Anticancer Properties of Aqueous Extracts from Leguminosae
Abstract
:1. Introduction
2. Discussion
2.1. Soluble Proteins from Leguminous and Anticancer Mechanisms
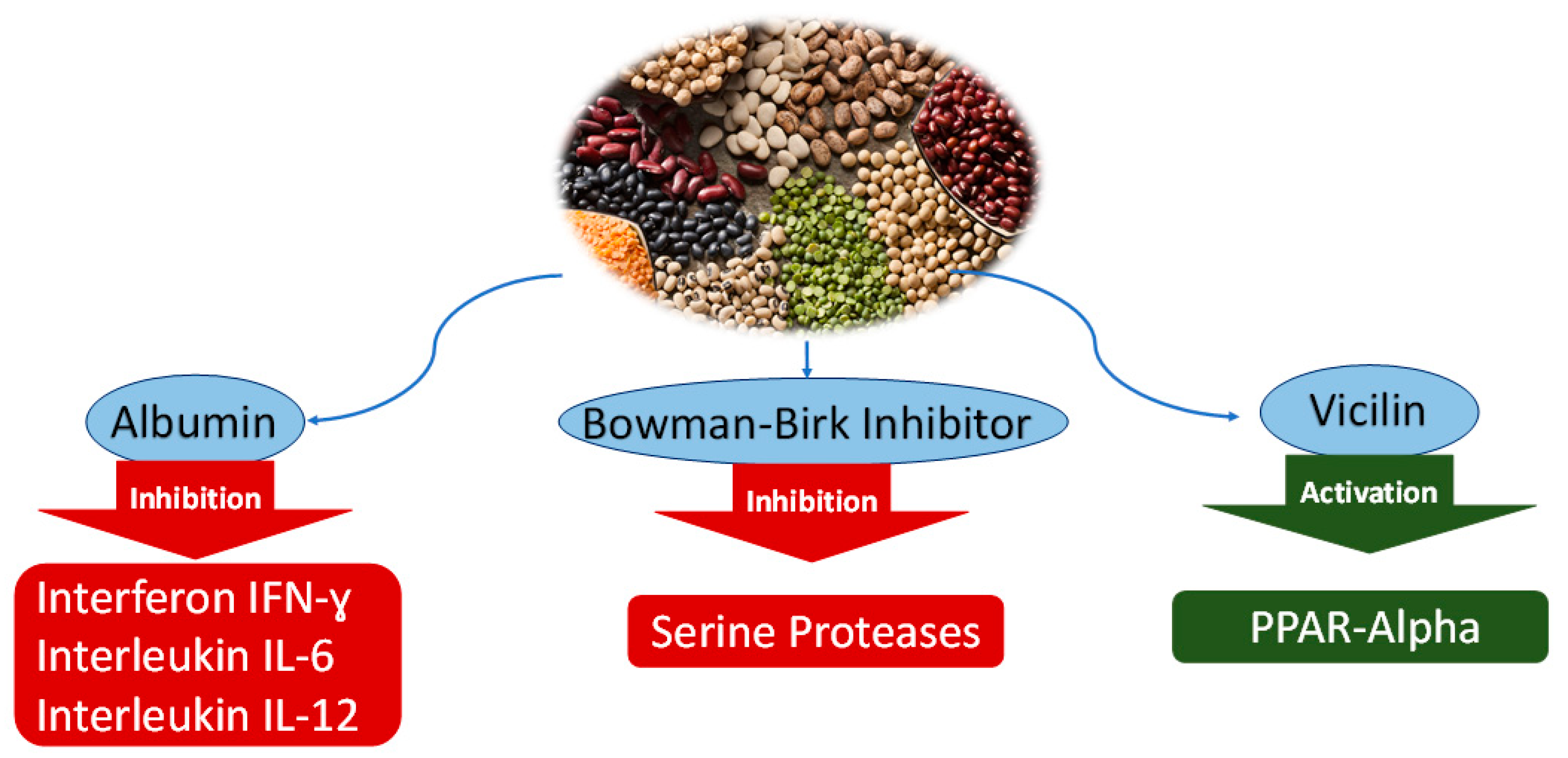
2.1.1. Albumin
2.1.2. Bowman-Birk Inhibitor
2.1.3. Vicilin
2.2. Saponins from Leguminous and Anticancer Mechanisms
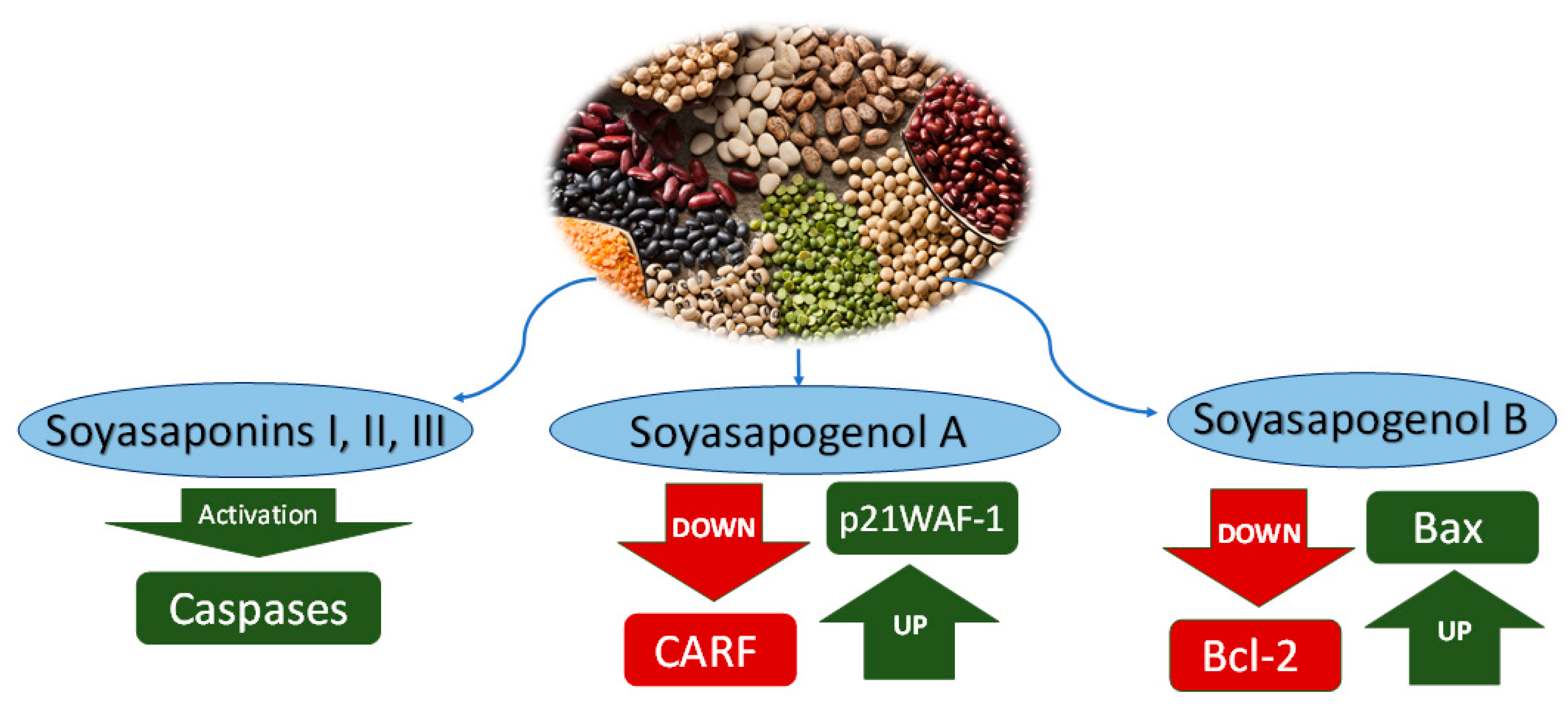
2.2.1. Saponins
2.2.2. Soyasapogenol A
2.2.3. Soyasapogenol B
2.3. Oligosaccharides from Leguminous and Anticancer Mechanisms
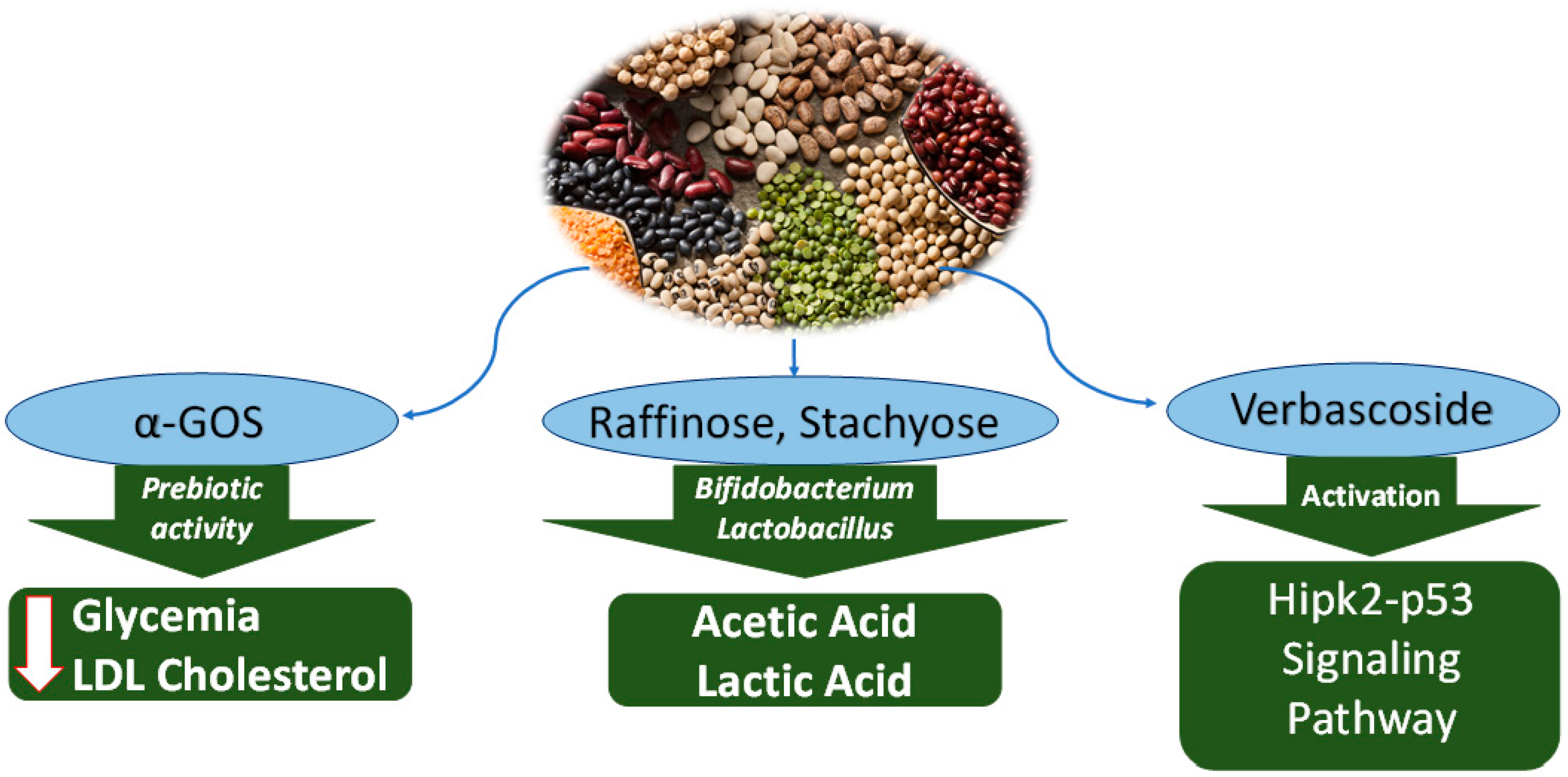
2.3.1. α Galactooligosaccharides (α-GOS)
2.3.2. Raffinose and Stachyose
2.3.3. Verbascoside
| Nutrient | Source | Anticancer Effect | Anticancer Mechanism | Cell Type | Key References |
|---|---|---|---|---|---|
| Bioactive Peptides and Protein | |||||
| Albumin | Cicer arietinum (Chickpeas) | Anti-inflammatory | Reduction of nitric oxide levels Inhibition of proinflammatory cytokines (interferon IFN-ɣ, interleukins IL-6, IL-12) | RAW 264.7 | [21,22] |
| Bowman-Birk Inhibitor | Glycine max (Soybeans) | Antiproliferative of colon cancer | Inhibition of serine proteases | HT29 | [22] |
| Vicilin (Globulin polypeptide) | Pisum sativum (Peas) | Protection against obesity-associated metabolic disorders | Activation of peroxisome proliferator-activated receptor (PPAR-γ) | 3T3-L1 | [26] |
| Saponins | |||||
| Soyasaponins I and III | Glycine max (Soybeans) | Cytotoxicity to cancer cells | Activation of caspases leading to apoptosis | p53 cancer cell SKOV-3 and Saos-2 | [34] |
| Soyasaponin II | Glycine max (Soybeans) | Cytotoxicity to cancer cells | Increased intracellular calcium, damaged mitochondrial functionality and cytochrome C release, leading to apoptosis | HeLa | [27] |
| Soyasapogenol A | Glycine max (Soybeans) | Cytotoxicity to cancer cells | Downregulation of CARF and upregulation of p21WAF-1 inducing repression of cell multiplication (fibrosarcoma, osteosarcoma, ovarian adenocarcinoma, breast adenocarcinoma) | p53 cancer cell SKOV-3 and Saos-2 | [34] |
| Soyasapogenol B | Glycine max (Soybeans) | Cytotoxicity to human colon cancer cells | Inhibition of protein kinase C (PKC) | Caco-2 cells | [49] |
| Oligosaccharides | |||||
| α-linked galactooligosaccharide (α-GOS). | Glycine max (Soybeans) | Protection against obesity-associated metabolic disorders | Lower fasting glycemia, free fatty acids, low-density lipoprotein (LDL), total cholesterol | In vivo (Mice) | [52] |
| Raffinose, Stachyose | Glycine max (Soybeans) | Prebiotic | Proliferation of Bifidobacterium and Lactobacillus, leading to production of acetate, lactate and other organic acids, thus lowering intestinal pH | In vivo (Mice) | [59] |
| Verbascoside | Cytotoxic to breast cancer | Activation of Hipk2-p53 signaling pathway, leading to apoptosis | 4T1 | [67] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Matthias, J.; Zielinski, C.E. Shaping the diversity of Th2 cell responses in epithelial tissues and its potential for allergy treatment. Eur. J. Immunol. 2019, 49, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021, 117, 139–147. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, B.; Kaur, A. Bioactive compounds of legume seeds. In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 645–665. [Google Scholar]
- Serventi, L. Upcycling Legume Water: From Wastewater to Food Ingredients; Springer Nature: Berlin, Germany, 2020. [Google Scholar]
- Erem, E.; Icyer, N.C.; Tatlisu, N.B.; Kilicli, M.; Kaderoglu, G.H.; Toker, Ö.S. A new trend among plant-based food ingredients in food processing technology: Aquafaba. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Bochenek, H.; Francis, N.; Santhakumar, A.B.; Blanchard, C.L.; Chinkwo, K.A. Chickpea Water and Chickpea Polyphenols Induce Apoptosis and Alleviate Cell Migration In Vitro in Human Colon Adenocarcinoma Cells. Preprints 2022, 2022060317. [Google Scholar] [CrossRef]
- Kusumah, J.; Real Hernandez, L.M.; Gonzalez de Mejia, E. Antioxidant potential of mung bean (Vigna radiata) albumin peptides produced by enzymatic hydrolysis analyzed by biochemical and in silico methods. Foods 2020, 9, 1241. [Google Scholar] [CrossRef]
- Finkina, E.I.; Bogdanov, I.V.; Ignatova, A.A.; Kanushkina, M.D.; Egorova, E.A.; Voropaev, A.D.; Stukacheva, E.A.; Ovchinnikova, T.V. Antifungal Activity, Structural Stability, and Immunomodulatory Effects on Human Immune Cells of Defensin from the Lentil Lens culinaris. Membranes 2022, 12, 855. [Google Scholar] [CrossRef]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Lunasin protease inhibitor concentrate decreases pro-inflammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis. Food Sci. Hum. Wellness 2022, 11, 1508–1514. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Cal, R.; Kennedy, K.; Wall, A.; Murphy, N.; Trajkovic, S.; O’Callaghan, S.; Adelfio, A.; Khaldi, N. Characterising the efficacy and bioavailability of bioactive peptides identified for attenuating muscle atrophy within a Vicia faba-derived functional ingredient. Curr. Res. Food Sci. 2021, 4, 224–232. [Google Scholar] [CrossRef]
- Collins, S.L.; McMillan, A.; Seney, S.; van der Veer, C.; Kort, R.; Sumarah, M.W.; Reid, G. Promising prebiotic candidate established by evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a lactobacillus-dominated vaginal microbiota. Appl. Environ. Microbiol. 2018, 84, e02200-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Cheng, Y.; Wang, T.; Tang, L.; Sun, Y.; Lu, X.; Yu, H. Soyasaponin Ab inhibits lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2016, 30, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Aisa, H.A.; Gao, Y.; Yili, A.; Ma, Q.; Cheng, Z. Beneficial role of chickpea (Cicer arietinum L.) functional factors in the intervention of metabolic syndrome and diabetes mellitus. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: Cambridge, MA, USA, 2019; pp. 615–627. [Google Scholar]
- Chen, P.X.; Zhang, H.; Marcone, M.F.; Pauls, K.P.; Liu, R.; Tang, Y.; Zhang, B.; Renaud, J.B.; Tsao, R. Anti-inflammatory effects of phenolic-rich cranberry bean (Phaseolus vulgaris L.) extracts and enhanced cellular antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2017, 38, 675–685. [Google Scholar] [CrossRef]
- Mahbub, R.; Francis, N.; Blanchard, C.; Santhakumar, A. The anti-inflammatory and antioxidant properties of chickpea hull phenolic extracts. Food Biosci. 2021, 40, 100850. [Google Scholar] [CrossRef]
- Supasatyankul, B.; Saisriyoot, M.; Klinkesorn, U.; Rattanaporn, K.; Sae-Tan, S. Extraction of Phenolic and Flavonoid Compounds from Mung Bean (Vigna radiata L.) Seed Coat by Pressurized Liquid Extraction. Molecules 2022, 27, 2085. [Google Scholar] [CrossRef] [PubMed]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef]
- Safavi, F.; Rostami, A. Role of serine proteases in inflammation: Bowman–Birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp. Mol. Pathol. 2012, 93, 428–433. [Google Scholar] [CrossRef]
- Singh, M.; Joseph, K.P. Erythrocytes sedimentation profiles under gravitational field as determined by He-Ne laser. VII. Influence of dextrans, albumin and saline on cellular aggregation and sedimentation rate. Biorheology 1987, 24, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.L.; Ferreira, E.S.; Silva, M.A.; Neves, V.A.; Demonte, A. The Vicilin protein (Vigna radiata L.) of mung bean as a functional food: Evidence of “in vitro” hypocholesterolemic activity. Nutr. Food Sci. 2017, 47, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, R.; Olías, R.; Clemente, A.; Rubio, L.A. A pea (Pisum sativum L.) seed vicilins hydrolysate exhibits PPARγ ligand activity and modulates adipocyte differentiation in a 3T3-L1 cell culture model. Foods 2020, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef]
- Zhou, Y.; Farooqi, A.A.; Xu, B. Comprehensive review on signaling pathways of dietary saponins in cancer cells suppression. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; Wang, C.; Yin, S.; Cheng, S. Recent advances in biotransformation of saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [Green Version]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins–polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Shi, J.; Xue, S.J.; Ma, Y.; Li, D.; Kakuda, Y.; Lan, Y. Kinetic study of saponins B stability in navy beans under different processing conditions. J. Food Eng. 2009, 93, 59–65. [Google Scholar] [CrossRef]
- Takahashi, S.; Hori, K.; Hokari, M.; Gotoh, T.; Sugiyama, T. Inhibition of human renin activity by saponins. Biomed. Res. 2010, 31, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, B.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, X.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-X.; Huang, G.-Q.; Zhang, S.-H. Soyasaponins inhibit the proliferation of Hela cells by inducing apoptosis. Exp. Toxicol. Pathol. 2007, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, J.K.; Yu, Z.; Hornicek, F.J.; Kan, Q.; Duan, Z. Expression and therapeutic implications of cyclin-dependent kinase 4 (CDK4) in osteosarcoma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1573–1582. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Roginsky, A.B.; Ding, X.-Z.; Woodward, C.; Ujiki, M.B.; Singh, B.; Bell, R.H., Jr.; Collin, P.; Adrian, T.E. Anti-pancreatic cancer effects of a polar extract from the edible sea cucumber, Cucumaria frondosa. Pancreas 2010, 39, 646–652. [Google Scholar] [CrossRef]
- Yu, C.Y.; Teng, C.L.J.; Hung, P.S.; Cheng, C.C.; Hsu, S.L.; Hwang, G.Y.; Tzeng, Y.M. Ovatodiolide isolated from Anisomeles indica induces cell cycle G2/M arrest and apoptosis via a ROS-dependent ATM/ATR signaling pathways. Eur. J. Pharmacol. 2018, 819, 16–29. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Zhang, Y.; Choi, C.-I.; Woodward, C.; Collin, P.; Steele, V.E.; Rao, C.V. Chemopreventive effects of Frondanol A5, a Cucumaria frondosa extract, against rat colon carcinogenesis and inhibition of human colon cancer cell growth. Cancer Prev. Res. 2010, 3, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.Y.; Chen, Y.H.; Chien, Y.W.; Huang, W.H.; Lin, S.H. Effect of soy saponin on the growth of human colon cancer cells. World J. Gastroenterol. 2010, 16, 3371. [Google Scholar] [CrossRef]
- Omar, A.; Kalra, R.S.; Putri, J.; Elwakeel, A.; Kaul, S.C.; Wadhwa, R. Soyasapogenol-A targets CARF and results in suppression of tumor growth and metastasis in p53 compromised cancer cells. Sci. Rep. 2020, 10, 6323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalra, R.S.; Chaudhary, A.; Yoon, A.; Bhargava, P.; Omar, A.; Garg, S.; Yun, C.-K.; Kaul, S.C.; Wadhwa, R. CARF enrichment promotes epithelial–mesenchymal transition via Wnt/β-catenin signaling: Its clinical relevance and potential as a therapeutic target. Oncogenesis 2018, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.T.; Singh, R.; Kalra, R.S.; Kaul, S.C.; Wadhwa, R. Collaborator of ARF (CARF) regulates proliferative fate of human cells by dose-dependent regulation of DNA damage signaling. J. Biol. Chem. 2014, 289, 18258–18269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.K.; Yaguchi, T.; Harada, J.I.; Hirano, T.; Wadhwa, R.; Kaul, S.C. CARF (collaborator of ARF) interacts with HDM2: Evidence for a novel regulatory feedback regulation of CARF-p53-HDM2-p21WAF1 pathway. Int. J. Oncol. 2008, 32, 663–671. [Google Scholar] [CrossRef]
- Li, M.; Zhao, M.; Wei, P.; Zhang, C.; Lu, W. Biosynthesis of soyasapogenol B by engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2021, 193, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.C.; Berhow, M.A.; Badger, T.M. Estrogenic and antiproliferative properties of soy sapogenols in human breast cancer cells in vitro. Food Chem. Toxicol. 2002, 40, 1767–1774. [Google Scholar] [CrossRef]
- Zhang, W.; Popovich, D.G. Effect of soyasapogenol A and soyasapogenol B concentrated extracts on Hep-G2 cell proliferation and apoptosis. J. Agric. Food Chem. 2008, 56, 2603–2608. [Google Scholar] [CrossRef]
- Salyer, J.; Eswaranandam, S.; Lee, S.O. Soyasaponin I, III, and soyasapogenol B inhibit proliferation and modulate PKC expression in caco-2 human colon cancer cells. J. Food Res. 2013, 2, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Chappuis, E.; Morel-Depeisse, F.; Bariohay, B.; Roux, J. Alpha-galacto-oligosaccharides at low dose improve liver steatosis in a high-fat diet mouse model. Molecules 2017, 22, 1725. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangola, M.P.; Jaiswal, S.; Kannan, U.; Gaur, P.M.; Båga, M.; Chibbar, R.N. Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry 2016, 125, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ni, K.; Tsuruta, T.; Nishino, N. Dietary casein and soy protein isolate modulate the effects of raffinose and fructooligosaccharides on the composition and fermentation of gut microbiota in rats. J. Food Sci. 2016, 81, H2093–H2098. [Google Scholar] [CrossRef]
- Ende, W.V.D. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolomeo, F.; Startek, J.B.; Van den Ende, W. Prebiotics to fight diseases: Reality or fiction? Phytother. Res. 2013, 27, 1457–1473. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-Q.; Wang, P.-F.; Qin, M.-J.; Qi, J.-J.; Sun, P.; Xu, D.-W.; Yang, S.-H.; Li, X.-E. Optimization of extraction methods with alkali and determination of stachyose, sucrose, and raffinose in fresh rehmannia (Rehmannia glutinosa Libosch) using high-performance liquid chromatography with evaporative light scattering detection. J. Med. Plants Res. 2013, 7, 2170–2176. [Google Scholar]
- de Fátima Viana, S.; Guimarães, V.M.; José, I.C.; e Oliveira, M.G.D.A.; Costa, N.M.B.; de Barros, E.G.; Moreira, M.A.; de Rezende, S.T. Hydrolysis of oligosaccharides in soybean flour by soybean α-galactosidase. Food Chem. 2005, 93, 665–670. [Google Scholar] [CrossRef]
- Tiwari, A.; Verma, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Stimuli-responsive polysaccharides for colon-targeted drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 547–566. [Google Scholar]
- Ma, C.; Huo, D.; You, Z.; Peng, Q.; Jiang, S.; Chang, H.; Zhang, J.; Zhang, H. Differential pattern of indigenous microbiome responses to probiotic Bifidobacterium lactis V9 consumption across subjects. Food Res. Int. 2020, 36, 109496. [Google Scholar] [CrossRef]
- Miyoshi, M.; Shiroto, A.; Kadoguchi, H.; Usami, M.; Hori, Y. Prebiotics improved the defecation status via changes in the microbiota and short-chain fatty acids in hemodialysis patients. Kobe J. Med. Sci. 2020, 66, E12–E21. [Google Scholar]
- Qian, Y.; Lynch, J.H.; Guo, L.; Rhodes, D.; Morgan, J.A.; Dudareva, N. Completion of the cytosolic post-chorismate phenylalanine biosynthetic pathway in plants. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef]
- Dai, Z.; Su, D.; Zhang, Y.; Sun, Y.; Hu, B.; Ye, H.; Jabbar, S.; Zeng, X. Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus). J. Agric. Food Chem. 2014, 62, 10727–10735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Feng, Y.; Jin, Y.; Liu, X.; Sui, H.; Chai, N.; Chen, X.; Liu, N.; Ji, Q.; Wang, Y.; et al. Verbascoside promotes apoptosis by regulating HIPK2–p53 signaling in human colorectal cancer. BMC Cancer 2014, 14, 747. [Google Scholar] [CrossRef] [PubMed]
- Daneshforouz, A.; Nazemi, S.; Gholami, O.; Kafami, M.; Amin, B. The cytotoxicity and apoptotic effects of verbascoside on breast cancer 4T1 cell line. BMC Pharmacol. Toxicol. 2021, 22, 72. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Chen, C.-H.; Hsieh, P.-F.; Lee, Y.-H.; Kuo, W.W.-T.; Wu, R.C.-Y.; Hung, C.H.; Yang, Y.L.; Lin, V.C. Verbascoside inhibits the epithelial-mesenchymal transition of prostate cancer cells through high-mobility group box 1/receptor for advanced glycation end-products/TGF-β pathway. Environ. Toxicol. 2021, 36, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
| Nutrient | Source | Bioactivity | Cell Type | References |
|---|---|---|---|---|
| Albumin | Vigna radiata (Mung beans) | Antioxidant | ABTS test ORAC test | [9] |
| Defensin | Lens culinaris (Lentils) | Antifungal, anti-inflammatory | Caco-2 cells ATCC HTB-37 | [10] |
| Lunasin protease inhibitor (Lunasin, Kunitz, Bowman–Birk) | Glycine max (Soybeans) | Anti-inflammatory Antiproliferation of cytokines | RAW 264.7 | [11] |
| Vicilin | Vicia faba (Faba beans) | Anti-inflammatory | C2C12 | [12] |
| Oligosaccharides | Numerous sources | Prebiotic | Human studies | [13] |
| Saponins | Glycine max (Soybeans) | Anti-inflammatory | Murine alveolar macrophages line MH-S | [14] |
| Cicer arietinum (Chickpeas) | Anti-proliferative | Human studies | [15] | |
| Phenolics | Phaseolus vulgari (Cranberry beans) | Anti-inflammatory Antioxidant | Caco-2 cells | [16] |
| Cicer arietinum (Chickpeas) | Anti-inflammatory Antioxidant | RAW 264.7 | [17] | |
| Vigna radiata (Mung beans) | Antioxidant | ABTS test | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serventi, L.; Cai, X.; Chen, R.; Dilrukshi, N.; Su, J.; Tuange, R.P.N.; Ham, E.E. Anticancer Properties of Aqueous Extracts from Leguminosae. Nutraceuticals 2022, 2, 323-334. https://doi.org/10.3390/nutraceuticals2040025
Serventi L, Cai X, Chen R, Dilrukshi N, Su J, Tuange RPN, Ham EE. Anticancer Properties of Aqueous Extracts from Leguminosae. Nutraceuticals. 2022; 2(4):323-334. https://doi.org/10.3390/nutraceuticals2040025
Chicago/Turabian StyleServenti, Luca, Xuanyi Cai, Ruitian Chen, Nadeesha Dilrukshi, Jingyi Su, Refi Priskila Novaleta Tuange, and Elizabeth Eilidh Ham. 2022. "Anticancer Properties of Aqueous Extracts from Leguminosae" Nutraceuticals 2, no. 4: 323-334. https://doi.org/10.3390/nutraceuticals2040025