently inhibiting lung cancer cell growth, migration and invasion associated with the disruption of Signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways [12].
Rosolic acid (RA), also known as aurin, is a polyphenol extracted from the rhizome of Plantago asiatica L.; several studies have reported its role in cytoprotection due to its remarkable ability of increasing HO-1 levels, especially in endothelial cells [13,14]. Nevertheless, to date, there has still been little literature focused on RA’s potential as a nanticancer agent [15].
Stilbenes are polyphenolic compounds that can be found in blueberries, grapes, red wine and some medicinal plants. Several pterostilbene formulations have been approved as nutraceuticals and dietary supplements, primarily for neurological and cardiovascular health support. Resveratrol and DMR have been considered exceptional anticancer agents thanks to their low toxicity and ability to regulate different signaling pathways involved in carcinogenesis. However, resveratrol has a very low bioavailability, which undermines its biological activity and efficacy, while DMR, being more lipophilic, exhibits a better bioavailability [16]. Indeed, DMR has been reported to exert stronger antiproliferative and apoptotic effects compared to resveratrol in human colon and cervical cancers [17,18]. In vitro and in vivo studies unraveled DMR’s ability to induce cell cycle arrest and apoptosis in lung cancer cell lines together with the inhibition of tumor growth in mouse xenograft models [19].
In order to investigate the potential antiproliferative effect that HO-1-inducing plant-derived compounds can exert on breast cancer, paeonol, RA and DMR were tested on MCF-7 and MDA-MB 231 cell lines.
2. Materials and Methods
2.1. Chemicals
The natural products selected and tested were purchased from a commercial vendor. 1-(2-Hydroxy-4-methoxyphenyl) ethanone (paeonol) was obtained from Tokyo Chemical Industry (TCI, Oxford UK), 4-[bis(4-hydroxyphenyl) methylidene] cyclohexa-2,5-dien-1-one (rosolic acid) and 4-(3,5-dimethoxystyryl) phenol (pterostilbene) were prepared from Fluorochem, and all were bought by Zentek srl (Milan, Italy). Trolox was obtained from Sigma Aldrich (St. Louis, MS, USA). Ferrostatin-1 was purchased from Sigma-aldrich (SML0583, St. Louis, MS, USA). All the compounds tested, excluding Trolox (water-soluble), were solubilized in Dimethylsulphoxide (DMSO), and a stock solution 1000× higher than the concentration to be tested was prepared. Subsequently, the stock solution was diluted 1:1000 in culture medium.
2.2. Evaluation of Free Radical Scavenger Activity (DPPH)
The free radical scavenging activity of paeonol, aurin and DMR was assessed via DPPH (2,2-diphenyl-1-picrylhydrazyl) assay. The reaction mixture consisted of 86 μM DPPH, different concentrations of the compounds (1, 10, 50, 100 µM) and ethanol (1 mL). Following a 10 min incubation at RT, the absorbance was measured at λ = 517 nm. The DPPH radical reduction (%) was expressed as a function of the molar ratio of the compound/DPPH free radical.
2.3. Cell Culture and Treatments
Experiments were performed on human breast cancer cell lines MDA-MB 231 (HTB-26, ATCC, Rockville, MD, USA) and MCF-7 (HTB-22, ATCC Rockville, MD, USA). Cell cultures were maintaned in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (HG) supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin–streptomycin and incubated at a constant temperature and CO2 levels (respectively, 37 °C and 5% CO2). Cells were treated 24 h after being plated in multiwell plates with different concentrations of paeonol, rosolic acid and DMR (1, 10, 50, 100 µM) for 72 h; additionally, co-treatments with Ferrostatin-1 (1 µM) and Trolox (100 µM) were tested. Each experimental condition was compared to the appropriate control group.
2.4. Cell Viability
Cell viability was evaluated after cells were plated in 96-well plates at a density of 7.0 × 103 cells/well in 100 µL of culture medium. Treatments were administered 24 h after plating cells using 1% FBS-supplemented DMEM, and after 72 h, 100 µL of 0.25 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (ACROS Organics-Antwerp, Belgium) were added to each well. Cells were then incubated for 2 h at 37 °C and 5% CO2. Following incubation, supernatant was discarded and DMSO (100 µL for each well) was added to dissolve formazan salts produced by active mitochondria. The measurement of absorbance (OD) at λ = 570 nm was obtained using a microplate reader (Biotek Synergy-HT, Winooski, VT, USA). At least two separate experiments were conducted in which eight replicate wells were used for each group.
2.5. Measurement of HO-1 Levels (ELISA)
In order to assess HO-1 levels, Simple Step ELISA (ab207621, Abcam, Cambridge, UK) was used, following the manufacturer’s instructions. The absorbance (OD) at λ = 450 nm was measured in a microplate reader (Biotek Synergy-HT, Winooski, VT, USA). The experiment was conducted in triplicate for each sample, and the results are expressed as pg/mL.
2.6. Measurement of Activated Caspase 3/7
CellEvent Caspase-3/7 Green ReadyProbes Reagent (R37111-Invitrogen, Waltham, MA, USA) was used as the indicator of activated caspase-3/7 in 96-well-plated and treated cells. Two drops of reagent were added to 1 mL of culture medium, and 100 µL/well were added according to the manufacturer’s instructions. Fluorescence was measured in a microplate reader (excitation, λ = 502 nm; emission, λ = 530 nm) after 30 min of incubation. Representative pictures were taken with a fluorescence microscope (EVOS Fl AMG).
3. Results and Discussion
Over the last decades, research has primarily focused on nutraceuticals and functional foods, highlighting the correlation between their consumption and health benefits. Although several compounds have been studied, their mechanism of action is still to be investigated. Phytochemicals may either function intrinsically as radical scavengers or as the immune system’s modulators [20].
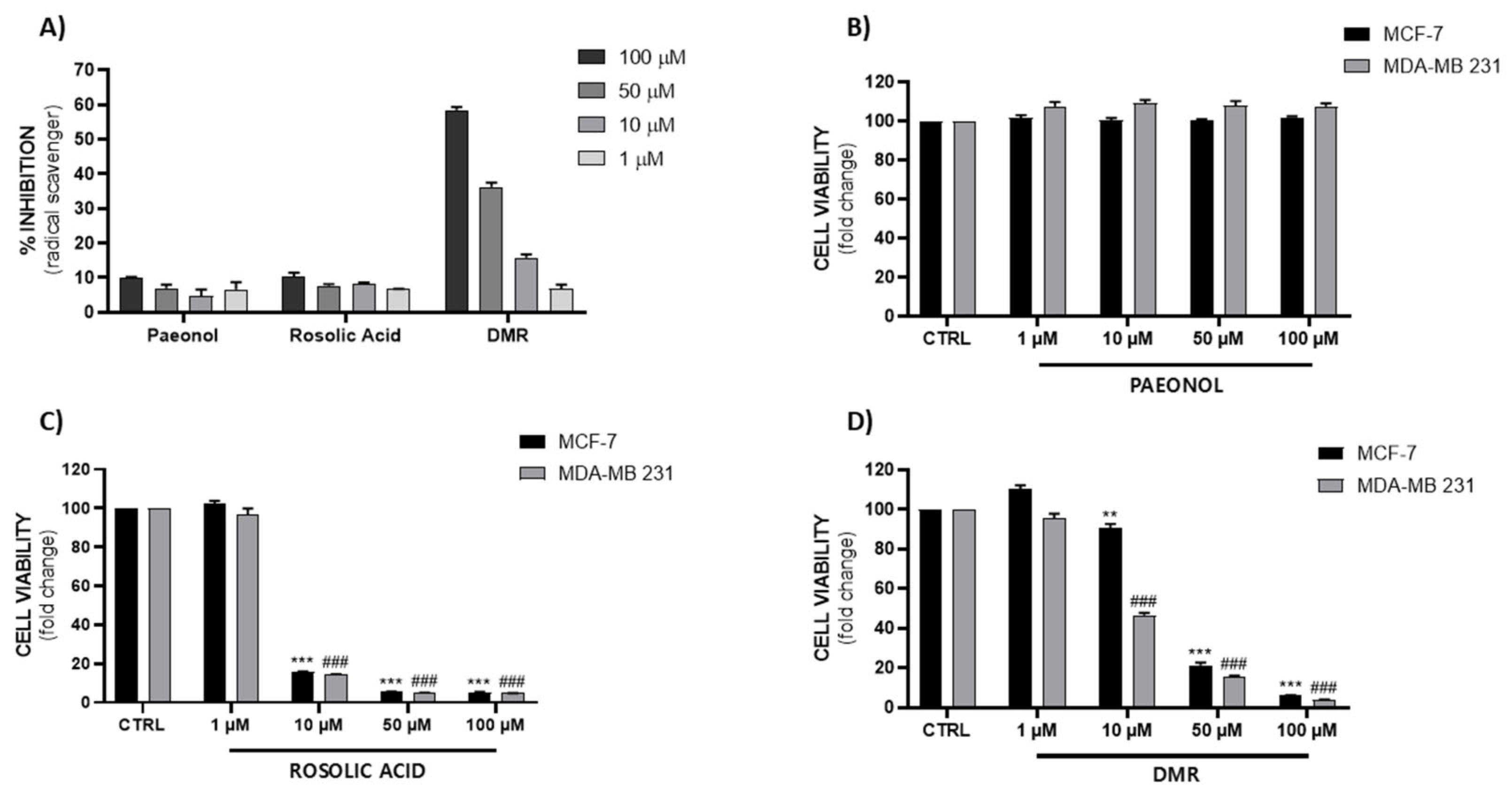
To evaluate the three compounds’ antioxidant properties, a DPPH assay was performed. Although the antioxidant activity of these compounds has already been reported [13,14,21,22,23,24,25], surprisingly, paeonol and RA showed a very low scavenger activity at the tested concentrations (1, 10, 50, 100 µM), while DMR displayed a significant scavenger activity in a dose-dependent manner (Figure 2A).

Figure 2.
(A) DPPH radical scavenging activity of paeonol, RA and DMR at different concentrations (1, 10, 50, 100 µM). Results are expressed as percentage (%) of inhibition rate ± SEM. (B–D) Evaluation of paeonol, RA and DMR cytotoxicity on MCF-7 and MDA-MB 231 cell lines after 72 h treatment (### p < 0.0005 vs. vehicle CTRL MDA-MB 231; ** p < 0.005, *** p < 0,0005 vs. vehicle CTRL MCF-7). Data are expressed as mean ± SEM.
Subsequently, in order to evaluate the antiproliferative effect of these compounds, we performed an MTT test on MCF-7 and MDA-MB 231, respectively an estrogen-dependent and epidermal growth factor (EGF) cell line and a hormone-independent one, also known as a triple negative breast cancer line. The three tested compounds showed very different response profiles. We did not observe any significant differences in cell viability following paeonol exposure (Figure 2B); however, Saahene et al. showed a cytotoxic effect at higher concentrations in breast cancer cells [26]. As reported in Figure 2C,D, RA and DMR showed a potent antiproliferative effect. In particular, rosolic acid can be considered a potent but non-selective compound compared to DMR, which showed a lower efficacy on MCF-7 compared to MDA-MB 231. As shown in Figure 2D, MDA-MB 231’s sensitivity to DMR was evident at 10 µM. Our results regarding rosolic acid’s cytotoxicity are in agreement with previous studies reporting that it is an apoptogenic inducer of proteotoxic and oxidative stress in human malignant melanoma cells [15].
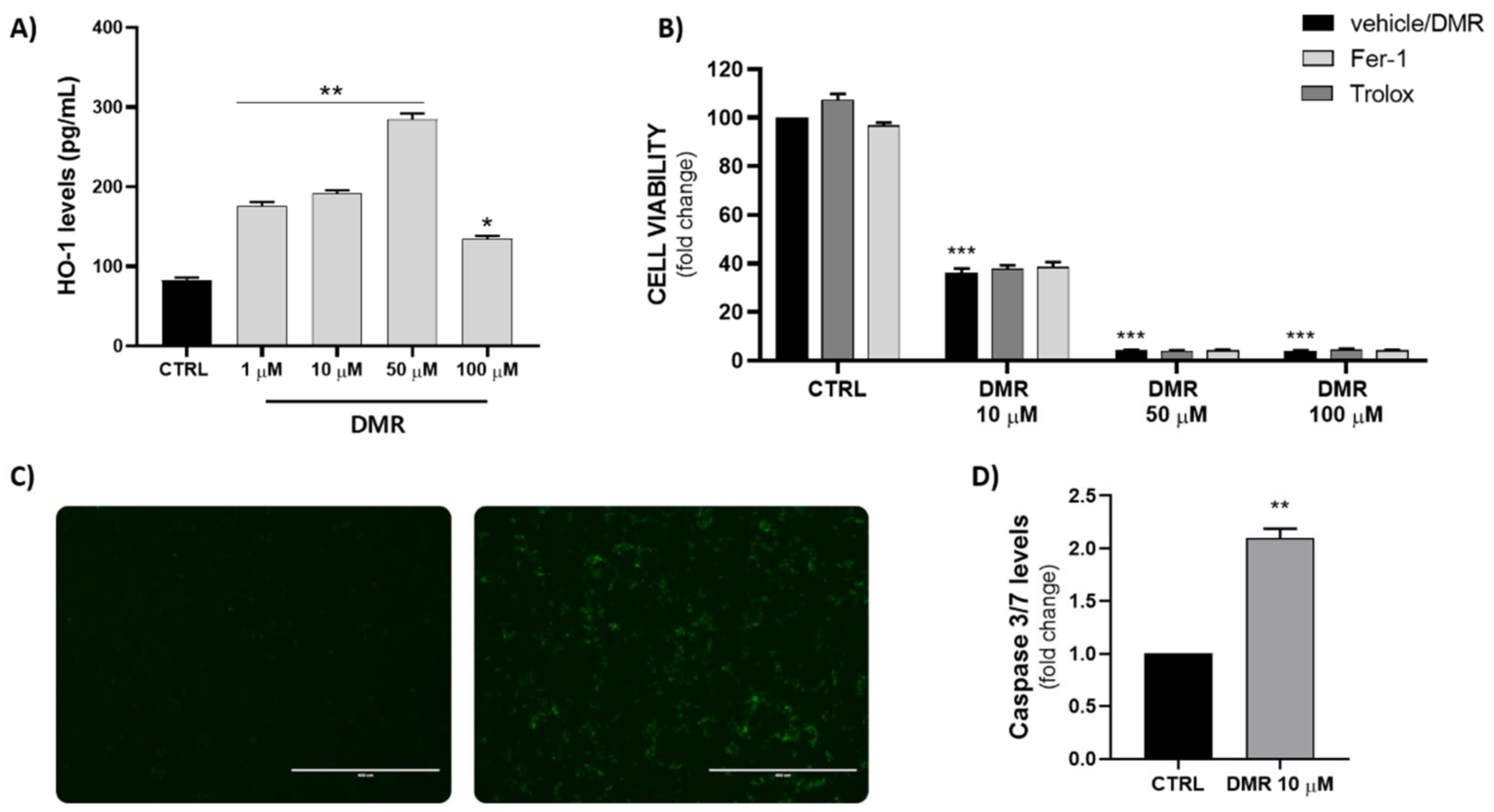
Based on the obtained preliminary results, we decided to explore the possible involvement of HO-1 in the DMR mechanism, since it represents the only selective compound. As shown in Figure 3A, DMR significantly increased HO-1 levels in a dose-dependent manner, though we observed a reduction at the highest concentration (100 µM), mainly caused by a strong reduction of cell viability. DMR was observed to already be an HO-1 inducer at 1 µM, which represents a non-toxic concentration; indeed, higher levels of HO-1 were associated with a lower cell viability after DMR treatment, suggesting its role in anti-proliferative mechanisms’ activation.

Figure 3.
(A) Measurement of HO-1 levels in MDA-MB 231 cells after treatment with different concentrations of DMR (* p < 0.05, ** p < 0.005 vs. CTRL). (B) Cell viability evaluation after DMR co-treatment with Trolox (100 µM) and Ferrostatin-1 (1 µM) (*** p < 0.0005 vs. CTRL). (C,D) Representative fluorescence images (scale bar: 400 µm) and measurement of caspase 3/7 levels following DMR treatment (** p < 0.005 vs. CTRL).
In order to investigate the mechanism of action in a deeper manner, we decided to evaluate the possible activation of ferroptosis. Recently, our group and others demonstrated that induction of HO-1 represents a possible trigger of ferroptosis in breast cancer cells [27,28,29]. However, co-treatment with Ferrostatin-1, a well-known inhibitor of ferroptosis, and Trolox, an inhibitor of lipid peroxidation, did not reverse the DMR cytotoxic effect, demonstrating that ferroptosis is not involved in DMR-induced cell death (Figure 3B).
We then decided to measure caspase 3/7 levels in order to evaluate apoptosis involvement. As shown in Figure 3C,D and in agreement with Tan et al. [19], DMR upregulated caspase-3/7 activity, suggesting the activation of apoptosis mechanism occurs in a caspase-dependent manner.
Gao et al. have recently investigated the toxicity of pterostilbene on endothelial cells (HUVEC). MTT results showed a lower cytotoxicity on non-cancerous cells compared to glioblastoma cell lines [30].
In conclusion, our preliminary results demonstrate that DMR can represent a possible adjuvant for breast cancer conventional therapy through the activation of caspase 3/7, as previously reported [30,31], and through the induction of HO-1 [32], which represents a potentially novel mechanism that needs to be further investigated.
Author Contributions
Conceptualization, V.C., V.S., M.N.M. and L.V.; methodology, V.C., I.B.; formal analysis, V.C., L.V.; resources, M.N.M.; data curation, V.C., L.V.; writing—original draft preparation, V.C., I.B.; writing—review and editing, L.V., V.S., M.N.M.; supervision, L.V.; funding acquisition, L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Catania, “Programma Ricerca di Ateneo UNICT 2020-22 linea 2”, “META” Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fallica, A.N.; Sorrenti, V.; D’Amico, A.G.; Salerno, L.; Romeo, G.; Intagliata, S.; Consoli, V.; Floresta, G.; Rescifina, A.; D’Agata, V.; et al. Discovery of Novel Acetamide-Based Heme Oxygenase-1 Inhibitors with Potent. J. Med. Chem. 2021, 64, 13373–13393. [Google Scholar] [CrossRef] [PubMed]
- Salerno, L.; Vanella, L.; Sorrenti, V.; Consoli, V.; Ciaffaglione, V.; Fallica, A.N.; Canale, V.; Zajdel, P.; Pignatello, R.; Intagliata, S. Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-FU/HO-1 hybrid): Design and preliminary. J. Enzym. Inhib. Med. Chem. 2021, 36, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.N.; Vukomanovic, D.; Vlahakis, J.Z.; Szarek, W.A.; Nakatsu, K.; Jia, Z. Structural insights into human heme oxygenase-1 inhibition by potent and selective azole-based compounds. J. R. Soc. Interface 2013, 10, 20120697. [Google Scholar] [CrossRef] [PubMed]
- Tertil, M.; Golda, S.; Skrzypek, K.; Florczyk, U.; Weglarczyk, K.; Kotlinowski, J.; Maleszewska, M.; Czauderna, S.; Pichon, C.; Kieda, C.; et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free Radic. Biol. Med. 2015, 89, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; D’Amico, A.G.; Barbagallo, I.; Consoli, V.; Grosso, S.; Vanella, L. Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems. Biomolecules 2021, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, L.; Sun, C.; Zhang, B.; Li, J.; Sun, J.; Zhang, Y.; Sun, D. Paeonol alleviates epirubicin-induced renal injury in mice by regulating Nrf2 and NF-κB pathways. Eur. J. Pharm. 2017, 795, 84–93. [Google Scholar] [CrossRef]
- Amin, K.N.; Palanisamy, R.; Sarada, D.V.L.; Ali, D.; Suzuki, T.; Ramkumar, K.M. Effect of Rosolic acid on endothelial dysfunction under ER stress in pancreatic microenvironment. Free Radic. Res. 2021, 55, 698–713. [Google Scholar] [CrossRef]
- Zeng, Q.; Lian, W.; Wang, G.; Qiu, M.; Lin, L.; Zeng, R. Pterostilbene induces Nrf2/HO-1 and potentially regulates NF-κB and JNK-Akt/mTOR signaling in ischemic brain injury in neonatal rats. 3 Biotech. 2020, 10, 192. [Google Scholar] [CrossRef]
- Lau, C.H.; Chan, C.M.; Chan, Y.W.; Lau, K.M.; Lau, T.W.; Lam, F.C.; Law, W.T.; Che, C.T.; Leung, P.C.; Fung, K.P.; et al. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine 2007, 14, 778–784. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Du, L.-D.; Lu, Y. Natural Small Molecule Drugs from Plants; Paeonol; Springer: Berlin/Heidelberg, Germany, 2018; pp. 439–444. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Hwang, E.; Yi, T.H. Paeonol extracted from Paeonia suffruticosa Andr. ameliorated UVB-induced skin photoaging via DLD/Nrf2/ARE and MAPK/AP-1 pathway. Phytother. Res. 2018, 32, 1741–1749. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.X.; Li, L.L.; Cao, Y.Z.; Geng, Y.D.; Feng, X.J.; Wang, A.Y.; Chen, Z.L.; Lu, Y.; Shen, A.Z. Paeonol Suppresses Proliferation and Motility of Non-Small-Cell Lung Cancer Cells by Disrupting STAT3/NF-κB Signaling. Front. Pharm. 2020, 11, 572616. [Google Scholar] [CrossRef] [PubMed]
- Naresh Amin, K.; Rajagru, P.; Sarkar, K.; Ganesh, M.R.; Suzuki, T.; Ali, D.; Kunka Mohanram, R. Pharmacological Activation of Nrf2 by Rosolic Acid Attenuates Endoplasmic Reticulum Stress in Endothelial Cells. Oxid. Med. Cell. Longev. 2021, 2021, 2732435. [Google Scholar] [CrossRef]
- Foresti, R.; Hoque, M.; Monti, D.; Green, C.J.; Motterlini, R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J. Pharm. Exp. Ther. 2005, 312, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Angela, L.D.; Qiao, S.; Lesson, J.L.; de la Vega, M.R.; Park, S.L.; Seanez, C.M.; Gokhale, V.; Cabello, C.M.; Wondrak, G.T. The quinone methide aurin is a heat shock response inducer that causes proteotoxic stress and Noxa-dependent apoptosis in malignant melanoma cells. J. Biol. Chem. 2015, 290, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: A side-by-side comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef]
- Tan, K.T.; Chen, P.W.; Li, S.; Ke, T.M.; Lin, S.H.; Yang, C.C. Pterostilbene inhibits lung squamous cell carcinoma growth. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of Pterostilbene against free radical mediated oxidative damage. BMC Complement Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Choi, S.H.; Gyawali, A.; Hyeon, S.J.; Kang, Y.S.; Ryu, H. Antioxidant and Neuroprotective Effects of Paeonol against Oxidative Stress and Altered Carrier-Mediated Transport System on NSC-34 Cell Lines. Antioxidants 2022, 11, 1392. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [PubMed]
- Roland, O.S.; Wang, J.; Wang, M.; Agbo, E.; Pang, D. The Antitumor Mechanism of Paeonol on CXCL4/CXCR3-B Signals in Breast Cancer Through Induction of Tumor Cell Apoptosis. Cancer Biother. Radiopharm. 2018, 33, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid. Med. Cell. Longev. 2020, 2020, 3469840. [Google Scholar] [CrossRef]
- Shi, H.; Hou, B.; Li, H.; Zhou, H.; Du, B. Cyclophosphamide Induces the Ferroptosis of Tumor Cells Through Heme Oxygenase-1. Front. Pharm. 2022, 13, 839464. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Pittalà, V.; Greish, K.; D’Amico, A.G.; Romeo, G.; Intagliata, S.; Salerno, L.; Vanella, L. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int. J. Mol. Sci. 2022, 23, 579. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene promotes mitochondrial apoptosis and inhibits proliferation in glioma cells. Sci. Rep. 2021, 11, 6381. [Google Scholar] [CrossRef]
- Moon, D.; McCormack, D.; McDonald, D.; McFadden, D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013, 180, 208–215. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).