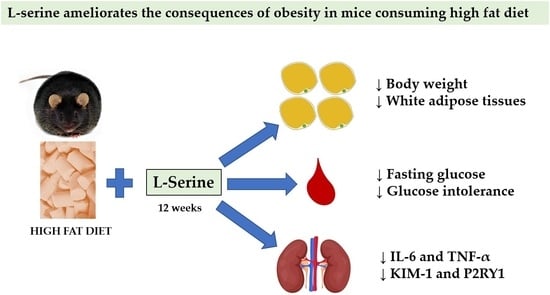
Twelve Weeks of Oral L-Serine Supplementation Improves Glucose Tolerance, Reduces Visceral Fat Pads, and Reverses the mRNA Overexpression of Renal Injury Markers KIM-1, IL-6, and TNF-α in a Mouse Model of Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diet, and L-Serine Supplementation
2.3. Glucose Tolerance Test
2.4. RT-qPCR Assay
2.5. Statistical Analysis
3. Results
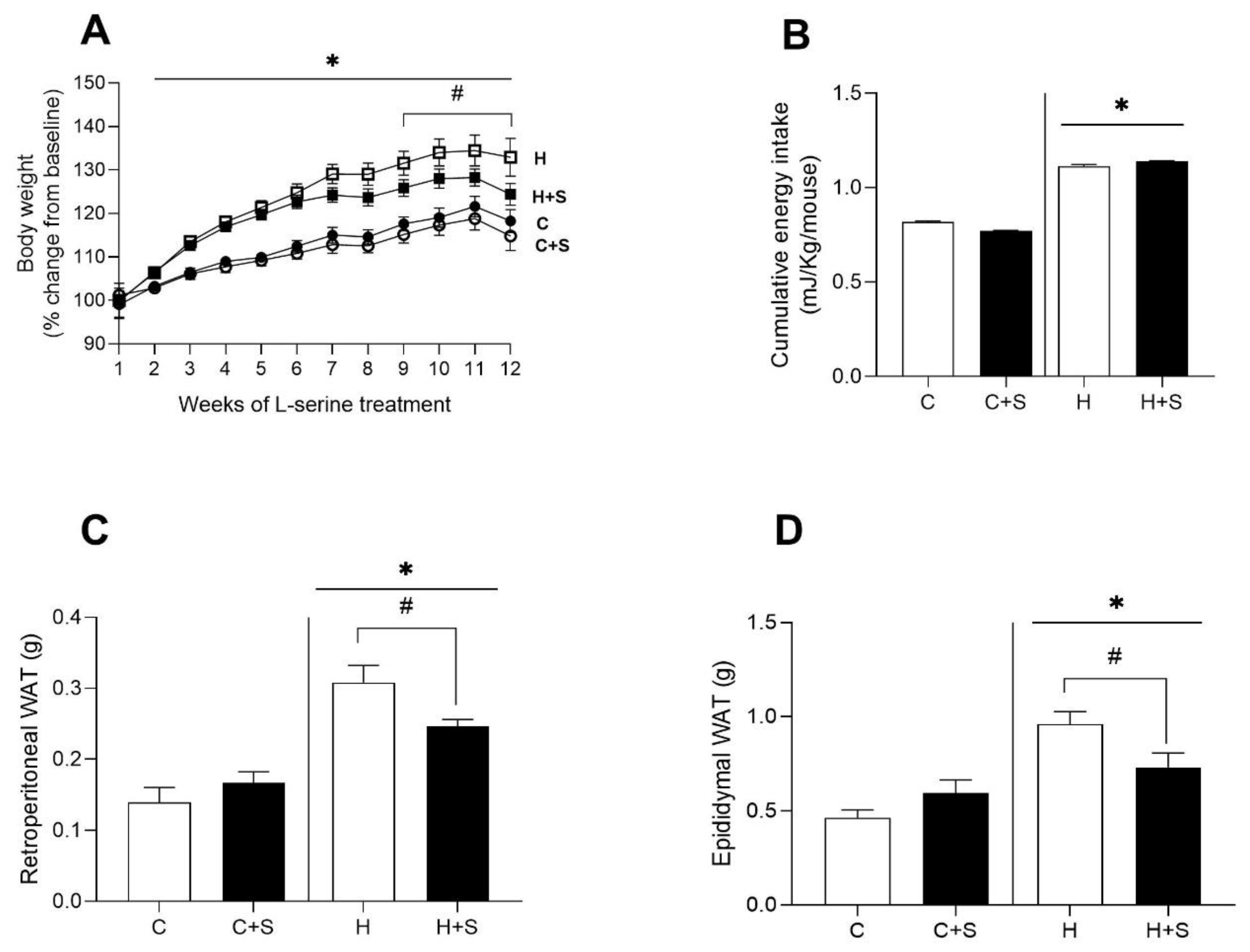
3.1. Effects of L-Serine Supplementation on Body Weight, Energy Intake, and Adiposity
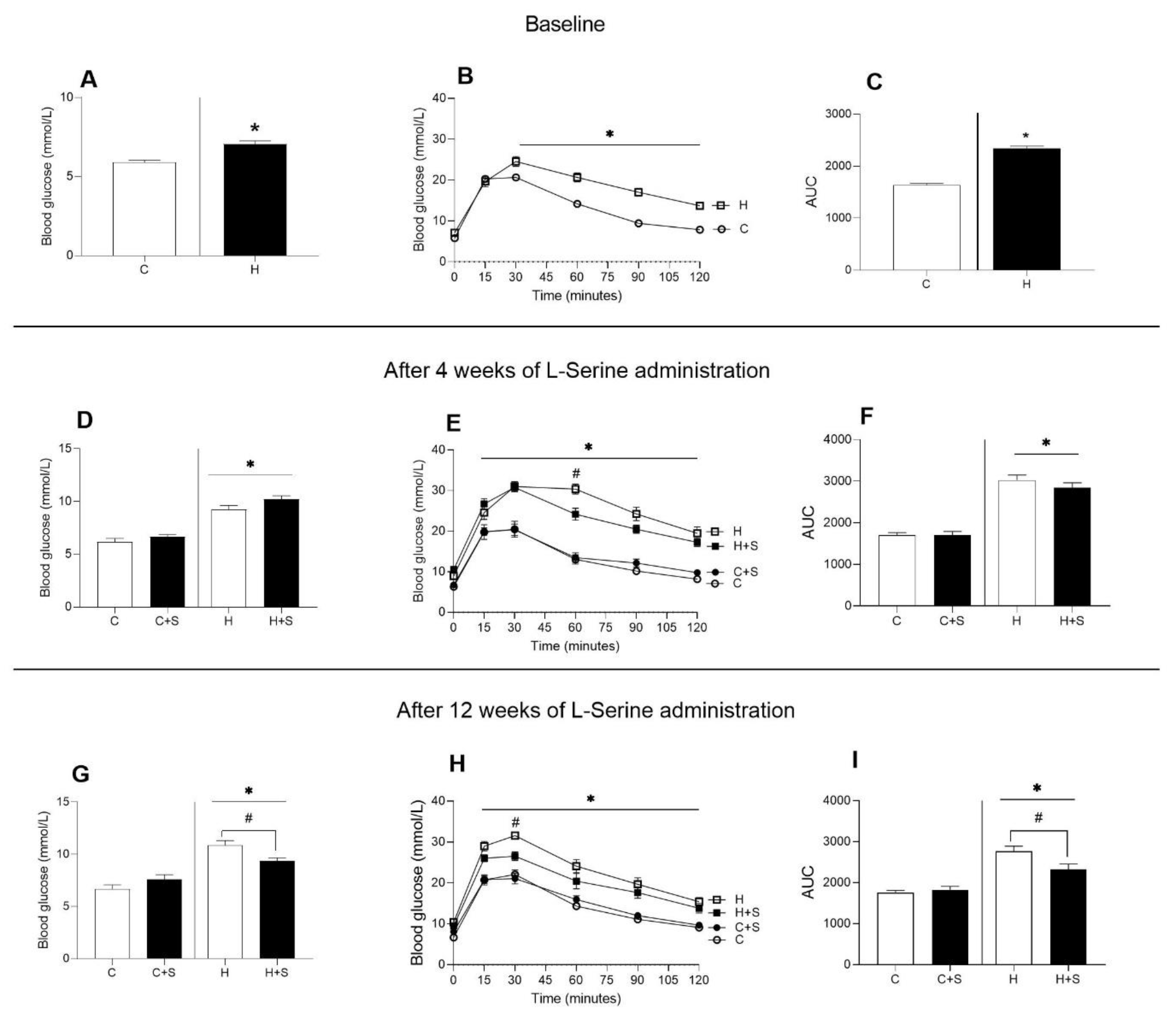
3.2. Effects of L-Serine Supplementation on Glucose Metabolism
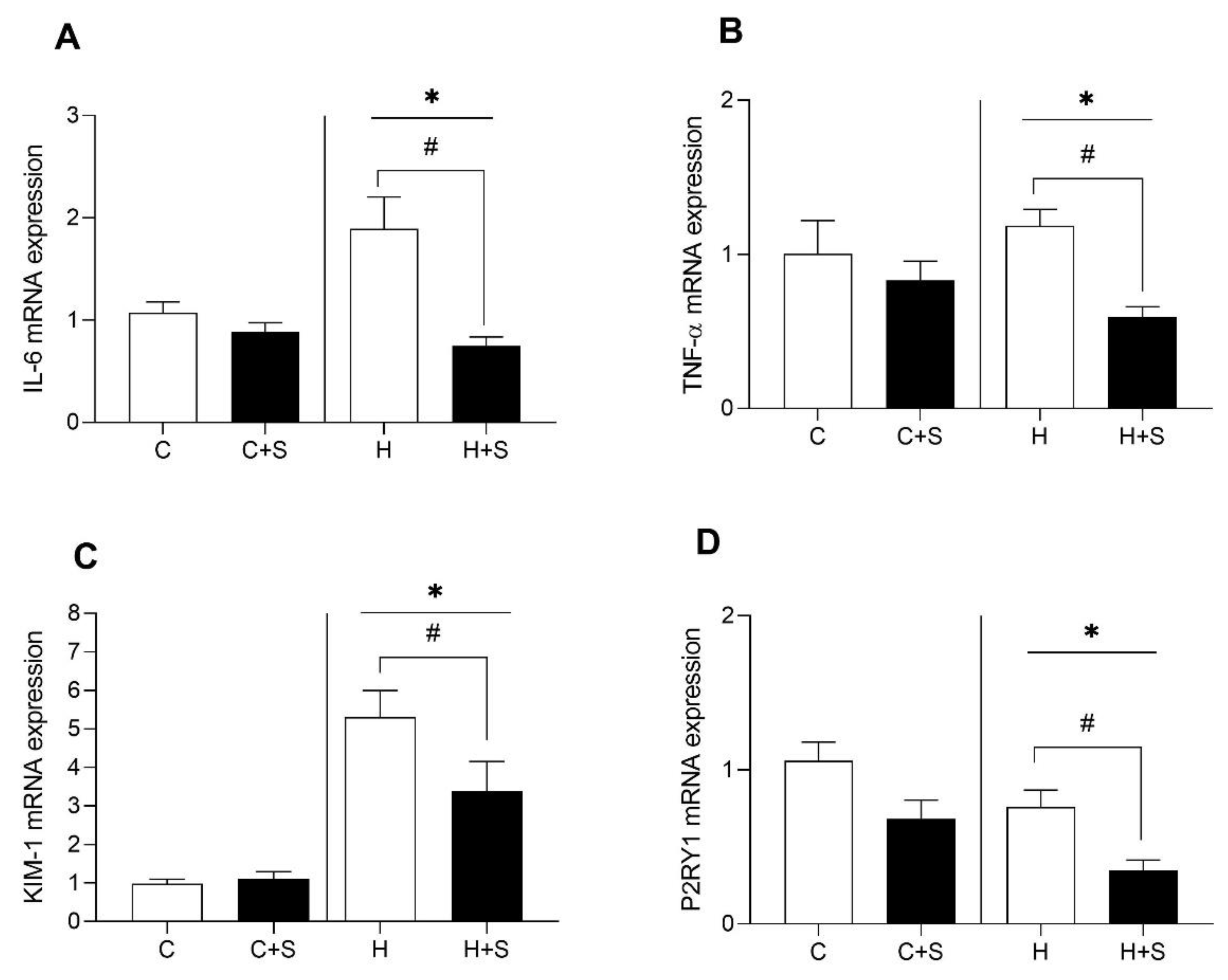
3.3. Effects of L-Serine Supplementation on Renal Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A.; Fruhbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- López-Gonzales, E.; Lehmann, L.; Ruiz-Ojeda, F.J.; Hernández-Bautista, R.; Altun, I.; Onogi, Y.; Khalil, A.E.; Liu, X.; Israel, A.; Ussar, S. L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis. Nutrients 2022, 14, 1922. [Google Scholar] [CrossRef]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef]
- Jiang, J.; Li, B.; He, W.; Huang, C. Dietary serine supplementation: Friend or foe? Curr. Opin. Pharmacol. 2021, 61, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Administration USFD. L-Serine GRAS 172.320. Substances Added to Food (formerly EAFUS), Food Ingredient and Packaging Inventories. 2021. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=SERINE (accessed on 20 October 2022).
- Holm, L.J.; Buschard, K. L-serine: A neglected amino acid with a potential therapeutic role in diabetes. APMIS 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Massa, G.; Guedens, W.; Louis, E.; Noben, J.-P.; Adriaensens, P. Metabolic profiling of type 1 diabetes mellitus in children and adolescents: A case–control study. Diabetol. Metab. Syndr. 2017, 9, 48. [Google Scholar] [CrossRef]
- Bertea, M.; Rütti, M.F.; Othman, A.; Marti-Jaun, J.; Hersberger, M.; von Eckardstein, A.; Hornemann, T. Deoxysphingoid bases as plasma markers in Diabetes mellitus. Lipids Health Dis. 2010, 9, 84. [Google Scholar] [CrossRef]
- Drábková, P.; Šanderová, J.; Kovařík, J.; KanĎár, R. An Assay of Selected Serum Amino Acids in Patients with Type 2 Diabetes Mellitus. Adv. Clin. Exp. Med. 2015, 24, 447–451. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; de Mutsert, R.; Rensen, P.C.N.; Prehn, C.; Adamski, J.; den Heijer, M.; le Cessie, S.; Suhre, K.; Rosendaal, F.R.; van Dijk, K.W. Type 2 diabetes is associated with postprandial amino acid measures. Arch. Biochem. Biophys. 2016, 589, 138–144. [Google Scholar] [CrossRef]
- Mwinyi, J.; Boström, A.; Fehrer, I.; Othman, A.; Waeber, G.; Marti-Soler, H.; Vollenweider, P.; Marques-Vidal, P.; Schiöth, H.B.; von Eckardstein, A.; et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS ONE 2017, 12, 0175776. [Google Scholar] [CrossRef]
- Alaa Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; von Eckardstein, A.; Hornemann, T. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2015, 3, 000073. [Google Scholar] [CrossRef]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.-J.; Frosch, M.P.; von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral l-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef] [PubMed]
- Fridman, V.; Suriyanarayanan, S.; Novak, P.; David, W.; Macklin, E.A.; McKenna-Yasek, D.; Walsh, K.; Aziz-Bose, R.; Oaklander, A.L.; Brown, R.; et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019, 92, e359–e370. [Google Scholar] [CrossRef] [PubMed]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated With Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Kimura, T.; Hesaka, A.; Isaka, Y. D-Amino acids and kidney diseases. Clin. Exp. Nephrol. 2020, 24, 404–410. [Google Scholar] [CrossRef]
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early alterations of plasma free amino acids in chronic renal failure. Clin. Chim. Acta 1990, 188, 101–108. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Kidney injury molecule-1. Curr. Opin. Crit. Care 2010, 16, 556–561. [Google Scholar] [CrossRef]
- Menzies, R.I.; Unwin, R.J.; Bailey, M.A. Renal P2 receptors and hypertension. Acta Physiol. 2015, 213, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Inscho, E.W. ATP, P2 receptors and the renal microcirculation. Purinergic Signal 2009, 5, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Tam, F.W.; Unwin, R.J.; Bailey, M.A. Purinergic signaling in kidney disease. Kidney Int. 2017, 91, 315–323. [Google Scholar] [CrossRef]
- Ahren, B.; Larsson, H.; Holst, J.J. Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1997, 82, 473–478. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The high-fat diet–fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53, S215–S219. [Google Scholar] [CrossRef]
- Ahrén, B.; Holst, J.J.; Mårtensson, H.; Balkan, B. Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur. J. Pharmacol. 2000, 404, 239–245. [Google Scholar] [CrossRef]
- Lin, S.; Thomas, T.; Storlien, L.; Huang, X. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. 2000, 24, 639. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Kontanis, E.J.; Reed, F.A. Evaluation of Real-Time PCR Amplification Efficiencies to Detect PCR Inhibitors. J. Forensic Sci. 2006, 51, 795–804. [Google Scholar] [CrossRef]
- Efficiency of Real-Time PCR. Available online: https://www.thermofisher.com/au/en/home/life-science/pcr/real-time-pcr/real-time-pcr-learning-center/real-time-pcr-basics/efficiency-real-time-pcr-qpcr.html (accessed on 14 April 2023).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, H.; He, L.; Wu, X.; Yin, Y. Long-Term l-Serine Administration Reduces Food Intake and Improves Oxidative Stress and Sirt1/NFκB Signaling in the Hypothalamus of Aging Mice. Front. Endocrinol. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.S.; Morley, J.E. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides 1984, 5, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Essner, R.A.; Smith, A.G.; Jamnik, A.A.; Ryba, A.R.; Trutner, Z.D.; Carter, M.E. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J. Neurosci. 2017, 37, 8678–8687. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.J.; Haupt-Jorgensen, M.; Larsen, J.; Giacobini, J.D.; Bilgin, M.; Buschard, K. L-serine supplementation lowers diabetes incidence and improves blood glucose homeostasis in NOD mice. PLoS ONE 2018, 13, e0194414. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Zuo, S.; Zhang, Y.; Wan, D.; Long, C.; Huang, P.; Wu, X.; Wu, C.; Liu, G.; et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta, Mol. Basis Dis 2018, 1864, 488–498. [Google Scholar] [CrossRef]
- Tran, D.; Myers, S.; McGowan, C.; Henstridge, D.; Eri, R.; Sonda, S.; Caruso, V. 1-Deoxysphingolipids, early predictors of type 2 diabetes, compromise the functionality of skeletal myoblasts. Front. Endocrinol. 2021, 1784. [Google Scholar] [CrossRef]
- Rotthier, A.; Auer-Grumbach, M.; Janssens, K.; Baets, J.; Penno, A.; Almeida-Souza, L.; Van Hoof, K.; Jacobs, A.; De Vriendt, E.; Schlotter-Weigel, B.; et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 2010, 87, 513–522. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Gengatharan, J.M.; Frizzi, K.E.; McGregor, G.H.; Martino, C.; Rahman, G.; Gonzalez, A.; Moreno, A.M.; Green, C.R.; Guernsey, L.S.; et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature 2023, 614, 118–124. [Google Scholar] [CrossRef]
- Xia, C.; Suriyanarayanan, S.; Gong, Y.; Fridman, V.; Selig, M.; Li, J.; Rutkove, S.; Hornemann, T.; Eichler, F. Long-term effects of l-serine supplementation upon a mouse model of diabetic neuropathy. J. Diabetes Complicat. 2023, 37, 108383. [Google Scholar] [CrossRef]
- Peers, I.S.; Ceuppens, P.R.; Harbron, C. In search of preclinical robustness. Nat. Rev. Drug Discov. 2012, 11, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Pellizzon, M.A.; Ricci, M.R. The common use of improper control diets in diet-induced metabolic disease research confounds data interpretation: The fiber factor. Nutr. Metab. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Hasselwander, S.; Li, H.; Xia, N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci. Rep. 2019, 9, 19556. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Martínez-Rojas, M.Á.; Caldiño-Bohn, R.I.; Pérez-Villalva, R.; Zambrano, E.; Castro-Rodríguez, D.C.; Bobadilla, N.A. Early triggers of moderately high-fat diet-induced kidney damage. Physiol. Rep. 2021, 9, e14937. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Lecomte, V.; Erlich, J.H.; Maloney, C.A.; Morris, M.J. Paternal High Fat Diet in Rats Leads to Renal Accumulation of Lipid and Tubular Changes in Adult Offspring. Nutrients 2016, 8, 521. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, H.; Zhuo, H.; Yu, C.; Liu, Y. High Fat Diet Induces Kidney Injury via Stimulating Wnt/β-Catenin Signaling. Front. Med. 2022, 9, 866. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Herbelin, A.; Nguyen, A.T.; Roux-Lombard, P.; Zingraff, J.; Moynot, A.; Verger, C.; Dahmane, D.; de Groote, D.; Jungers, P.; et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J. Immunol. 1995, 154, 882–892. [Google Scholar] [CrossRef]
- Chen, X.; Gong, Q.; Wang, C.-Y.; Zhang, K.; Ji, X.; Chen, Y.X.; Yu, X.-J. High-Fat Diet Induces Distinct Metabolic Response in Interleukin-6 and Tumor Necrosis Factor-α Knockout Mice. J. Interferon Cytokine Res. 2016, 36, 580–588. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Interleukin-18 and diabetic nephropathy: A review. J. Cell. Physiol. 2019, 234, 5674–5682. [Google Scholar] [CrossRef]
- Barbier, L.; Ferhat, M.; Salamé, E.; Robin, A.; Herbelin, A.; Gombert, J.-M.; Silvain, C.; Barbarin, A. Interleukin-1 Family Cytokines: Keystones in Liver Inflammatory Diseases. Front. Immunol. 2019, 10, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, B.; Renk, S.; Lang, K.; Daniel, C.; Freund, M.; Léon, C.; Amann, K.U.; Gachet, C.; Hugo, C.P. P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2007, 18, 494–505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, D.; Ishaq, M.; Yang, C.; Ahmad, T.; Ronci, M.; Zuccarini, M.; Myers, S.; McGowan, C.; Eri, R.; Henstridge, D.C.; et al. Twelve Weeks of Oral L-Serine Supplementation Improves Glucose Tolerance, Reduces Visceral Fat Pads, and Reverses the mRNA Overexpression of Renal Injury Markers KIM-1, IL-6, and TNF-α in a Mouse Model of Obesity. Nutraceuticals 2023, 3, 262-273. https://doi.org/10.3390/nutraceuticals3020021
Tran D, Ishaq M, Yang C, Ahmad T, Ronci M, Zuccarini M, Myers S, McGowan C, Eri R, Henstridge DC, et al. Twelve Weeks of Oral L-Serine Supplementation Improves Glucose Tolerance, Reduces Visceral Fat Pads, and Reverses the mRNA Overexpression of Renal Injury Markers KIM-1, IL-6, and TNF-α in a Mouse Model of Obesity. Nutraceuticals. 2023; 3(2):262-273. https://doi.org/10.3390/nutraceuticals3020021
Chicago/Turabian StyleTran, Duyen, Muhammad Ishaq, Cheng Yang, Tauseef Ahmad, Maurizio Ronci, Mariachiara Zuccarini, Stephen Myers, Courtney McGowan, Rajaraman Eri, Darren C. Henstridge, and et al. 2023. "Twelve Weeks of Oral L-Serine Supplementation Improves Glucose Tolerance, Reduces Visceral Fat Pads, and Reverses the mRNA Overexpression of Renal Injury Markers KIM-1, IL-6, and TNF-α in a Mouse Model of Obesity" Nutraceuticals 3, no. 2: 262-273. https://doi.org/10.3390/nutraceuticals3020021
APA StyleTran, D., Ishaq, M., Yang, C., Ahmad, T., Ronci, M., Zuccarini, M., Myers, S., McGowan, C., Eri, R., Henstridge, D. C., Sonda, S., & Caruso, V. (2023). Twelve Weeks of Oral L-Serine Supplementation Improves Glucose Tolerance, Reduces Visceral Fat Pads, and Reverses the mRNA Overexpression of Renal Injury Markers KIM-1, IL-6, and TNF-α in a Mouse Model of Obesity. Nutraceuticals, 3(2), 262-273. https://doi.org/10.3390/nutraceuticals3020021