Study of the Antioxidant Potential of UV-Treated Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Total Phenolic and Flavonoid Contents
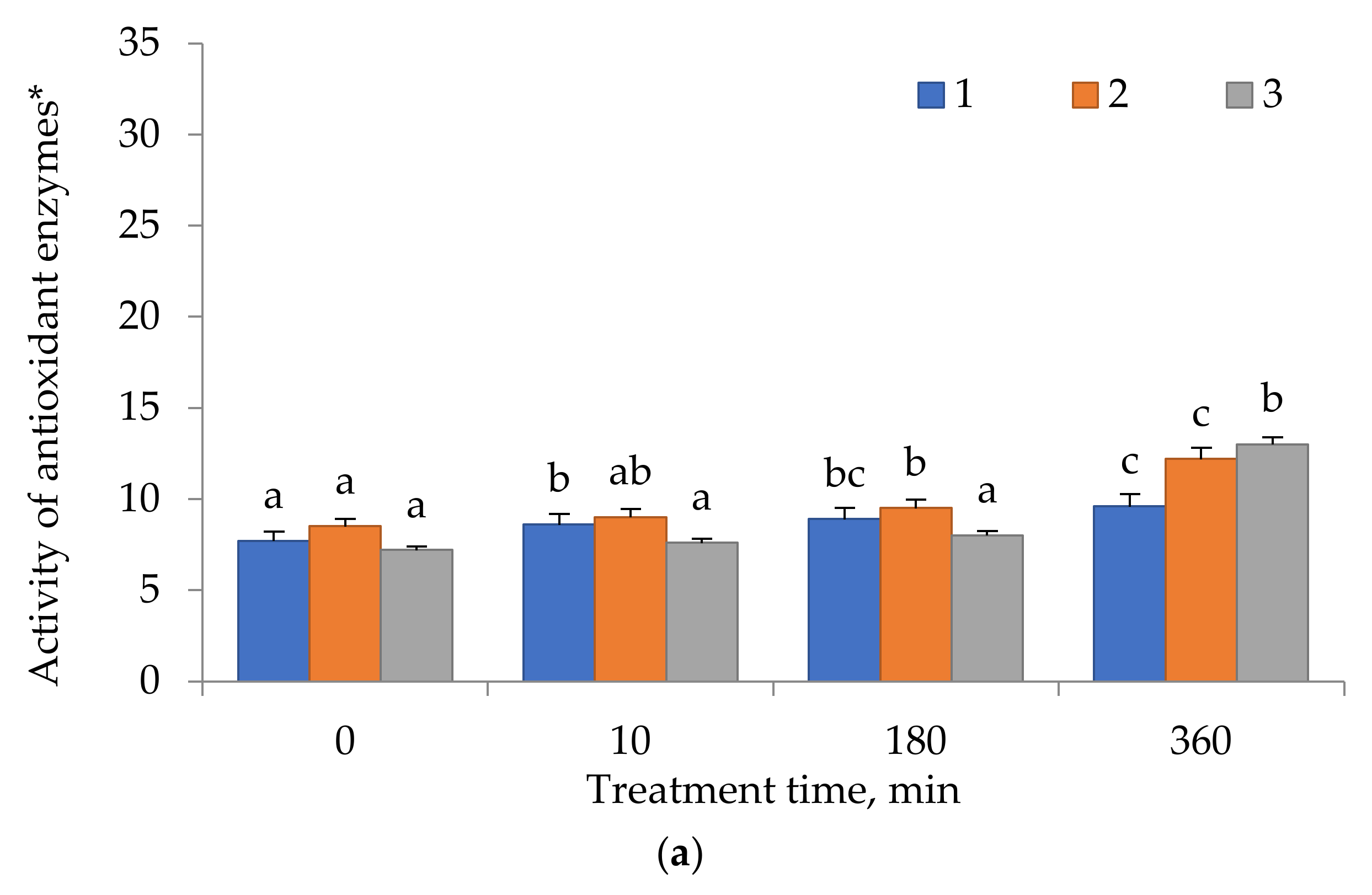
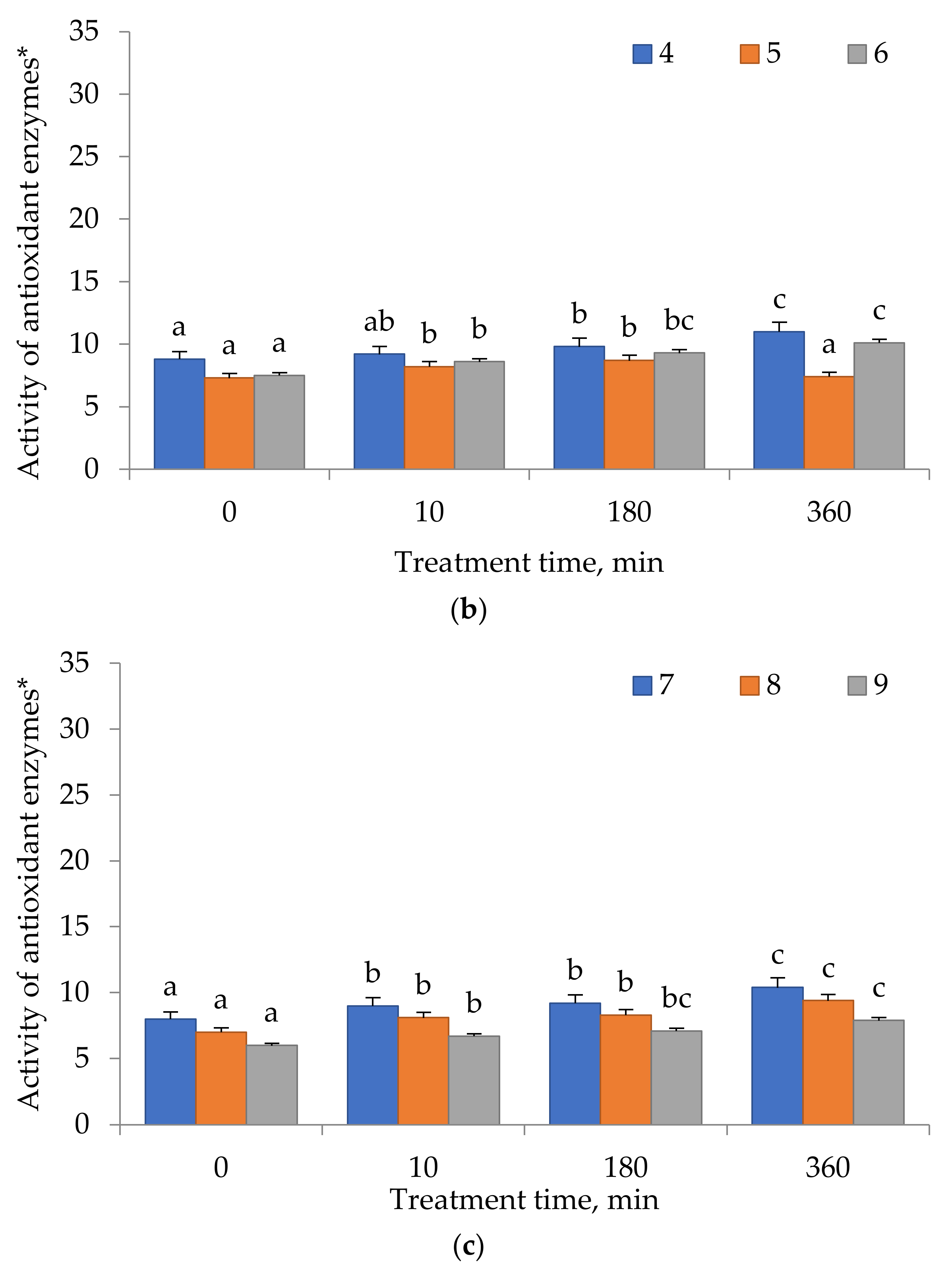
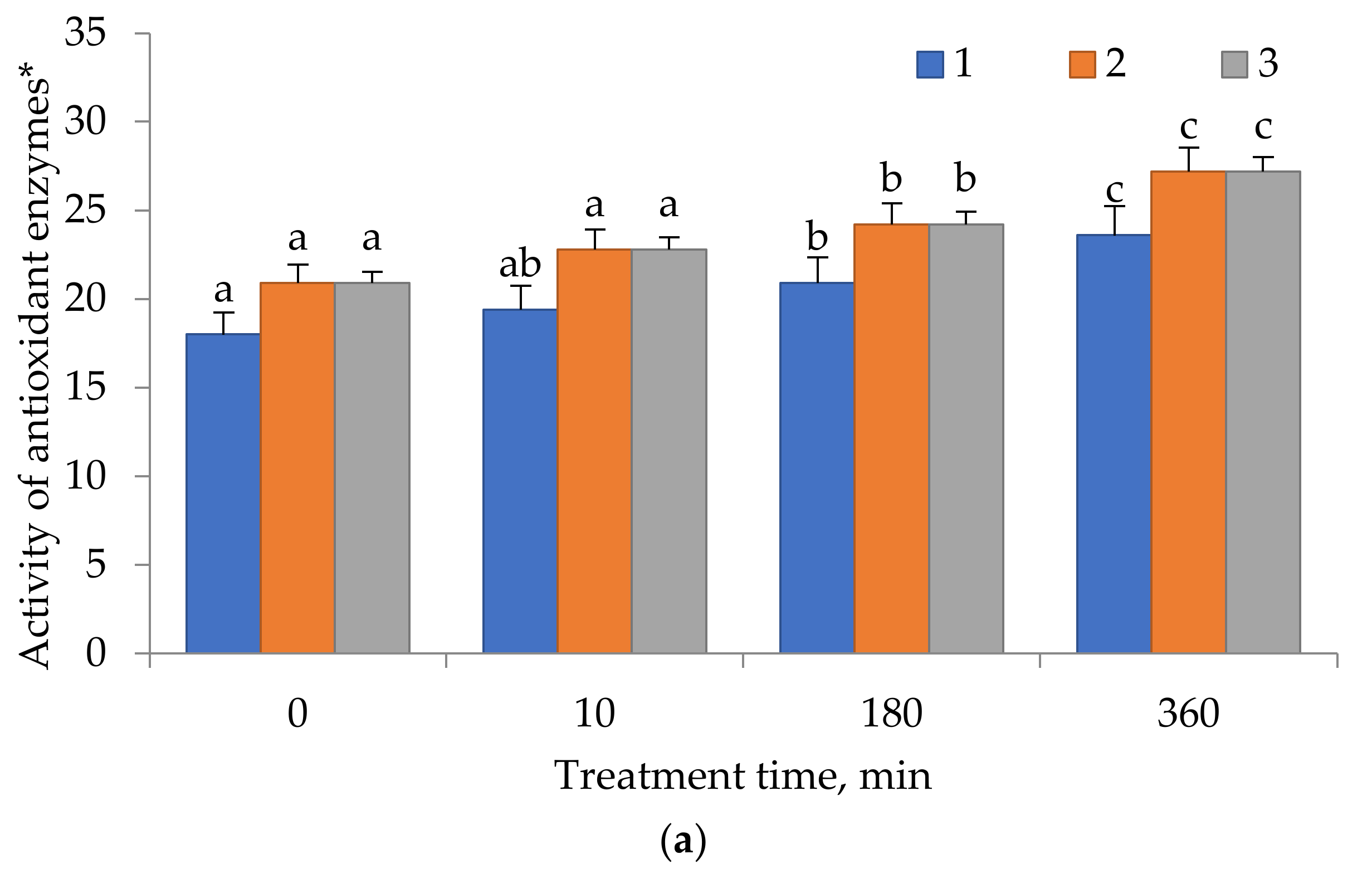
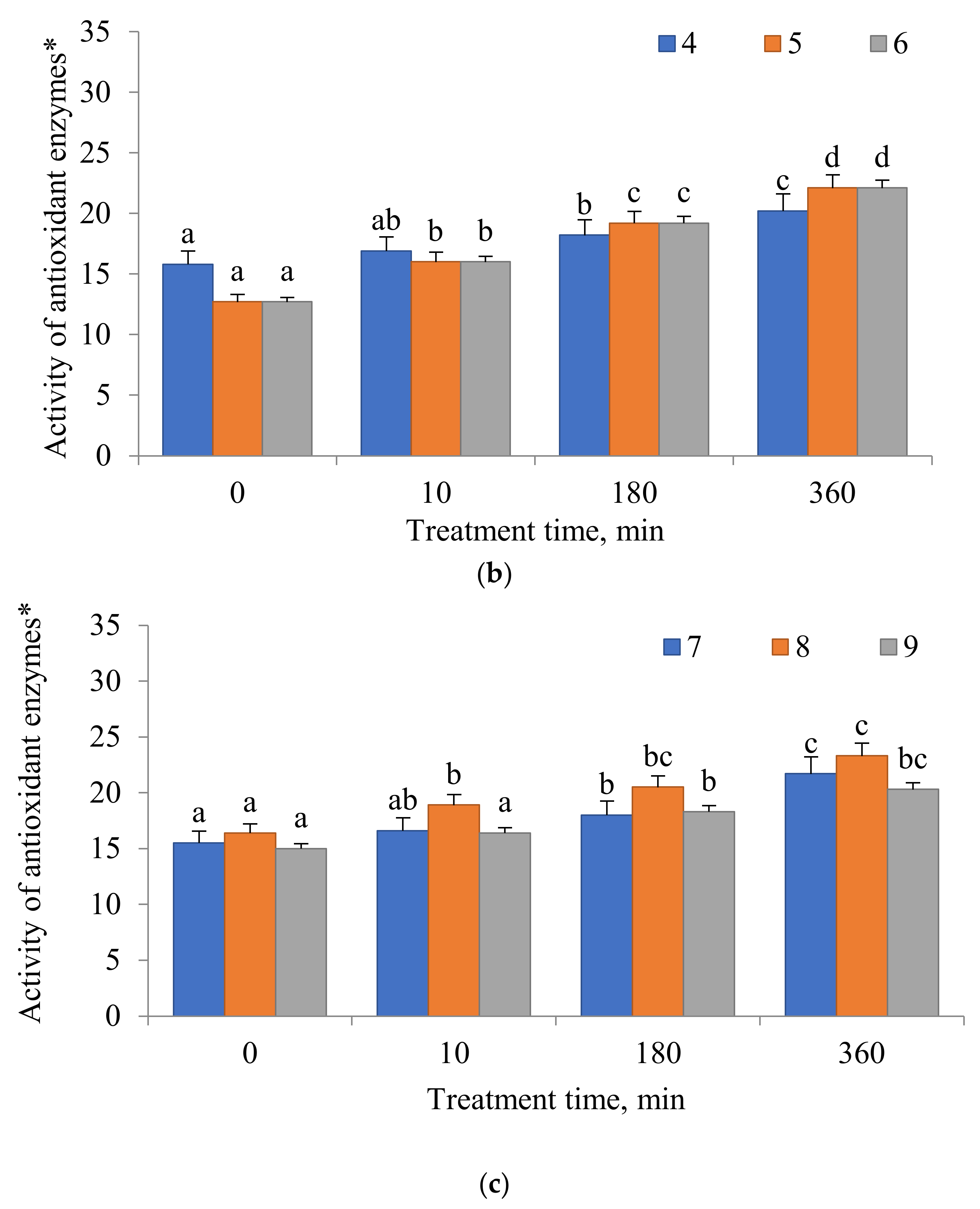
2.4. Antioxidant Enzyme Activity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apaka, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2011, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Büyükkormaz, Ç.; Küçükbay, F.Z. Kumquat fruit and leaves extracted with different solvents: Phenolic content and antioxidant activity. Foods Raw Mater. 2022, 10, 51–66. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Sukhikh, S.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T.; Kriger, O. Effects of Biopreservatives Combined with Modified Atmosphere Packaging on the Quality of Apples and Tomatoes. Pol. J. Food Nutr. Sci. 2019, 69, 289–296. [Google Scholar] [CrossRef]
- Parada, R.B.; Marguet, E.; Campos, C.A.; Vallejo, M. Improving the nutritional properties of Brassica L. vegetables by spontaneous fermentation. Foods Raw Mater. 2022, 10, 97–105. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Sukor, R. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [PubMed]
- Dyshlyuk, L.; Babich, O.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T. The effect of postharvest ultraviolet irradiation on the content of antioxidant compounds and the activity of antioxidant enzymes in tomato. Heliyon 2020, 6, e03288. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Leng, C.; Yang, R. UV-B treatment enhances phenolic acids accumulation and antioxidant capacity of barley seedlings. LWT 2022, 153, 112445. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Wang, P. Effects of V-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Darré, M.; Vicente, A.R.; Cisneros-Zevallos, L.; Artés-Hernández, F. Postharvest ultraviolet radiation in fruit and vegetables: Applications and factors modulating its efficacy on bioactive compounds and microbial growth. Foods 2022, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.Y.; Murray, V. The influence of DNA methylation on the sequence specificity of UVB-and UVC-induced DNA damage. J. Photochem. Photobiol. B Biol. 2021, 221, 112225. [Google Scholar] [CrossRef] [PubMed]
- Sarkany, R.P.E. Ultraviolet Radiation and the Skin. In Earth Systems and Environmental Sciences; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2018. [Google Scholar] [CrossRef]
- Sun, M.-F.; Jiang, C.-L.; Kong, Y.-S.; Luo, J.-L.; Yin, P.; Guo, G.-Y. Recent Advances in Analytical Methods for Determination of Polyphenols in Tea: A Comprehensive Review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Mirecki, R.M.; Teramura, A.H. Effects of ultraviolet-B irradiance on soybean: The dependence of plant-sensitivity on the photosynthetic photon flux-density during and after leaf expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Belaya, N.I.; Bely, A.V. Natural Phenols in the Reaction with Hydrazyl Radical; HouseLLC NPP Foliant: Donetsk, Ukraine, 2018. [Google Scholar]
- Zagoskina, N.V. Plant phenolome and its regulation. In Phenolic Compounds: Fundamental and Applied Aspects, Proceedings of the XI International Symposium, Moscow, Russia, 11–15 April 2022; Pero Publishing House: Moscow, Russia, 2022. [Google Scholar]
- Mansourbahmani, S.; Ghareyazie, B.; Kalatejari, S.; Mohammadi, R.S.; Zarinnia, V. Effect of post-harvest V-C irradiation and calcium chloride on enzymatic activity and decay of tomato (Lycopersicon esculentum L.) fruit during storage. J. Integr. Agric. 2017, 16, 2093–2100. [Google Scholar] [CrossRef]
- Panjai, L.; Noga, G.; Fiebig, A.; Hunsche, M. Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Sci. Hortic. 2017, 226, 97–103. [Google Scholar] [CrossRef]
- Santin, M.; Lucini, L.; Castagna, A.; Chiodelli, G.; Hauser, M.-T.; Ranieri, A. Post-harvest UV-B radiation modulates metabolite profile in peach fruit. Postharvest Biol. Technol. 2018, 139, 127–134. [Google Scholar] [CrossRef]
- Liu, C.; Han, X.; Cai, L.; Lu, X.; Ying, T.; Jiang, Z. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant apacity in tomato fruit during storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Baenas, N.; Iniesta, C.; González-Barrio, R.; Nuñez-Gómez, V.; Periago, M.J.; García-Alonso, F.J. Post-Harvest Use of Ultraviolet Light (UV) and Light Emitting Diode (LED) to Enhance Bioactive Compounds in Refrigerated Tomatoes. Molecules 2021, 26, 1847. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.A. The dependence of the growth and productivity of the cucumber plant on the spectral composition of optical radiation. Innov. Agric. 2019, 2, 207–213. (In Russian) [Google Scholar]
- Promyou, S.; Supapvanich, S. Effect of ultraviolet-C (UV-C) illumination on postharvest quality and bioactive compounds in yellow bell pepper fruit (Capsicum annuum L.) during storage. Afr. J. Agric. Res. 2012, 7, 4084–4096. [Google Scholar]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.A.; Aparicio-Fernández, X.; Ávila-Sosa, R.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]


| Operation Modes | Total Content of Phenolic Compounds (mg/kg) | Total Content of Flavonoids (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| min | nm | A | B | C | A | B | C |
| Control | 254 ± 18 a | 231 ± 16 a | 244 ± 17 a | 61 ± 4 a | 66 ± 5 a | 63 ± 4 a | |
| 10 | 353 | 267 ± 19 a | 245 ± 17 a | 254 ± 18 a | 65 ± 5 a | 67 ± 5 a | 66 ± 5 a |
| 365 | 292 ± 21 b | 263 ± 18 b | 271 ± 19 b | 68 ± 5 a | 75 ± 5 b | 72 ± 5 b | |
| 400 | 277 ± 19 b | 250 ± 18 a | 261 ± 18 a | 67 ± 5 b | 70 ± 5 a | 67 ± 5 a | |
| 180 | 353 | 277 ± 20 b | 252 ± 18 b | 266 ± 19 a | 68 ± 5 b | 70 ± 5 a | 69 ± 5 a |
| 365 | 325 ± 23 c | 305 ± 21 c | 305 ± 21 c | 72 ± 5 b | 79 ± 6 b | 77 ± 5 c | |
| 400 | 287 ± 20 b | 259 ± 18 b | 281 ± 20 b | 69 ± 5 b | 74 ± 5 b | 72 ± 5 b | |
| 360 | 353 | 285 ± 20 b | 256 ± 18 b | 276 ± 19 b | 70 ± 5 b | 73 ± 5 b | 71 ± 5 a |
| 365 | 402 ± 28 c | 358 ± 25 c | 327 ± 23 c | 79 ± 6 c | 91 ± 6 c | 86 ± 6 d | |
| 400 | 297 ± 21 b | 266 ± 19 b | 298 ± 21 c | 73 ± 5 b | 77 ± 5 b | 75 ± 5 b | |
| Operation Modes | Total Content of Phenolic Compounds (mg/kg) | Total Content of Flavonoids (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| min | nm | A | B | C | A | B | C |
| Control | 544 ± 38 a | 521 ± 37 a | 487 ± 34 a | 85 ± 6 a | 93 ± 7 a | 90 ± 6 a | |
| 10 | 353 | 571 ± 10 a | 552 ± 39 a | 492 ± 34 a | 88 ± 6 a | 97 ± 7 a | 92 ± 6 a |
| 365 | 625 ± 44 b | 589 ± 41 b | 560 ± 39 b | 98 ± 7 b | 107 ± 8 b | 97 ± 7 a | |
| 400 | 582 ± 41 a | 563 ± 39 a | 531 ± 37 a | 90 ± 6 a | 99 ± 7 a | 95 ± 7 a | |
| 180 | 353 | 593 ± 42 a | 578 ± 41 b | 512 ± 36 a | 90 ± 6 a | 102 ± 7 a | 98 ± 7 b |
| 365 | 680 ± 48 c | 656 ± 46 c | 624 ± 44 c | 111 ± 8 c | 124 ± 9 c | 106 ± 7 c | |
| 400 | 631 ± 44 b | 584 ± 41 b | 575 ± 40 b | 95 ± 7 b | 109 ± 8 b | 102 ± 7 b | |
| 360 | 353 | 620 ± 44 b | 594 ± 42 b | 526 ± 37 a | 92 ± 6 a | 106 ± 7 b | 103 ± 7 b |
| 365 | 859 ± 60 d | 818 ± 57 d | 755 ± 53 d | 116 ± 8 c | 129 ± 9 c | 115 ± 8 c | |
| 400 | 658 ± 46 c | 615 ± 43 b | 599 ± 42 b | 100 ± 7 b | 116 ± 8 b | 106 ± 8 c | |
| Operation Modes | Total Content of Phenolic Compounds (mg/kg) | Total Content of Flavonoids (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| min | nm | A | B | C | A | B | C |
| Control | 222 ± 16 a | 226 ± 16 a | 226 ± 16 a | 63 ± 4 a | 65 ± 5 a | 62 ± 4 a | |
| 10 | 353 | 230 ± 16 a | 242 ± 17 a | 242 ± 17 a | 66 ± 5 a | 68 ± 5 a | 65 ± 4 a |
| 365 | 255 ± 18 b | 262 ± 18 b | 256 ± 18 b | 69 ± 5 a | 74 ± 5 b | 70 ± 5 b | |
| 400 | 235 ± 16 a | 246 ± 17 a | 247 ± 17 a | 67 ± 5 a | 70 ± 5 a | 66 ± 5 a | |
| 180 | 353 | 242 ± 17 a | 253 ± 18 b | 253 ± 18 b | 68 ± 5 a | 72 ± 5 b | 67 ± 5 a |
| 365 | 286 ± 20 b | 289 ± 20 b | 285 ± 20 b | 78 ± 5 b | 77 ± 5 b | 71 ± 5 b | |
| 400 | 250 ± 18 a | 257 ± 18 b | 258 ± 18 b | 70 ± 5 b | 73 ± 5 b | 69 ± 5 b | |
| 360 | 353 | 246 ± 17 a | 260 ± 18 b | 262 ± 18 b | 70 ± 5 a | 74 ± 5 b | 68 ± 5 b |
| 365 | 337 ± 23 c | 330 ± 23 c | 335 ± 23 c | 87 ± 6 c | 82 ± 6 c | 79 ± 6 c | |
| 400 | 259 ± 18 b | 264 ± 19 b | 269 ± 19 b | 64 ± 5 a | 76 ± 5 b | 71 ± 5 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, S.; Prosekov, A. Study of the Antioxidant Potential of UV-Treated Vegetables. Nutraceuticals 2022, 2, 289-299. https://doi.org/10.3390/nutraceuticals2040022
Ivanova S, Prosekov A. Study of the Antioxidant Potential of UV-Treated Vegetables. Nutraceuticals. 2022; 2(4):289-299. https://doi.org/10.3390/nutraceuticals2040022
Chicago/Turabian StyleIvanova, Svetlana, and Alexander Prosekov. 2022. "Study of the Antioxidant Potential of UV-Treated Vegetables" Nutraceuticals 2, no. 4: 289-299. https://doi.org/10.3390/nutraceuticals2040022
APA StyleIvanova, S., & Prosekov, A. (2022). Study of the Antioxidant Potential of UV-Treated Vegetables. Nutraceuticals, 2(4), 289-299. https://doi.org/10.3390/nutraceuticals2040022








