Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration
Abstract
:1. Introduction
2. Materials and Methods
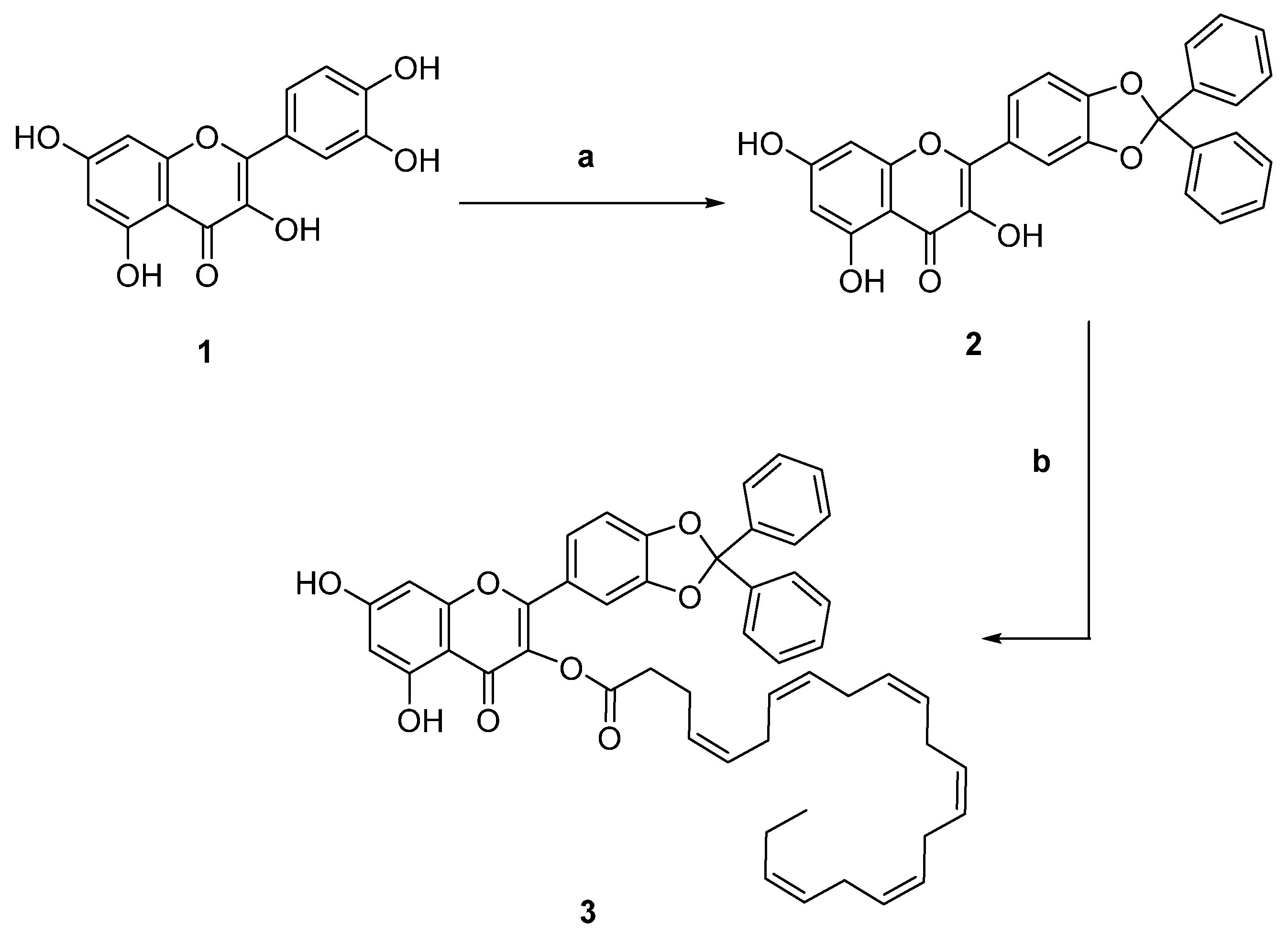
2.1. Chemistry
2.1.1. Synthesis of 2-(2,2-Diphenylbenzo[d][1,3]dioxol-5-yl)-3,5,7-trihydroxy-4H-chromen-4-one (2)
2.1.2. Synthesis of 2-(2,2-Diphenylbenzo[d][1,3]dioxol-5-yl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl (4Z,7Z,10Z,13Z,16Z,19Z)-Docosa-4,7,10,13,16,19-hexaenoate (3)
2.2. Disposable Phenolic Equivalents by Folin–Ciocalteu Procedure
2.3. Total Antioxidant Activity
2.4. Scavenging Activity against DPPH Radicals
2.5. Scavenging Activity agaist ABTS Radical Cations
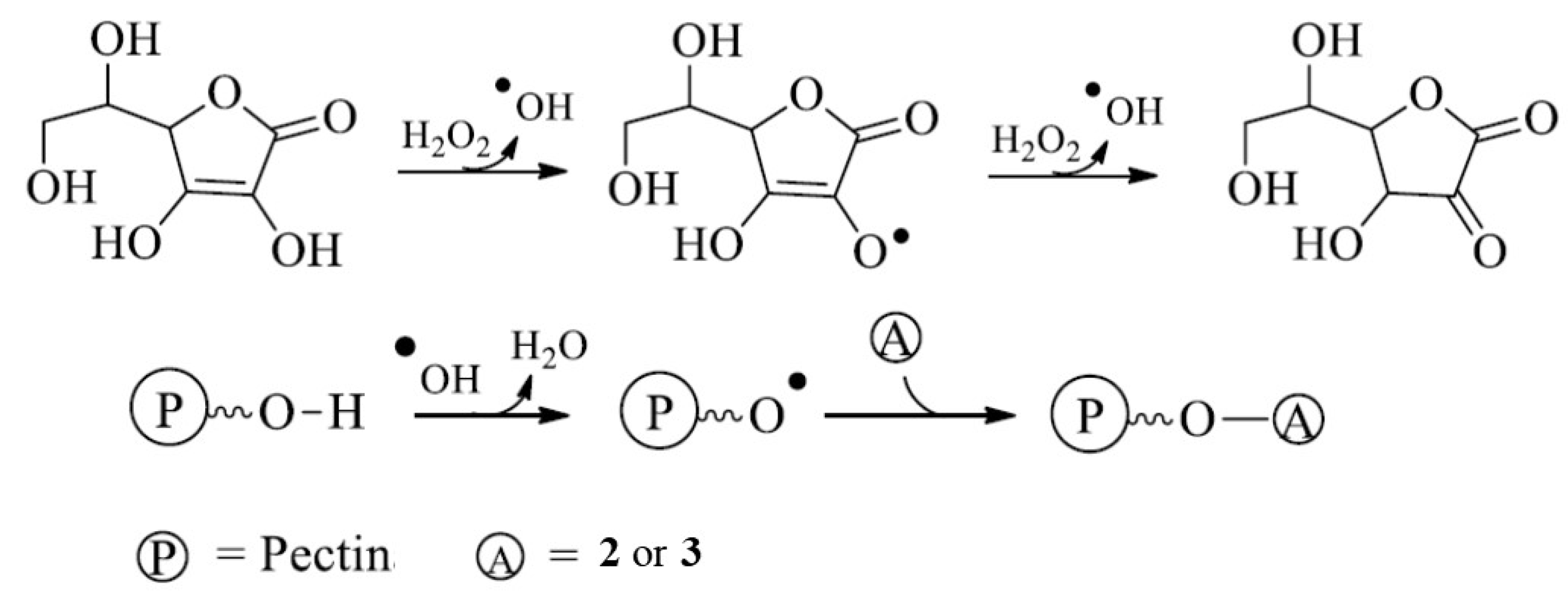
2.6. Synthesis of Conjugates
2.7. Cell Culture
2.8. Neutral Red Uptake (NRU)
2.9. Cell Viability Assay
2.10. Wound-Healing Scratch Assay
2.11. Statistical Analysis
3. Results
3.1. Antioxidant Performances
3.2. Toxicity Evaluation
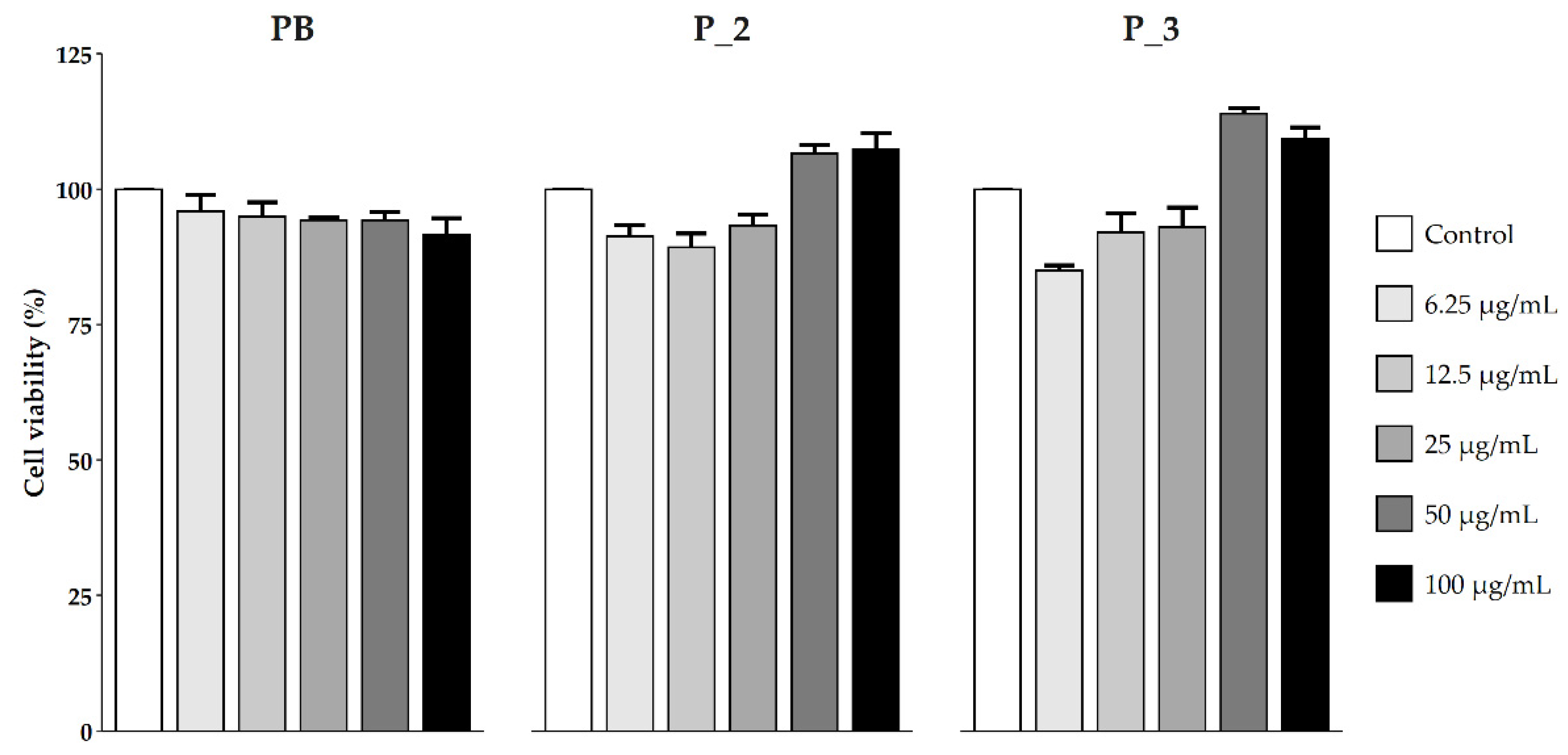
3.2.1. NRU Test
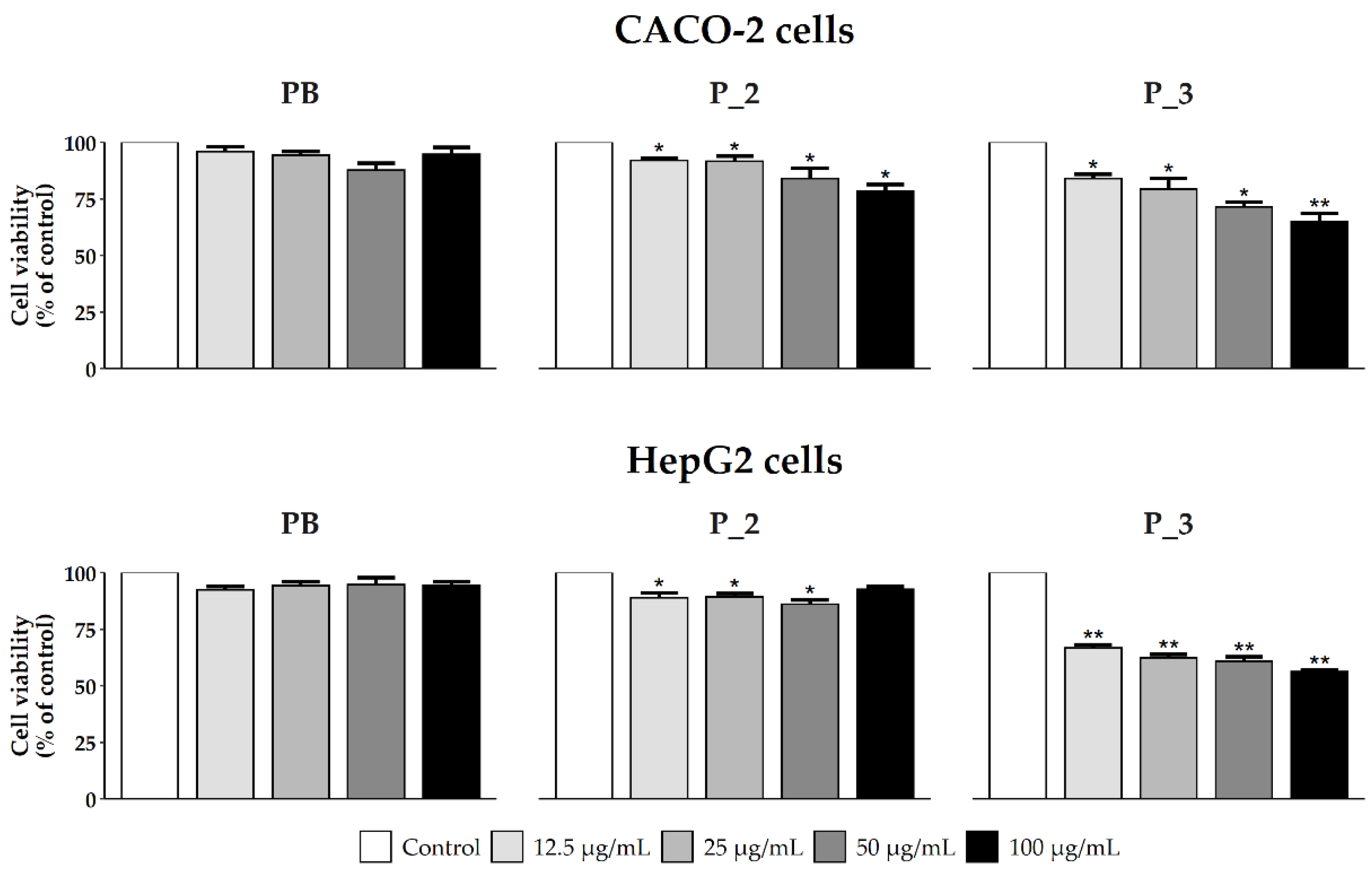
3.2.2. Cell Viability
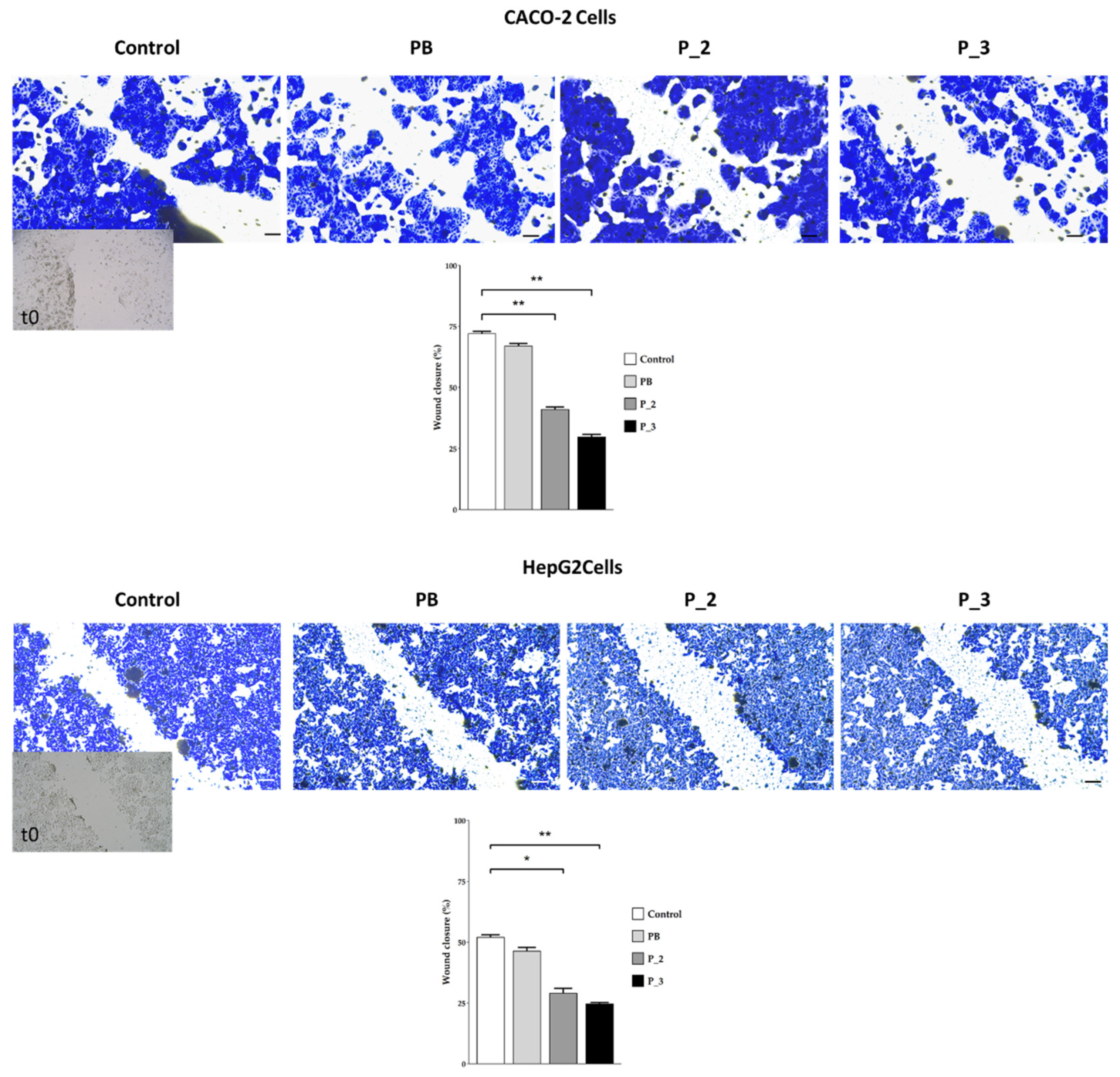
3.2.3. Cells’ Migratory Capability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carullo, G.; Ahmed, A.; Fusi, F.; Sciubba, F.; Di Cocco, M.E.; Restuccia, D.; Spizzirri, U.G.; Saponara, S.; Aiello, F. Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceuticals 2020, 13, 87. [Google Scholar] [CrossRef]
- Carullo, G.; Ahmed, A.; Trezza, A.; Spiga, O.; Brizzi, A.; Saponara, S.; Fusi, F.; Aiello, F. A multitarget semi-synthetic derivative of the flavonoid morin with improved in vitro vasorelaxant activity: Role of CaV1.2 and KCa1.1 channels. Biochem. Pharmacol. 2021, 185, 114429. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Mazzotta, S.; Koch, A.; Hartmann, K.M.; Friedrich, O.; Gilbert, D.F.; Vega-Holm, M.; Schneider-Stock, R.; Aiello, F. New oleoyl hybrids of natural antioxidants: Synthesis and in vitro evaluation as inducers of apoptosis in colorectal cancer cells. Antioxidants 2020, 9, 1077. [Google Scholar] [CrossRef]
- Mazzotta, S.; Governa, P.; Borgonetti, V.; Marcolongo, P.; Nanni, C.; Gamberucci, A.; Manetti, F.; Pessina, F.; Carullo, G.; Brizzi, A.; et al. Pinocembrin and its linolenoyl ester derivative induce wound healing activity in HaCaT cell line potentially involving a GPR120/FFA4 mediated pathway. Bioorg. Chem. 2021, 108, 104657. [Google Scholar] [CrossRef]
- Carullo, G.; Ahmed, A.; Trezza, A.; Spiga, O.; Brizzi, A.; Saponara, S.; Fusi, F.; Aiello, F. Design, synthesis and pharmacological evaluation of ester-based quercetin derivatives as selective vascular KCa1.1 channel stimulators. Bioorg. Chem. 2020, 105, 104404. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Matencio, A.; Pedrazzo, A.R.; Cecone, C.; Appleton, S.L.; Monfared, Y.K.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Addressing the insufficient availability of epa and dha to meet current and future nutritional demands. Nutrients 2021, 13, 2855. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida, A.; Alshammari, M.K.; Kamal, M.; Diwan, A.; Asdaq, S.M.B.; Alshehri, S. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants 2022, 11, 876. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can nutraceuticals assist treatment and improve COVID-19 symptoms? Nat. Prod. Res. 2022, 36, 2672–2691. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Collado, M.C.; Manzoni, P. Phytonutrient and nutraceutical action against COVID-19: Current review of characteristics and benefits. Nutrients 2021, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2022. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Badolato, M.; Carullo, G.; Perri, M.; Cione, E.; Manetti, F.; Di Gioia, M.L.; Brizzi, A.; Caroleo, M.C.; Aiello, F. Quercetin/oleic acid-based G-protein-coupled receptor 40 ligands as new insulin secretion modulators. Future Med. Chem. 2017, 9, 1873–1885. [Google Scholar] [CrossRef]
- Carullo, G.; Perri, M.; Manetti, F.; Aiello, F.; Caroleo, M.C.; Cione, E. Quercetin-3-oleoyl derivatives as new GPR40 agonists: Molecular docking studies and functional evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1761–1764. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Abduvakhidov, A.; Caputo, P.; Crupi, P.; Muraglia, M.; Oliviero Rossi, C.; Clodoveo, M.L.; Aiello, F.; Restuccia, D. Kefir Enriched with Carob (Ceratonia siliqua L.) Leaves Extract as a New Ingredient during a Gluten-Free Bread-Making Process. Fermentation 2022, 8, 305. [Google Scholar] [CrossRef]
- Restuccia, D.; Giorgi, G.; Gianfranco Spizzirri, U.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; De Cicco, L.; Crispini, A.; Scarpelli, F.; Restuccia, D.; Aiello, F. Synthesis and characterization of a (+)-catechin and L-(+)-ascorbic acid cocrystal as a new functional ingredient for tea drinks. Heliyon 2019, 5, e02291. [Google Scholar] [CrossRef]
- Carullo, G.; Spizzirri, U.G.; Loizzo, M.R.; Leporini, M.; Sicari, V.; Aiello, F.; Restuccia, D. Valorization of red grape (Vitis vinifera cv. Sangiovese) pomace as functional food ingredient. Ital. J. Food Sci. 2020, 32, 367–385. [Google Scholar] [CrossRef]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Oliviero Rossi, C.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of New Baking (+)-Catechin Based Leavening Agents: Effects on Rheology, Sensory and Antioxidant Features during Muffin Preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef]
- Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. Neutral Red Uptake Cytotoxicity Tests for Estimating Starting Doses for Acute Oral Toxicity Tests. Curr. Protoc. Toxicol. 2008, 36, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients 2022, 14, 2323. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Mishra, A.; Clark, J.H.; Vij, A.; Daswal, S. Synthesis of graft copolymers of xyloglucan and acrylonitrile. Polym. Adv. Technol. 2008, 19, 99–104. [Google Scholar] [CrossRef]
- Pan, J.-Q.; Liu, N.C.; Lau, W.W.Y. Preparation and properties of new antioxidants with higher MW. Polym. Degrad. Stab. 1998, 62, 165–170. [Google Scholar] [CrossRef]
- Kitagawa, M.; Tokiwa, Y. Polymerization of vinyl sugar ester using ascorbic acid and hydrogen peroxide as a redox reagent. Carbohydr. Polym. 2006, 64, 218–223. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Muniglia, L.; Humeau, C.; Jasniewski, J. Physico-chemical characterization of pectin grafted with exogenous phenols. Food Hydrocoll. 2016, 60, 486–493. [Google Scholar] [CrossRef]
- Wang, C.; Cai, W.-D.; Yao, J.; Wu, L.-X.; Li, L.; Zhu, J.; Yan, J.-K. Conjugation of ferulic acid onto pectinaffected the physicochemical, functionaland antioxidant properties. J. Sci. Food Agric. 2020, 100, 5352–5362. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, Y.; Zhang, X. Quercetin suppresses the migration and invasion in human colon cancer caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn. Mag. 2016, 12, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE 2017, 12, e0172838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Nair, M.G. An Efficient and Economical MTT Assay for Determining the Antioxidant Activity of Plant Natural Product Extracts and Pure Compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Palanca, P.; Fondevila, F.; Méndez-Blanco, C.; Tuñón, M.J.; González-Gallego, J.; Mauriz, J.L. Antitumor effects of quercetin in hepatocarcinoma in vitro and in vivo models: A systematic review. Nutrients 2019, 11, 2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019, 8, 4806–4820. [Google Scholar] [CrossRef] [PubMed]
| Disposable Phenolic Groups (meq Q g−1) | Total Antioxidant Activity (meq CT g−1) | IC50 (mg mL−1) | ||
|---|---|---|---|---|
| DPPH Radical | ABTS Radical | |||
| 2 | 0.770 ± 0.011 a | 0.866 ± 0.021 a | 0.042 ± 0.005 b | 0.153 ± 0.011 b |
| 3 | 0.542 ± 0.010 b | 0.580 ± 0.012 b | 0.226 ± 0.015 a | 0.530 ± 0.025 a |
| Disposable Phenolic Groups (meq Q/g) | Total Antioxidant Activity (meq CT/g) | IC50 (mg mL−1) | ||
|---|---|---|---|---|
| DPPH Radical | ABTS Radical | |||
| P_2 | 0.243 ± 0.011 a | 0.241 ± 0.008 a | 0.480 ± 0.031 a | 31.1% at 0.9 a mg mL−1 |
| P_3 | 0.051 ± 0.002 b | 0.159 ± 0.009 b | 44.1% at 1.3 b mg mL−1 | 16.5% at 0.9 b mg mL−1 |
| PB | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, G.; Spizzirri, U.G.; Malivindi, R.; Rago, V.; Motta, M.F.; Lofaro, D.; Restuccia, D.; Aiello, F. Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration. Nutraceuticals 2022, 2, 278-288. https://doi.org/10.3390/nutraceuticals2040021
Carullo G, Spizzirri UG, Malivindi R, Rago V, Motta MF, Lofaro D, Restuccia D, Aiello F. Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration. Nutraceuticals. 2022; 2(4):278-288. https://doi.org/10.3390/nutraceuticals2040021
Chicago/Turabian StyleCarullo, Gabriele, Umile Gianfranco Spizzirri, Rocco Malivindi, Vittoria Rago, Marisa Francesca Motta, Danilo Lofaro, Donatella Restuccia, and Francesca Aiello. 2022. "Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration" Nutraceuticals 2, no. 4: 278-288. https://doi.org/10.3390/nutraceuticals2040021
APA StyleCarullo, G., Spizzirri, U. G., Malivindi, R., Rago, V., Motta, M. F., Lofaro, D., Restuccia, D., & Aiello, F. (2022). Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration. Nutraceuticals, 2(4), 278-288. https://doi.org/10.3390/nutraceuticals2040021










