Study of the Cosmetic Potential Uses of Plants from Mayotte as Skin Care Agents through the Screening of Their Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Extracts Preparation
2.3. Lipoxygenase Inhibition Evaluation
2.4. DPPH-Reducing Potency Evaluation
2.5. Anti-Tyrosinase Activity Evaluation
2.6. Statistical Analysis
3. Results and Discussion
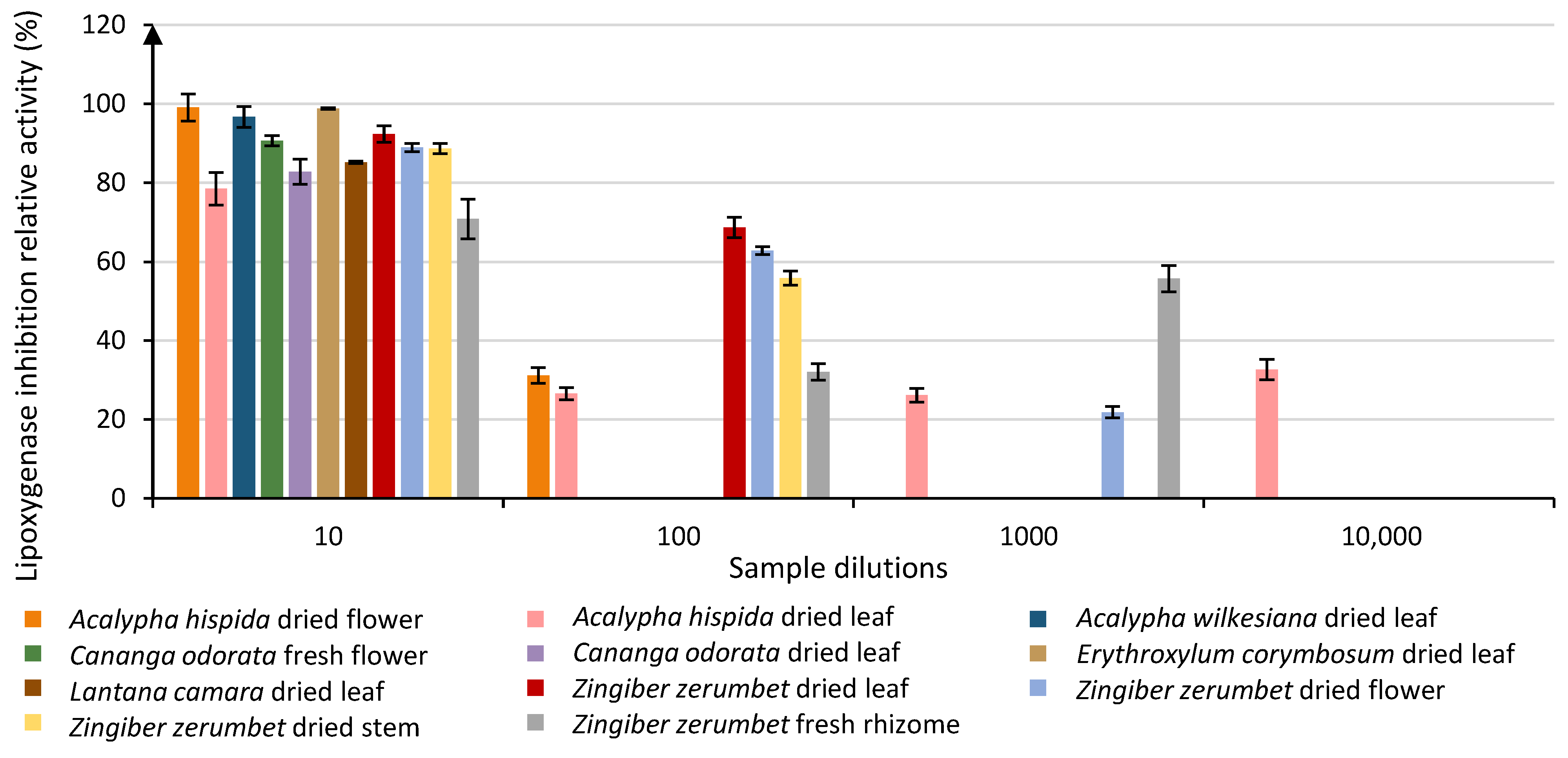
3.1. Anti-Lipoxygenase Activity Evaluation
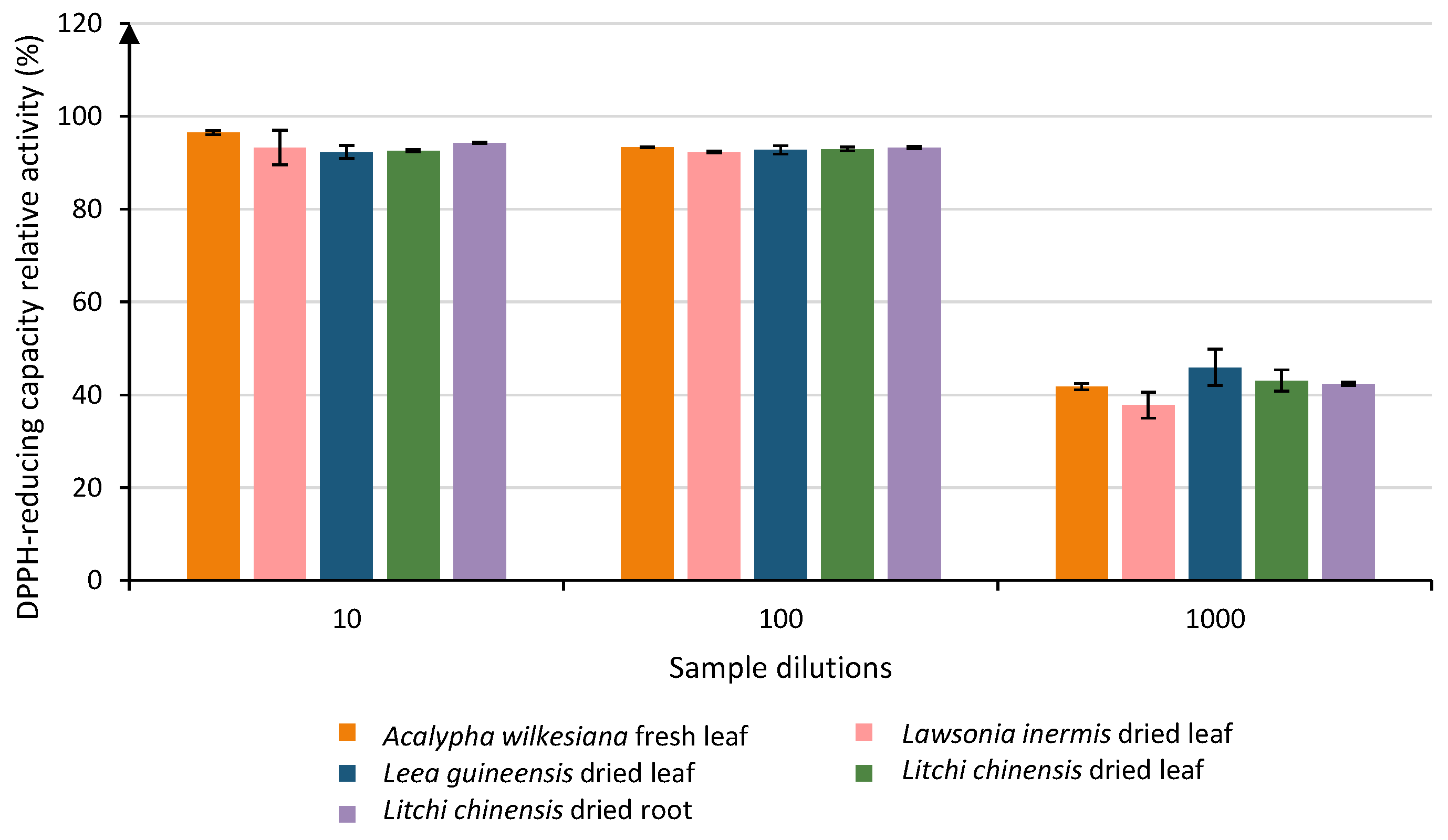
3.2. Antioxidant Activity (DPPH Test)
3.3. Anti-Tyrosinase Activity
3.4. Comparison of the Different Activities
| Sample | Status of the Plant Species in Mayotte | Anti-Lipoxygenase Activity | DPPH-Reducing Agent | Anti-Tyrosinase Activity |
|---|---|---|---|---|
| Acalypha hispida dried flower | Cultivated [27] | ++ | ++ | n/a |
| Acalypha hispida dried leaf | +++ | ++ | n/a | |
| Acalypha wilkesiana dried leaf | Cultivated [27] | n/a | ++ | n/a |
| Acalypha wilkesiana fresh leaf | n/a | ++ | + | |
| Cananga odorata dried leaf | Cultivated [10] | n/a | ++ | n/a |
| Cananga odorata fresh flower | n/a | ++ | n/a | |
| Erythroxylum corymbosum dried leaf | Indigenous [28] | n/a | ++ | n/a |
| Lantana camara dried leaf | Exotic [10] | n/a | ++ | n/a |
| Lawsonia inermis dried leaf | Cultivated [10] | n/a | ++ | n/a |
| Leea guineensis dried fruit | Indigenous [10] | n/a | ++ | +++ |
| Leea guineensis dried leaf | n/a | +++ | n/a | |
| Litchi chinensis dried leaf | Cultivated [10] | + | +++ | +++ |
| Litchi chinensis dried root | n/a | +++ | +++ | |
| Persea americana dried root | Cultivated [10] | n/a | ++ | + |
| Persea americana fresh pit | n/a | ++ | + | |
| Persea americana dried pit | + | ++ | n/a | |
| Zingiber zerumbet dried flower | Exotic [10] | +++ | + | n/a |
| Zingiber zerumbet dried leaf | ++ | n/a | n/a | |
| Zingiber zerumbet dried stem | ++ | n/a | n/a | |
| Zingiber zerumbet fresh rhizome | +++ | n/a | n/a |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurib-Fakim, A. Traditional roles and future prospects for medicinal plants in health care. Asian Biotechnol. Dev. Rev. 2011, 13, 77–83. [Google Scholar]
- Pascal, O. Plantes et forêts de Mayotte. In Collection Patrimoines Naturels; French National Museum: Paris, France, 2002. [Google Scholar]
- Liszkowski, H.D. Mayotte et les Comores: Escales sur la Route des Indes aux XVe et XVIIIe Siècles. In Collection Mémoires; Editions du Baobab: Mamoudzou, France, 2000. [Google Scholar]
- Saive, M.; Genva, M.; Istasse, T.; Frederich, M.; Maes, C.; Fauconnier, M.-L. Identification of a Proanthocyanidin from Litchi Chinensis Sonn. Root with Anti-Tyrosinase and Antioxidant Activity. Biomolecules 2020, 10, 1347. [Google Scholar] [CrossRef]
- Conco, W.Z. The African Bantu traditional practice of medicine: Some preliminary observations. Soc. Sci. Med. 1972, 6, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A. How Islam changed medicine. Br. Med. J. 2005, 331, 1486–1487. [Google Scholar] [CrossRef] [Green Version]
- Soidrou, S.H.; Mohamedb, N.A.; Abdellah Faraha, S.O.; Said Hassaneb, D.B. Ethnopharmacoligical investigation of five plants used in comorian folkloric medicine. Int. J. Phytopharm. 2013, 4, 230–236. [Google Scholar]
- Kaou, A.M.; Mahiou-Leddet, V.; Hutter, S.; Aïnouddine, S.; Hassani, S.; Yahaya, I.; Azas, N.; Ollivier, E. Antimalarial activity of crude extracts from nine African medicinal plants. J. Ethnopharmacol. 2008, 116, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Boullet, V. Index de la flore Vasculaire de Mayotte (Trachéophytes): Statuts, Menaces et Protections. Version 2016.1. Conservatoire Botanique National de Mascarin, Antenne de Mayotte, Coconi. Available online: http://floremaore.cbnm.org (accessed on 9 September 2022).
- Saive, M.; Frederich, M.; Fauconnier, M.-L. Plants used in traditional medicine and cosmetics in Mayotte Island (France): An ethnobotanical study. Indian J. Tradit. Knowl. 2018, 17, 645–653. [Google Scholar]
- Rogers, J.; Harding, C.; Mayo, A.; Banks, J.; Rawlings, A. Stratum corneum lipids: The effect of ageing and the seasons. Arch. Dermatol. Res. 1996, 288, 765–770. [Google Scholar] [CrossRef]
- Edwards, S.; Nebel, S.; Heinrich, M. Questionnaire surveys: Methodological and epistemological problems for field-based ethnopharmacologists. J. Ethnopharmacol. 2005, 100, 30–36. [Google Scholar] [CrossRef]
- Weller, S.C. Cultural consensus theory: Applications and frequently asked questions. Field Methods 2007, 19, 339–368. [Google Scholar] [CrossRef]
- Charles, P.; Elliott, M.J.; Davis, D.; Potter, A.; Kalden, J.R.; Antoni, C.; Ferdinand, C.; Smolen, J.S.; Eberl, G.; Feldmann, M.; et al. Regulation of Cytokines, Cytokine Inhibitors, and Acute-Phase Proteins Following Anti-TNF-α Therapy in Rheumatoid Arthritis. J. Immunol. 1999, 163, 1521–1528. [Google Scholar] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Tanoh, E.A.; Nea, F.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, F.Z.; Fauconnier, M. Antioxidant and Lipoxygenase Inhibitory Activities and Zanthoxylum psammophilum Ake Assi. Molecules 2019, 24, 2445. [Google Scholar] [CrossRef] [Green Version]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- National Association of Testing Authorities, Australia (NATA). Guidelines for the Validation and Verification of Quantitative and Qualitative Test Methods; National Association of Testing Authorities: Sydney, Australia, 2012. [Google Scholar]
- National Association of Testing Authorities, Australia (NATA). Technical Note 17 Guidelines for the Validation and Verification of Quantitative and Qualitative Test Methods; National Association of Testing Authorities: Sydney, Australia, 2013. [Google Scholar]
- Sengul, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2015, 24, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, T.Y.; Huang, S.K.H.; Lee, C.J.; Tsai, P.W.; Wang, C.C. Antinociceptive and anti-inflammatory effects of zerumbone against mono-iodoacetate-induced arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, I.M.; Cardiel, J.M.; Levin, G.A. Nomenclatural review of Acalypha (Euphorbiaceae) of the Western Indian Ocean Region (Madagascar, the Comoros Archipelago, the Mascarene Islands and the Seychelles Archipelago). PhytoKeys 2018, 116, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Pascal, O.; Labat, J.-N.; Pignal, M.; Soumille, O. Systematics and Geography of Plants. In Plant Systematics and Phytogeography for the Understanding of African Biodiversity; Botanic Garden Meise: Meise, Belgium, 2001; Volume 71, p. 1101. [Google Scholar]

| Species | Plant Organs | In Vitro Tests | ||
|---|---|---|---|---|
| Anti-Lipoxygenase Activity | DPPH-Reducing Potency | Anti-Tyrosinase Activity | ||
| Acalypha hispida | Dried flower | x | x | x |
| Dried leaf | x | x | x | |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Acalypha wilkesiana | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Adansonia digitata | Dried seed | x | x | x |
| Dried wood | x | x | x | |
| Aloes mayottensis | Mucilage | x | x | x |
| Dried leaf | x | x | x | |
| Fresh leaf | x | x | x | |
| Cananga odorata | Dried flower | x | x | x |
| Dried leaf | x | x | x | |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh flower | x | x | x | |
| Erythroxylum corymbosum | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Erythroxylum lanceum | Dried leaf | x | x | x |
| Dried root | x | |||
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Kalanchoe pinnata | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried stem | x | x | ||
| Fresh leaf | x | x | ||
| Lantana camara | Dried fruit | x | x | x |
| Dried leaf | x | x | ||
| Dried root | x | x | ||
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Lawsonia inermis | Dried leaf | x | x | |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Leea guineensis | Dried fruit | x | x | x |
| Dried leaf | x | x | x | |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Litchi chinensis | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Litsea glutinosa | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried wood | x | |||
| Fresh leaf | x | x | ||
| Myristica fragrance | Dried seed | x | x | x |
| Pandanus mayottensis | Dried aerial root | x | x | x |
| Dried fruit | x | x | x | |
| Dried leaf | x | x | ||
| Dried wood | x | x | x | |
| Fresh fruit | x | x | x | |
| Fresh leaf | x | x | x | |
| Paullinia pinnata | Dried aerial root | x | x | x |
| Dried leaf | x | x | ||
| Dried liana | x | x | x | |
| Dried root | x | x | x | |
| Fresh leaf | x | x | x | |
| Persea americana | Dried leaf | x | x | x |
| Dried pit | x | x | x | |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Fresh pit | x | x | x | |
| Sesamum indicum | Dried seeds | x | x | x |
| Syzygium aromaticum | Dried leaf | x | x | |
| Dried root | x | x | ||
| Dried wood | x | x | ||
| Fresh leaf | x | x | ||
| Tamarindus indica | Dried leaf | x | x | x |
| Dried root | x | x | x | |
| Dried wood | x | x | x | |
| Fresh leaf | x | x | x | |
| Zingiber zerumbet | Dried flower | x | x | x |
| Dried leaf | x | x | x | |
| Dried rhizom | x | x | ||
| Floral water | x | x | x | |
| Flower stem | x | x | x | |
| Fresh flower | x | x | x | |
| Fresh leaf | x | x | x | |
| Fresh rhizom | x | x | x | |
| Stem | x | x | x | |
| Samples | Lipoxygenase Inhibition Activity (%) | Grouping Information Based on Tukey’s Method | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution 10 | ||||||||||||||||||
| Acalypha hispida dried flower | 99.05 | A | ||||||||||||||||
| Erythroxylum corymbosum dried leaf | 98.83 | A | ||||||||||||||||
| Acalypha wilkesiana dried leaf | 96.70 | A | B | |||||||||||||||
| Zingiber zerumbet dried leaf | 92.36 | A | B | C | ||||||||||||||
| Cananga odorata fresh flower | 90.64 | A | B | C | D | |||||||||||||
| Zingiber zerumbet dried flower | 88.92 | B | C | D | ||||||||||||||
| Zingiber zerumbet stem | 88.66 | B | C | D | E | |||||||||||||
| Lantana camara dried leaf | 85.19 | C | D | E | F | |||||||||||||
| Cananga odorata dried leaf | 82.82 | D | E | F | G | |||||||||||||
| Leea guineensis dried root | 79.94 | E | F | G | H | |||||||||||||
| Acalypha hispida dried leaf | 78.51 | F | G | H | I | |||||||||||||
| Erythroxylum lanceum dried leaf | 74.49 | G | H | I | ||||||||||||||
| Litchi chinensis dried wood | 72.50 | H | I | |||||||||||||||
| Zingiber zerumbet fresh rhizome | 70.85 | I | J | |||||||||||||||
| Cananga odorata dried root | 62.69 | J | K | |||||||||||||||
| Zingiber zerumbet fresh leaf | 60.23 | K | L | |||||||||||||||
| Lawsonia inermis dried root | 58.54 | K | L | |||||||||||||||
| Persea americana dried pit | 51.57 | L | M | |||||||||||||||
| Zingiber zerumbet flower stem | 45.04 | M | N | |||||||||||||||
| Litsea glutinosa dried root | 42.55 | N | ||||||||||||||||
| Paullinia pinnata dried root | 37.88 | N | O | |||||||||||||||
| Zingiber zerumbet fresh flower | 33.70 | O | P | |||||||||||||||
| Acalypha hispida dried root | 33.62 | O | P | |||||||||||||||
| Erythroxylum corymbosum fresh leaf | 31.22 | O | P | |||||||||||||||
| Acalypha wilkesiana dried root | 30.30 | O | P | |||||||||||||||
| Paullinia pinnata dried liana | 27.87 | P | ||||||||||||||||
| Tamarindus indica dried root | 9.21 | Q | ||||||||||||||||
| Dilution 100 | ||||||||||||||||||
| Zingiber zerumbet dried leaf | 68.73 | A | ||||||||||||||||
| Zingiber zerumbet dried flower | 62.84 | A | ||||||||||||||||
| Zingiber zerumbet stem | 55.90 | B | ||||||||||||||||
| Myristica fragrance dried seed | 38.19 | C | ||||||||||||||||
| Zingiber zerumbet fresh rhizome | 32.03 | C | D | |||||||||||||||
| Acalypha hispida dried flower | 31.15 | D | ||||||||||||||||
| Acalypha hispida dried leaf | 26.54 | D | ||||||||||||||||
| Litchi chinensis dried leaf | 16.18 | E | ||||||||||||||||
| Persea americana dried pit | 13.92 | E | ||||||||||||||||
| Dilution 1000 | ||||||||||||||||||
| Zingiber zerumbet fresh rhizome | 55.73 | A | ||||||||||||||||
| Acalypha hispida dried leaf | 26.13 | B | ||||||||||||||||
| Zingiber zerumbet dried flower | 21.81 | B | ||||||||||||||||
| Samples | Antioxidant Activity (%) | Grouping Information Based on Tukey’s Method | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution 10 | ||||||||||||||||||||||||||
| Erythroxylum corymbosum dried leaf | 99.97 | A | ||||||||||||||||||||||||
| Acalypha hispida fresh flower | 99.96 | A | B | |||||||||||||||||||||||
| Erythroxylum lanceum dried root | 99.94 | A | B | |||||||||||||||||||||||
| Lantana camara fresh leaf | 99.91 | A | B | |||||||||||||||||||||||
| Erythroxylum corymbosum dried wood | 99.89 | A | B | |||||||||||||||||||||||
| Erythroxylum corymbosum fresh leaf | 99.68 | A | B | C | ||||||||||||||||||||||
| Acalypha hispida fresh leaf | 99.21 | A | B | C | D | |||||||||||||||||||||
| Erythroxylum lanceum dried leaf | 99.04 | A | B | C | D | E | ||||||||||||||||||||
| Acalypha wilkesiana dried root | 97.83 | A | B | C | D | E | F | |||||||||||||||||||
| Erythroxylum lanceum dried wood | 97.36 | A | B | C | D | E | F | G | ||||||||||||||||||
| Myristica fragrans dried seed | 97.02 | A | B | C | D | E | F | G | H | |||||||||||||||||
| Acalypha wilkesiana fresh leaf | 96.49 | A | B | C | D | E | F | G | H | I | ||||||||||||||||
| Cananga odorata dried root | 96.35 | A | B | C | D | E | F | G | H | I | ||||||||||||||||
| Paullinia pinnata dried leaf | 96.29 | A | B | C | D | E | F | G | H | I | ||||||||||||||||
| Cananga odorata dried flower | 96.10 | A | B | C | D | E | F | G | H | I | J | |||||||||||||||
| Adansonia digitata dried wood | 95.55 | A | B | C | D | E | F | G | H | I | J | K | ||||||||||||||
| Leea guineensis dried root | 94.86 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Syzygium aromaticum fresh leaf | 94.86 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Leea guineensis dried fruit | 94.51 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Persea americana dried root | 94.50 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Paullinia pinnata dried liana | 94.50 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Litchi chinensis dried root | 94.28 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Acalypha hispida dried wood | 94.28 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Leea guineensis fresh leaf | 94.25 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Kalanchoe pinnata fresh leaf | 94.21 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Litchi chinensis dried wood | 94.00 | A | B | C | D | E | F | G | H | I | J | K | L | |||||||||||||
| Persea americana dried pit | 93.68 | B | C | D | E | F | G | H | I | J | K | L | ||||||||||||||
| Paullinia pinnata dried aerial root | 93.50 | C | D | E | F | G | H | I | J | K | L | |||||||||||||||
| Lawsonia inermis fresh leaf | 93.33 | D | E | F | G | H | I | J | K | L | ||||||||||||||||
| Lawsonia inermis dried leaf | 93.27 | D | E | F | G | H | I | J | K | L | ||||||||||||||||
| Pandanus mayottensis dried fruit | 93.14 | D | E | F | G | H | I | J | K | L | ||||||||||||||||
| Syzygium aromaticum dried wood | 93.05 | D | E | F | G | H | I | J | K | L | ||||||||||||||||
| Paullinia pinnata dried root | 92.91 | E | F | G | H | I | J | K | L | M | ||||||||||||||||
| Litchi chinensis dried leaf | 92.64 | F | G | H | I | J | K | L | M | |||||||||||||||||
| Lantana camara dried leaf | 92.40 | F | G | H | I | J | K | L | M | |||||||||||||||||
| Leea guineensis dried leaf | 92.31 | F | G | H | I | J | K | L | M | |||||||||||||||||
| Litchi chinensis fresh leaf | 92.19 | F | G | H | I | J | K | L | M | |||||||||||||||||
| Leea guineensis dried wood | 92.18 | F | G | H | I | J | K | L | M | |||||||||||||||||
| Persea americana fresh pit | 91.99 | F | G | H | I | J | K | L | M | N | ||||||||||||||||
| Paullinia pinnata fresh leaf | 91.90 | F | G | H | I | J | K | L | M | N | ||||||||||||||||
| Persea americana fresh leaf | 91.87 | F | G | H | I | J | K | L | M | N | ||||||||||||||||
| Litsea glutinosa dried bark | 91.31 | G | H | I | J | K | L | M | N | |||||||||||||||||
| Pandanus mayottensis fresh fruit | 91.05 | H | I | J | K | L | M | N | ||||||||||||||||||
| Acalypha wilkesiana dried leaf | 90.98 | H | I | J | K | L | M | N | ||||||||||||||||||
| Persea americana dried leaf | 90.95 | H | I | J | K | L | M | N | ||||||||||||||||||
| Cananga odorata dried leaf | 90.87 | H | I | J | K | L | M | N | ||||||||||||||||||
| Lawsonia inermis dried root | 90.31 | I | J | K | L | M | N | |||||||||||||||||||
| Lawsonia inermis dried wood | 90.30 | I | J | K | L | M | N | |||||||||||||||||||
| Acalypha hispida dried flower | 89.82 | J | K | L | M | N | ||||||||||||||||||||
| Cananga odorata fresh flower | 89.78 | K | L | M | N | |||||||||||||||||||||
| Zingiber zerumbet fresh flower | 89.61 | K | L | M | N | |||||||||||||||||||||
| Persea americana dried wood | 88.91 | L | M | N | O | |||||||||||||||||||||
| Litsea glutinosa fresh leaf | 86.66 | M | N | O | P | |||||||||||||||||||||
| Tamarindus indica dried leaf | 85.80 | N | O | P | Q | |||||||||||||||||||||
| Kalanchoe pinnata dried leaf | 82.38 | O | P | Q | R | |||||||||||||||||||||
| Tamarindus indica fresh leaf | 80.39 | P | Q | R | ||||||||||||||||||||||
| Acalypha hispida dried leaf | 80.13 | Q | R | |||||||||||||||||||||||
| Litsea glutinosa dried leaf | 78.64 | R | ||||||||||||||||||||||||
| Pandanus mayottensis dried root | 64.81 | S | ||||||||||||||||||||||||
| Litsea glutinosa dried wood | 62.24 | S | ||||||||||||||||||||||||
| Litsea glutinosa dried root | 60.13 | S | ||||||||||||||||||||||||
| Zingiber zerumbet dried leaf | 59.18 | S | T | |||||||||||||||||||||||
| Tamarindus indica dried wood | 58.58 | S | T | |||||||||||||||||||||||
| Aloes mayottensis dried root | 53.21 | T | U | |||||||||||||||||||||||
| Lantana camara dried fruit | 51.92 | U | ||||||||||||||||||||||||
| Erythroxylum lanceum fresh leaf | 50.25 | U | ||||||||||||||||||||||||
| Zingiber zerumbet dried flower | 49.18 | U | ||||||||||||||||||||||||
| Pandanus mayottensis dried leaf | 47.57 | U | ||||||||||||||||||||||||
| Aloes Mayottensis dried leaf | 31.24 | V | ||||||||||||||||||||||||
| Zingiber zerumbet fresh rhizome | 26.45 | V | W | |||||||||||||||||||||||
| Pandanus mayottensis fresh leaf | 21.87 | W | X | |||||||||||||||||||||||
| Zingiber zerumbet fresh leaf | 21.67 | W | X | |||||||||||||||||||||||
| Kalanchoe pinnata dried root | 18.23 | X | Y | |||||||||||||||||||||||
| Cananga odorata dried wood | 16.65 | X | Y | |||||||||||||||||||||||
| Lantana camara dried root | 16.39 | X | Y | |||||||||||||||||||||||
| Acalypha wilkesiana dried wood | 15.35 | Y | ||||||||||||||||||||||||
| Lantana camara dried wood | 12.50 | Y | ||||||||||||||||||||||||
| Dilution 100 | ||||||||||||||||||||||||||
| Acalypha hispida fresh leaf | 98.76 | A | ||||||||||||||||||||||||
| Erythroxylum corymbosum fresh leaf | 98.54 | A | B | |||||||||||||||||||||||
| Acalypha hispida dried flower | 96.86 | A | B | C | ||||||||||||||||||||||
| Erythroxylum corymbosum dried leaf | 95.77 | A | B | C | D | |||||||||||||||||||||
| Acalypha wilkesiana dried leaf | 94.99 | A | B | C | D | E | ||||||||||||||||||||
| Leea guineensis dried fruit | 94.61 | A | B | C | D | E | F | |||||||||||||||||||
| Leea guineensis dried root | 94.55 | A | B | C | D | E | F | |||||||||||||||||||
| Syzygium aromaticum fresh leaf | 94.36 | A | B | C | D | E | F | |||||||||||||||||||
| Leea guineensis fresh leaf | 93.92 | A | B | C | D | E | F | |||||||||||||||||||
| Lawsonia inermis fresh leaf | 93.91 | A | B | C | D | E | F | |||||||||||||||||||
| Acalypha wilkesiana dried root | 93.71 | A | B | C | D | E | F | |||||||||||||||||||
| Litchi chinensis dried wood | 93.47 | A | B | C | D | E | F | |||||||||||||||||||
| Persea americana dried root | 93.34 | A | B | C | D | E | F | |||||||||||||||||||
| Acalypha wilkesiana fresh leaf | 93.30 | A | B | C | D | E | F | |||||||||||||||||||
| Acalypha hispida fresh flower | 93.27 | A | B | C | D | E | F | |||||||||||||||||||
| Litchi chinensis dried root | 93.26 | A | B | C | D | E | F | |||||||||||||||||||
| Litchi chinensis dried leaf | 92.95 | A | B | C | D | E | F | G | ||||||||||||||||||
| Leea guineensis dried leaf | 92.76 | A | B | C | D | E | F | G | ||||||||||||||||||
| Persea americana dried leaf | 92.58 | A | B | C | D | E | F | G | ||||||||||||||||||
| Persea americana fresh pit | 92.32 | B | C | D | E | F | G | |||||||||||||||||||
| Lawsonia inermis dried leaf | 92.31 | B | C | D | E | F | G | |||||||||||||||||||
| Syzygium aromaticum dried wood | 91.79 | C | D | E | F | G | ||||||||||||||||||||
| Lantana camara dried leaf | 89.71 | D | E | F | G | |||||||||||||||||||||
| Paullinia pinnata dried root | 89.39 | D | E | F | G | |||||||||||||||||||||
| Paullinia pinnata dried leaf | 89.20 | E | F | G | ||||||||||||||||||||||
| Persea americana fresh leaf | 89.12 | E | F | G | ||||||||||||||||||||||
| Acalypha hispida dried leaf | 88.36 | F | G | H | ||||||||||||||||||||||
| Lawsonia inermis dried root | 86.63 | G | H | I | ||||||||||||||||||||||
| Paullinia pinnata dried aerial root | 82.12 | H | I | |||||||||||||||||||||||
| Cananga odorata dried leaf | 81.67 | I | ||||||||||||||||||||||||
| Paullinia pinnata dried liana | 73.33 | J | ||||||||||||||||||||||||
| Persea americana dried pit | 65.31 | K | ||||||||||||||||||||||||
| Litchi chinensis fresh leaf | 62.58 | K | ||||||||||||||||||||||||
| Lantana camara fresh leaf | 55.48 | L | ||||||||||||||||||||||||
| Erythroxylum lanceum dried wood | 52.26 | L | M | |||||||||||||||||||||||
| Adansonia digitata dried wood | 49.57 | L | M | |||||||||||||||||||||||
| Tamarindus indica dried leaf | 48.75 | M | ||||||||||||||||||||||||
| Kalanchoe pinnata fresh leaf | 40.24 | N | ||||||||||||||||||||||||
| Erythroxylum corymbosum dried wood | 38.45 | N | O | |||||||||||||||||||||||
| Erythroxylum lanceum dried root | 37.71 | N | O | P | ||||||||||||||||||||||
| Persea americana dried wood | 36.16 | N | O | P | ||||||||||||||||||||||
| Cananga odorata fresh flower | 35.01 | N | O | P | ||||||||||||||||||||||
| Myristica fragrans dried seed | 33.76 | O | P | |||||||||||||||||||||||
| Paullinia pinnata fresh leaf | 31.38 | P | Q | |||||||||||||||||||||||
| Leea guineensis dried wood | 26.62 | Q | R | |||||||||||||||||||||||
| Pandanus mayottensis fresh fruit | 23.15 | R | ||||||||||||||||||||||||
| Kalanchoe pinnata dried leaf | 22.45 | R | ||||||||||||||||||||||||
| Pandanus mayottensis dried fruit | 20.57 | R | S | |||||||||||||||||||||||
| Erythroxylum corymbosum dried root | 20.55 | R | S | T | ||||||||||||||||||||||
| Litsea glutinosa fresh leaf | 14.33 | S | T | U | ||||||||||||||||||||||
| Pandanus mayottensis dried root | 13.67 | T | U | |||||||||||||||||||||||
| Aloes Mayottensis dried leaf | 12.01 | U | V | |||||||||||||||||||||||
| Erythroxylum lanceum fresh leaf | 9.69 | U | V | |||||||||||||||||||||||
| Pandanus mayottensis dried leaf | 8.07 | U | V | W | ||||||||||||||||||||||
| Zingiber zerumbet dried flower | 6.81 | V | W | |||||||||||||||||||||||
| Cananga odorata dried wood | 3.13 | W | ||||||||||||||||||||||||
| Dilution 1000 | ||||||||||||||||||||||||||
| Leea guineensis dried leaf | 45.91 | A | ||||||||||||||||||||||||
| Litchi chinensis dried leaf | 43.10 | A | ||||||||||||||||||||||||
| Litchi chinensis dried root | 42.39 | A | B | |||||||||||||||||||||||
| Acalypha wilkesiana fresh leaf | 41.78 | A | B | |||||||||||||||||||||||
| Lawsonia inermis dried leaf | 37.80 | B | C | |||||||||||||||||||||||
| Syzygium aromaticum fresh leaf | 36.53 | C | ||||||||||||||||||||||||
| Acalypha hispida dried flower | 35.97 | C | ||||||||||||||||||||||||
| Acalypha hispida fresh leaf | 27.45 | D | ||||||||||||||||||||||||
| Acalypha hispida fresh flower | 26.83 | D | ||||||||||||||||||||||||
| Leea guineensis fresh leaf | 26.48 | D | E | |||||||||||||||||||||||
| Lawsonia inermis fresh leaf | 26.38 | D | E | |||||||||||||||||||||||
| Leea guineensis dried fruit | 26.07 | D | E | |||||||||||||||||||||||
| Persea americana dried leaf | 26.02 | D | E | |||||||||||||||||||||||
| Erythroxylum corymbosum dried leaf | 22.67 | D | E | F | ||||||||||||||||||||||
| Tamarindus indica dried leaf | 21.83 | E | F | |||||||||||||||||||||||
| Syzygium aromaticum dried wood | 18.49 | F | G | |||||||||||||||||||||||
| Acalypha wilkesiana dried leaf | 17.86 | F | G | |||||||||||||||||||||||
| Acalypha hispida dried leaf | 16.39 | G | ||||||||||||||||||||||||
| Persea americana dried pit | 7.81 | H | ||||||||||||||||||||||||
| Erythroxylum corymbosum dried wood | 3.06 | H | I | |||||||||||||||||||||||
| Leea guineensis dried wood | 2.65 | I | ||||||||||||||||||||||||
| Samples | Anti-Tyrosinase Activity (%) | Grouping Information Based on Tukey’s Method | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution 10 | |||||||||||||||||||||||
| Litchi chinensis dried leaf | 98.57 | A | |||||||||||||||||||||
| Leea guineensis dried fruit | 93.28 | A | B | ||||||||||||||||||||
| Leea guineensis fresh leaf | 91.53 | A | B | C | |||||||||||||||||||
| Leea guineensis dried root | 89.82 | A | B | C | D | ||||||||||||||||||
| Syzygium aromaticum dried leaf | 89.74 | A | B | C | D | ||||||||||||||||||
| Lawsonia inermis dried root | 87.69 | B | C | D | |||||||||||||||||||
| Syzygium aromaticum fresh leaf | 86.22 | B | C | D | |||||||||||||||||||
| Litchi chinensis dried root | 84.83 | B | C | D | E | ||||||||||||||||||
| Persea americana dried leaf | 82.05 | C | D | E | |||||||||||||||||||
| Persea americana dried root | 80.10 | D | E | ||||||||||||||||||||
| Lawsonia inermis dried leaf | 80.07 | D | E | ||||||||||||||||||||
| Litchi chinensis dried wood | 75.61 | E | F | ||||||||||||||||||||
| Paullinia pinnata dried leaf | 68.99 | F | G | ||||||||||||||||||||
| Kalanchoe pinnata fresh leaf | 66.18 | F | G | H | |||||||||||||||||||
| Adansonia digitata dried wood | 64.91 | G | H | I | |||||||||||||||||||
| Paullinia pinnata dried root | 63.91 | G | H | I | |||||||||||||||||||
| Persea americana fresh leaf | 62.84 | G | H | I | |||||||||||||||||||
| Litchi chinensis fresh leaf | 62.36 | G | H | I | |||||||||||||||||||
| Persea americana fresh pit | 60.80 | G | H | I | J | ||||||||||||||||||
| Acalypha hispida fresh leaf | 60.20 | G | H | I | J | ||||||||||||||||||
| Lawsonia inermis fresh leaf | 59.63 | G | H | I | J | ||||||||||||||||||
| Acalypha wilkesiana fresh leaf | 59.01 | H | I | J | |||||||||||||||||||
| Paullinia pinnata dried aerial root | 56.23 | H | I | J | K | ||||||||||||||||||
| Lantana camara fresh leaf | 54.99 | I | J | K | |||||||||||||||||||
| Paullinia pinnata dried liana | 51.78 | J | K | L | |||||||||||||||||||
| Persea americana dried pit | 51.64 | J | K | L | |||||||||||||||||||
| Tamarindus indica fresh leaf | 51.33 | J | K | L | |||||||||||||||||||
| Erythroxylum lanceum dried wood | 48.76 | K | L | M | |||||||||||||||||||
| Persea americana dried wood | 47.22 | K | L | M | N | ||||||||||||||||||
| Litsea glutinosa dried root | 44.81 | L | M | N | |||||||||||||||||||
| Pandanus mayottensis dried aerial root | 44.07 | L | M | N | O | ||||||||||||||||||
| Erythroxylum corymbosum dried root | 43.89 | L | M | N | O | P | |||||||||||||||||
| Acalypha hispida dried flower | 43.69 | L | M | N | O | P | Q | ||||||||||||||||
| Syzygium aromaticum dried wood | 43.47 | L | M | N | O | P | Q | ||||||||||||||||
| Cananga odorata fresh flower | 40.55 | M | N | O | P | Q | R | ||||||||||||||||
| Acalypha hispida dried root | 40.17 | M | N | O | P | Q | R | ||||||||||||||||
| Aloes mayottensis mucilage | 39.15 | M | N | O | P | Q | R | S | |||||||||||||||
| Zingiber zerumbet dried rhizome | 38.77 | N | O | P | Q | R | S | ||||||||||||||||
| Paullinia pinnata fresh leaf | 34.12 | O | P | Q | R | S | T | ||||||||||||||||
| Tamarindus indica dried root | 34.08 | P | Q | R | S | T | |||||||||||||||||
| Cananga odorata dried root | 33.85 | Q | R | S | T | ||||||||||||||||||
| Zingiber zerumbet fresh leaf | 31.72 | R | S | T | U | ||||||||||||||||||
| Zingiber zerumbet flower stem | 30.59 | R | S | T | U | ||||||||||||||||||
| Zingiber zerumbet stem | 29.61 | S | T | U | |||||||||||||||||||
| Erythroxylum corymbosum dried wood | 29.40 | S | T | U | |||||||||||||||||||
| Syzygium aromaticum dried root | 28.46 | T | U | V | |||||||||||||||||||
| Cananga odorata dried wood | 27.51 | T | U | V | |||||||||||||||||||
| Zingiber zerumbet fresh rhizome | 26.71 | T | U | V | |||||||||||||||||||
| Zingiber zerumbet floral water | 26.14 | T | U | V | |||||||||||||||||||
| Zingiber zerumbet dried flower | 24.76 | T | U | V | |||||||||||||||||||
| Pandanus mayottensis fresh leaf | 22.70 | U | V | ||||||||||||||||||||
| Aloes mayottensis dried leaf | 18.85 | V | |||||||||||||||||||||
| Dilution 100 | |||||||||||||||||||||||
| Litchi chinensis dried leaf | 51.36 | A | |||||||||||||||||||||
| Leea guineensis dried fruit | 45.45 | B | |||||||||||||||||||||
| Litchi chinensis dried root | 42.29 | B | |||||||||||||||||||||
| Persea americana dried root | 27.49 | C | |||||||||||||||||||||
| Syzygium aromaticum dried leaf | 27.28 | C | |||||||||||||||||||||
| Persea americana fresh pit | 18.69 | D | |||||||||||||||||||||
| Acalypha wilkesiana fresh leaf | 16.26 | D | |||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genva, M.; Lheureux, L.; Saive, M.; Maes, C.; Fauconnier, M.-L. Study of the Cosmetic Potential Uses of Plants from Mayotte as Skin Care Agents through the Screening of Their Biological Activities. Nutraceuticals 2022, 2, 420-440. https://doi.org/10.3390/nutraceuticals2040031
Genva M, Lheureux L, Saive M, Maes C, Fauconnier M-L. Study of the Cosmetic Potential Uses of Plants from Mayotte as Skin Care Agents through the Screening of Their Biological Activities. Nutraceuticals. 2022; 2(4):420-440. https://doi.org/10.3390/nutraceuticals2040031
Chicago/Turabian StyleGenva, Manon, Laura Lheureux, Matthew Saive, Chloé Maes, and Marie-Laure Fauconnier. 2022. "Study of the Cosmetic Potential Uses of Plants from Mayotte as Skin Care Agents through the Screening of Their Biological Activities" Nutraceuticals 2, no. 4: 420-440. https://doi.org/10.3390/nutraceuticals2040031
APA StyleGenva, M., Lheureux, L., Saive, M., Maes, C., & Fauconnier, M.-L. (2022). Study of the Cosmetic Potential Uses of Plants from Mayotte as Skin Care Agents through the Screening of Their Biological Activities. Nutraceuticals, 2(4), 420-440. https://doi.org/10.3390/nutraceuticals2040031