Extraction, Chemical Composition, Antiradical Capacity, and Photoprotective Effect of Inonotus obliquus from Eastern Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Betulin and Betulinic Acid Content
2.3. ICP Analysis
2.4. Elemental Analysis
2.5. Antioxidant Activity
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Photoprotective Effects
2.9. Statistical Analysis
3. Results
3.1. Extraction
3.2. Betulinic Acid and Betulin Content
3.3. ICP/MS Analysis
3.4. Elemental Analysis
3.5. Antioxidant Activity
3.6. Total Flavonoid Content
3.7. Total Phenolic Content
3.8. Photoprotective Effects
4. Discussion
4.1. Extraction
4.2. Betulinic Acid and Betulin Content
4.3. ICP/MS Analysis
4.4. Elemental Analysis
4.5. Antioxidant Activity
4.6. Photoprotective Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plasek, B.; Lakner, Z.; Kasza, G.; Temesi, Á. Consumer Evaluation of the Role of Functional Food Products in Disease Prevention and the Characteristics of Target Groups. Nutrients 2019, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Roosen, J.; Bruhn, M.; Mecking, R.-A.; Drescher, L.S. Consumer Demand for Personalized Nutrition and Functional Food. Int. J. Vitam. Nutr. Res. 2008, 78, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, A.; McCluskey, J.J.; Wahl, T.I. Consumer Response to Information about a Functional Food Product: Apples Enriched with Antioxidants. Can. J. Agric. Econ./Rev. Can. D’agroecon. 2009, 57, 325–341. [Google Scholar] [CrossRef]
- Teratanavat, R.; Hooker, N.H. Consumer Valuations and Preference Heterogeneity for a Novel Functional Food. J. Food Sci. 2006, 71, S533–S541. [Google Scholar] [CrossRef]
- Salleh, H.S.; Mohd Noor, A.; Nik Mat, N.H.; Yusof, Y.; Mohamed, W.N. Consumer-Behavioural Intention Towards the Consumption of Functional Food in Malaysia: Their Profiles and Behaviours. Int. Bus. Econ. Res. J. 2015, 14, 727. [Google Scholar] [CrossRef]
- Kraus, A. Development of Functional Food with the Participation of the Consumer. Motivators for Consumption of Functional Products: Development of Functional Food. Int. J. Consum. Stud. 2015, 39, 2–11. [Google Scholar] [CrossRef]
- Topolska, K.; Radzki, R.P.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Leszczyńska, T.; Cieślik, E. Fructan-Enriched Diet Increases Bone Quality in Female Growing Rats at Calcium Deficiency. Plant Foods Hum. Nutr. 2018, 73, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Topolska, K.; Bieńko, M.; Filipiak-Florkiewicz, A.; Radzki, R.; Cieślik, E. The Effect of Fructan-Enriched Diet on Bone Turnover Parameters in Ovariectomized Rats under Calcium Restriction. Ann. Agric. Environ. Med. 2020, 27, 219–224. [Google Scholar] [CrossRef]
- Bekoglu, F.B.; Ergen, A.; Inci, B. The Impact of Attitude, Consumer Innovativeness and Interpersonal Influence on Functional Food Consumption. Int. Bus. Res. 2016, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Ren, K.; Lu, S.; Yang, S.; Sun, D. Progress of Research on Inonotus obliquus. Chin. J. Integr. Med. 2009, 15, 156–160. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus Obliquus—From Folk Medicine to Clinical Use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Robak, J.; Szczepkowski, A.; Gunia-Krzyżak, A.; Popiół, J.; Piotrowska, J.; Rospond, B.; Szewczyk, A.; Kała, K.; Muszyńska, B. Comparison of Bioactive Secondary Metabolites and Cytotoxicity of Extracts from Inonotus obliquus Isolates from Different Host Species. Molecules 2023, 28, 4907. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Zheng, J.-J.; Qu, C.; Xiao, Y.; Li, F.-F.; Jin, Q.-X.; Li, H.-H.; Meng, F.-P.; Jin, G.-H.; Jin, D. Inonotus obliquus Polysaccharide Ameliorates Dextran Sulphate Sodium Induced Colitis Involving Modulation of Th1/Th2 and Th17/Treg Balance. Artif. Cells Nanomed. Biotechnol. 2019, 47, 757–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandaykin, M.E.; Zmitrovich, I.V. Review on Chaga Medicinal Mushroom, Inonotus obliquus (Higher Basidiomycetes): Realm of Medicinal Applications and Approaches on Estimating Its Resource Potential. Int. J. Med. Mushrooms 2015, 17, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zheng, W. Deciphering the Antitumoral Potential of the Bioactive Metabolites from Medicinal Mushroom Inonotus obliquus. J. Ethnopharmacol. 2021, 265, 113321. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Yang, L.; Mei, W.-L.; Chen, H.-Q.; Cai, C.-H.; Li, W.; Dong, W.-H.; Chen, Z.-B.; Dai, H.-F. Phenolic Compounds from the Sclerotia of Inonotus obliquus. Nat. Prod. Res. 2022, 36, 2413–2417. [Google Scholar] [CrossRef]
- Chung, M.J.; Chung, C.K.; Jeong, Y.; Ham, S.S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 3, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Kou, R.-W.; Han, R.; Gao, Y.-Q.; Li, D.; Yin, X.; Gao, J.-M. Anti-Neuroinflammatory Polyoxygenated Lanostanoids from Chaga Mushroom Inonotus obliquus. Phytochemistry 2021, 184, 112647. [Google Scholar] [CrossRef]
- Gracheva, N.V.; Golovanchikov, A.B. Studies of the intensification of the extraction of biologically active substances from chaga using direct current electric fields. Pharm. Chem. J. 2011, 44, 608–610. [Google Scholar] [CrossRef]
- Nomura, M.; Tatsuo, T.; Uesugi, A.; Tanaka, R.; Kobayashi, S. Inotodiol, a Lanostane Triterpenoid, from Inonotus obliquus Inhibits Cell Proliferation through Caspase-3-Dependent Apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.-P.; Bouchart, V.; Heutte, N.; Eldin De Pécoulas, P.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef] [Green Version]
- Mu, H.; Zhang, A.; Zhang, W.; Cui, G.; Wang, S.; Duan, J. Antioxidative Properties of Crude Polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 2012, 13, 9194–9206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glamočlija, J.; Ćirić, A.; Nikolić, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; Van Griensven, L.J.L.D. Chemical Characterization and Biological Activity of Chaga (Inonotus obliquus), a Medicinal “Mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bao, C.; Zhang, J. Inotodiol Suppresses Proliferation of Breast Cancer in Rat Model of Type 2 Diabetes Mellitus via Downregulation of β-Catenin Signaling. Biomed. Pharmacother. 2018, 99, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Keyes, E.; Werth, V.P.; Brod, B. Potential Allergenicity of Commonly Sold High SPF Broad Spectrum Sunscreens in the United States; from the Perspective of Patients with Autoimmune Skin Disease. Int. J. Women’s Dermatol. 2019, 5, 227–232. [Google Scholar] [CrossRef]
- Kittiwannachot, P.; Borisut, P.; Wanasawas, P.; Ponpanich, L.; Rattanasuk, O.; Chulasiri, M. Antimutagenic Potentials of Hydroalcoholicherbal Extracts towards UV-Induced Mutation. Thai J. Toxic. 2010, 23, 27–34. [Google Scholar]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of Some Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047. [Google Scholar] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ko, M.-J.; Nam, H.-H.; Chung, M.-S. Subcritical Water Extraction of Bioactive Compounds from Orostachys Japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 10890. [Google Scholar] [CrossRef]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and Determination of Total Polyphenols and Antioxidant Capacity of Red Coffee (Coffea arabica L.) Pulp of Wet Processing Plants. Chem. Biol. Technol. Agric. 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Diffey, B.L.; Robson, J.A. New Substrate to Measure Sunscreen Protection Factors throughout the Ultraviolet Spectrum. J. Soc. Cosm. Chem. 1989, 40, 127–133. [Google Scholar]
- Matts, P.J.; Alard, V.; Brown, M.W.; Ferrero, L.; Gers-Barlag, H.; Issachar, N.; Moyal, D.; Wolber, R. The COLIPA in vitro UVA method: A standard and reproducible measure of sunscreen UVA protection. Int. J. Cosmet. Sci. 2010, 32, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, L.; Pissavini, M.; Marguerie, S.; Zastrow, L. Sunscreen in Vitro Spectroscopy: Application to UVA Protection Assessment and Correlation with in Vivo Persistent Pigment Darkening. Int. J. Cosmet. Sci. 2002, 24, 63–70. [Google Scholar] [CrossRef]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In Vitro Assessment of the Broad-Spectrum Ultraviolet Protection of Sunscreen Products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springsteen, A.; Yurek, R.; Frazier, M.; Carr, K.F. In Vitro Measurement of Sun Protection Factor of Sunscreens by Diffuse Transmittance. Anal. Chim. Acta 1999, 380, 155–164. [Google Scholar] [CrossRef]
- Khan, M.A. Sun protection factor determination studies of some sunscreen formulations use in cosmetics for their selection. J. Drug Deliv. Ther. 2018, 8, 149–151. [Google Scholar] [CrossRef]
- Fathia, A.M.; Randah, O.M. Sunscreen Cream Formulation with Natural Ingredients, Including Arabic Gum and Beeswax Foundation. Sirte Univ. Sci. J. 2019, 9, 1–11. [Google Scholar]
- Jitka, V.; Tinková, E.; Biedermann, D.; Pavel, K.; Jitka, U.; Alena Rajnochová, S. Skin Protective Activity of Silymarin And its Flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, H.; Zhang, W.; Li, Y.; Fan, Z.; Jiang, H.; Luo, J. Identification of LncRNA-MRNA Regulatory Module to Explore the Pathogenesis and Prognosis of Melanoma. Front. Cell Dev. Biol. 2020, 8, 615671. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives. Molecules 2019, 24, 3546. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, Oleanolic and Betulinic Acids: Antibacterial Spectra and Selectivity Indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Li, J.; Goto, M.; Yang, X.; Morris-Natschke, S.L.; Huang, L.; Chen, C.-H.; Lee, K.-H. Fluorinated Betulinic Acid Derivatives and Evaluation of Their Anti-HIV Activity. Bioorganic Med. Chem. Lett. 2016, 26, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Yong, T.; Chen, S.; Liang, D.; Zuo, D.; Diao, X.; Deng, C.; Wu, Y.; Hu, H.; Xie, Y.; Chen, D. Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling. Int. J. Mol. Sci. 2018, 19, 3222. [Google Scholar] [CrossRef] [Green Version]
- Strohm, D.; Ellinger, S.; Leschik-Bonnet, E.; Maretzke, F.; Heseker, H. Revised Reference Values for Potassium Intake. Ann. Nutr. Metab. 2017, 71, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M. Potassium and Health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic Composition and Amino Acid Contents of Mushrooms Cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms: Chemical Composition of Edible Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M. Nutritional and Medicinal Use of Cactus Pear (Opuntia spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
- Zambonin, L.; Caliceti, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S.; Landi, L.; Prata, C. Dietary Phenolic Acids Act as Effective Antioxidants in Membrane Models and in Cultured Cells, Exhibiting Proapoptotic Effects in Leukaemia Cells. Oxidative Med. Cell. Longev. 2012, 2012, 839298. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, Nutritionals and Antioxidant Properties of Two Prickly Pear Cactus Cultivars (Opuntia Ficus Indica Mill.) Growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Adesegun, S.A.; Fajana, A.; Orabueze, C.I.; Coker, H.A.B. Evaluation of Antioxidant Properties of Phaulopsis fascisepala CBCl. (Acanthaceae). Evid.-Based Complement. Altern. Med. 2009, 6, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Rafat Husain, S.; Cillard, J.; Cillard, P. Hydroxyl Radical Scavenging Activity of Flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Dcrozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and Free Radical Scavenging Mechanisms of Inhibitory Action of Rutin and Quercetin in Lipid Peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Torel, J.; Cillard, J.; Cillard, P. Antioxidant Activity of Flavonoids and Reactivity with Peroxy Radical. Phytochemistry 1986, 25, 383–385. [Google Scholar] [CrossRef]
- Kyyamova, G.I.; Khabibrakhmanova, V.R.; Sysoeva, M.A. Hydrophobic Constituents Extracted from Chaga by Ethylacetate. Pharm. Chem. J. 2018, 51, 1085–1087. [Google Scholar] [CrossRef]
- Kyoung Ju, H.; Chung, H.W.; Hong, S.-S.; Park, J.H.; Lee, J.; Kwon, S.W. Effect of Steam Treatment on Soluble Phenolic Content and Antioxidant Activity of the Chaga Mushroom (Inonotus obliquus). Food Chem. 2010, 119, 619–625. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus obliquus (Chaga Mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Rawat, S.; Bhatt, I.D.; Rawal, R.S. Total Phenolic Compounds and Antioxidant Potential of Hedychium Spicatum Buch. Ham. Ex D. Don in West Himalaya, India. J. Food Compos. Anal. 2011, 24, 574–579. [Google Scholar] [CrossRef]
- Dutra, E.A.; Oliveira, D.A.G.D.C.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of Sun Protection Factor (SPF) of Sunscreens by Ultraviolet Spectrophotometry. Rev. Bras. Cienc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus Monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Ludriksone, L.; Elsner, P. Adverse Reactions to Sunscreens. Curr. Probl. Dermatol. 2021, 55, 223–235. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
- Imam, S.; Azhar, I.; Mahmood, Z.A. In-vitro evaluation of sun protection factor of a cream formulation prepared from extracts of Musa accuminata (L.), Psidium gujava (L.) and Pyrus communis (L.). Asian J. Pharm. Clin. Res. 2015, 8, 234–237. [Google Scholar]
- Department of Health and Human Services. Food and Drug Administration, Sunscreen Drug Products for Over-the-Counter Human Use. Fed. Regist. 2019, 84, 6204–6275. [Google Scholar]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective Characteristics of Natural Antioxidant Polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Mazzei, J.L.; Evangelista, H.; Marques, M.R.C.; Ferraz, E.R.A.; Felzenszwalb, I. Protection against UV-Induced Oxidative Stress and DNA Damage by Amazon Moss Extracts. J. Photochem. Photobiol. B Biol. 2018, 183, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.D.; Costa, R.Y.S.; Guedes, A.A.S.; Silva, L.C.R.C.E.; Chinalia, F.A. Guava-Fruit Extract Can Improve the UV-Protection Efficiency of Synthetic Filters in Sun Cream Formulations. J. Photochem. Photobiol. B Biol. 2019, 201, 111639. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Rodrigues, A.L.M.; De Queiróz, D.B.; Vieira, I.G.P.; Neto, J.F.C.; Junior, J.T.C.; Tintino, S.R.; De Morais, S.M.; Coutinho, H.D.M. Photoprotective Potential of Medicinal Plants from Cerrado Biome (Brazil) in Relation to Phenolic Content and Antioxidant Activity. J. Photochem. Photobiol. B Biol. 2018, 189, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In Vitro Antioxidant Activity and Solar Protection Factor of Blackberry and Raspberry Extracts in Topical Formulation. J. Cosmet. Dermatol. 2019, 18, 539–544. [Google Scholar] [CrossRef]
- Fardiyah, Q.; Ersam, T.; Suyanta; Slamet, A.; Suprapto; Kurniawan, F. New Potential and Characterization of Andrographis paniculata L. Ness Plant Extracts as Photoprotective Agent. Arab. J. Chem. 2020, 13, 8888–8897. [Google Scholar] [CrossRef]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective Effects of Spirulina Platensis Extract against UVB Irradiated Human Skin Fibroblasts. S. Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; Baby, A.R.; Grombone Guaratini, M.T.; Moreno, P.R.H. In Vitro Antioxidant and Photoprotective Activity of Five Native Brazilian Bamboo Species. Ind. Crop. Prod. 2019, 130, 208–215. [Google Scholar] [CrossRef]
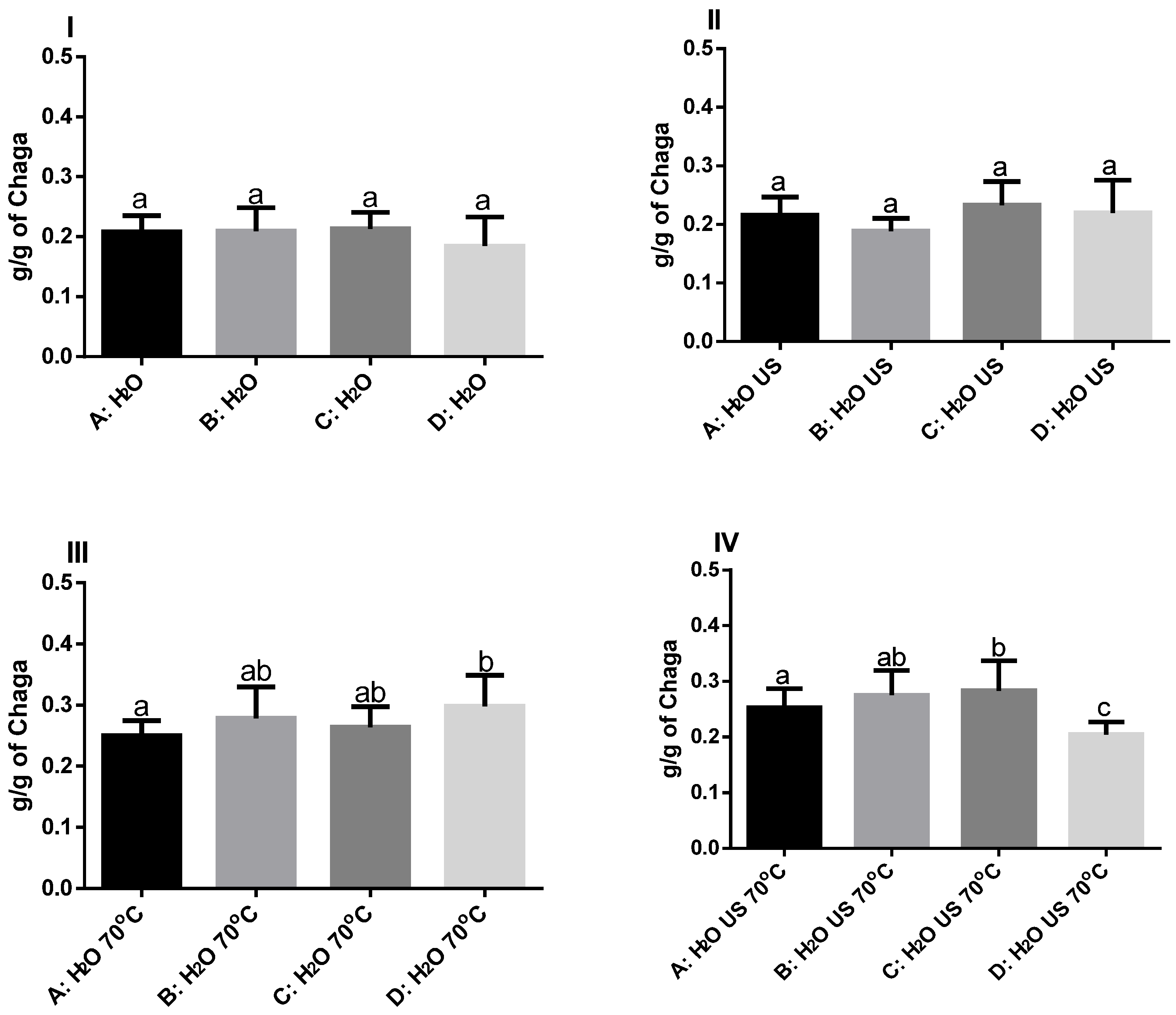


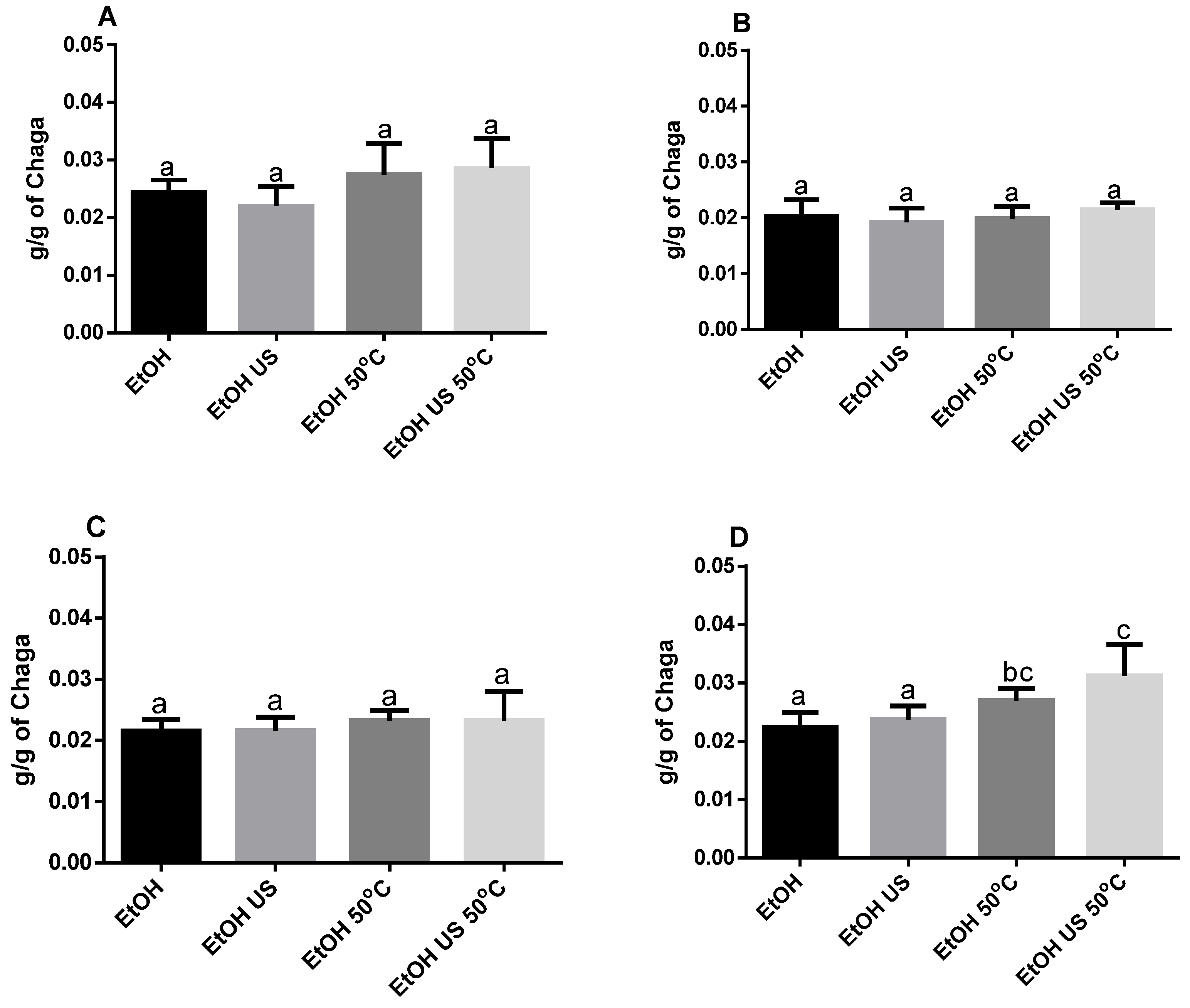






| Saison/Extraction Conditions | Betulinic Acid (ng/mL) | Betulin (ng/mL) |
|---|---|---|
| A/H2O | 40.6 | nd a |
| A/H2O US | 160.2 | nd |
| A/H2O 70 °C | 150.1 | nd |
| A/H2O US 70 °C | 133.9 | nd |
| B/H2O | 13.6 | nd |
| B/H2O US | 405.7 | nd |
| B/H2O 70 °C | 246.2 | nd |
| B/H2O US 70 °C | 229.4 | nd |
| C/H2O | 360.8 | nd |
| C/H2O US | 351.3 | nd |
| C/H2O 70 °C | 346.8 | 0.6 |
| C/H2O US 70 °C | 369.7 | nd |
| D/H2O | 11.7 | nd |
| D/H2O US | 334.5 | nd |
| D/H2O 70 °C | 384.5 | nd |
| D/H2O US 70 °C | 389.0 | nd |
| Saison/Extraction Conditions | Betulinic Acid (ng/mL) | Betulin (ng/mL) |
|---|---|---|
| A/EtOH | 22.62 | 13.45 |
| A/EtOH US | 28.13 | 7.41 |
| A/EtOH 50 °C | 22.10 | 4.75 |
| A/EtOH US 50 °C | 7.57 | 4.02 |
| B/EtOH | 12.94 | 3.97 |
| B/EtOH US | 4.833 | 70.87 |
| B/EtOH 50 °C | 15.85 | 26.19 |
| B/EtOH US 50 °C | 7.66 | 9.16 |
| C/EtOH | 21.93 | 53.93 |
| C/EtOH US | 16.29 | 53.93 |
| C/EtOH 50 °C | 8.21 | 10.61 |
| C/EtOH US 50 °C | 16.88 | 87.47 |
| D/EtOH | 29.2 | 90.49 |
| D/EtOH US | 23.94 | 68.45 |
| D/EtOH 50 °C | 25.42 | 51.73 |
| D/EtOH US 50 °C | 13.21 | 27.90 |
| Season | %C | %N | %S | C/N a |
|---|---|---|---|---|
| A | 43.44 | 0.43 | 0.075 | 118 |
| B | 42.97 | 0.40 | 0.045 | 125 |
| C | 42.38 | 0.39 | 0.040 | 127 |
| D | 44.87 | 0.40 | Bd b | 131 |
| Season/Extraction Conditions | %C | %N | %S | C/N a |
|---|---|---|---|---|
| A/H2O | 51.12 | 0.36 | 0.070 | 166 |
| A/H2O US | 43.80 | 0.35 | 0.055 | 146 |
| A/H2O 70 °C | 44.30 | 0.35 | 0.040 | 148 |
| A/H2O US 70 °C | 42.27 | 0.42 | 0.040 | 117 |
| B/H2O | 41.79 | 0.37 | 0.12 | 132 |
| B/H2O US | 40.50 | 0.35 | 0.065 | 135 |
| B/H2O 70 °C | 45.69 | 0.35 | 0.045 | 152 |
| B/H2O US 70 °C | 41.33 | 0.34 | 0.025 | 142 |
| C/H2O | 22.86 | 0.22 | 0.010 | 121 |
| C/H2O US | 42.09 | 0.35 | 0.020 | 140 |
| C/H2O 70 °C | 46.80 | 0.37 | 0.040 | 148 |
| C/H2O US 70 °C | 43.90 | 0.38 | 0.030 | 135 |
| D/H2O | 41.14 | 0.28 | 0.070 | 171 |
| D/H2O US | 44.71 | 0.36 | 0.19 | 145 |
| D/H2O 70 °C | 46.42 | 0.36 | 0.045 | 150 |
| D/H2O US 70 °C | 55.23 | 0.40 | 0.055 | 161 |
| Season/Extraction Conditions | %C | %N | %S | C/N a |
|---|---|---|---|---|
| A/EtOH | 62.79 | 0.51 | bd b | 144 |
| A/EtOH US | 43.67 | 0.38 | 0.02 | 134 |
| A/EtOH 50 °C | 45.72 | 0.41 | bd | 130 |
| A/EtOH US 50 °C | 45.08 | 0.34 | 0.07 | 155 |
| B/EtOH | 64.38 | 0.36 | bd | 209 |
| B/EtOH US | 64.79 | 0.28 | bd | 270 |
| B/EtOH 50 °C | 44.39 | 0.41 | 0.01 | 126 |
| B/EtOH US 50 °C | 61.31 | 0.32 | bd | 224 |
| C/EtOH | 60.71 | 0.31 | 0.03 | 228 |
| C/EtOH US | 62.08 | 0.34 | bd | 213 |
| C/EtOH 50 °C | 62.19 | 0.31 | bd | 234 |
| C/EtOH US 50 °C | 65.87 | 0.29 | bd | 265 |
| D/EtOH | 63.40 | 0.28 | bd | 264 |
| D/EtOH US | 62.38 | 0.26 | 0.03 | 280 |
| D/EtOH 50 °C | 63.63 | 0.26 | bd | 286 |
| D/EtOH US 50 °C | 61.75 | 0.23 | bd | 313 |
| Season/Extraction Conditions | mg QcEq/g Chaga |
|---|---|
| A/H2O | 187.44 ± 4.14 |
| A/H2O US | 219.16 ± 3.19 |
| A/H2O 70 °C | 347.61 ± 2.44 |
| A/H2O US 70 °C | 320.45 ± 8.47 |
| B/H2O | 256.57 ± 8.31 |
| B/H2O US | 246.89 ± 8.93 |
| B/H2O 70 °C | 373.02 ± 9.61 |
| B/H2O US 70 °C | 340.55 ± 9.58 |
| C/H2O | 302.26 ± 4.79 |
| C/H2O US | 412.49 ± 9.86 |
| C/H2O 70 °C | 402.47 ± 8.30 |
| C/H2O US 70 °C | 603.68 ± 7.75 |
| D/H2O | 368.07 ± 5.66 |
| D/H2O US | 380.87 ± 1.66 |
| D/H2O 70 °C | 453.27 ± 1.29 |
| D/H2O US 70 °C | 520.70 ± 3.98 |
| Season/Extraction Conditions | mg QcEq/g Chaga |
|---|---|
| A/EtOH | 26.18 ± 1.37 |
| A/EtOH US | 14.28 ± 0.93 |
| A/EtOH 50 °C | 11.86 ± 0.51 |
| A/EtOH US 50 °C | 17.27 ± 2.43 |
| B/EtOH | 6.23 ± 1.20 |
| B/EtOH US | 7.62 ± 0.64 |
| B/EtOH 50 °C | 9.64 ± 1.04 |
| B/EtOH US 50 °C | 9.01 ± 0.85 |
| C/EtOH | 13.96 ± 1.56 |
| C/EtOH US | 15.32 ± 4.42 |
| C/EtOH 50 °C | 17.89 ± 1.70 |
| C/EtOH US 50 °C | 23.98 ± 5.26 |
| D/EtOH | 26.85 ± 1.82 |
| D/EtOH US | 22.85 ± 2.50 |
| D/EtOH 50 °C | 30.25 ± 0.51 |
| D/EtOH US 50 °C | 37.41 ± 0.96 |
| Season/Extraction Conditions | mg GAEq/g Chaga |
|---|---|
| A/H2O | 523.50 ± 7.25 |
| A/H2O US | 545.60 ± 2.06 |
| A/H2O 70 °C | 789.22 ± 5.55 |
| A/H2O US 70 °C | 923.63 ± 32.6 |
| B/H2O | 603.03 ± 3.12 |
| B/H2O US | 532.22 ± 4.72 |
| B/H2O 70 °C | 974.10 ± 2.11 |
| B/H2O US 70 °C | 919.03 ± 4.00 |
| C/H2O | 582.27 ± 2.24 |
| C/H2O US | 872.87 ± 4.38 |
| C/H2O 70 °C | 951.61 ± 6.91 |
| C/H2O US 70 °C | 1181.6 ± 7.16 |
| D/H2O | 578.76 ± 2.15 |
| D/H2O US | 822.26 ± 3.44 |
| D/H2O 70 °C | 1050.9 ± 4.57 |
| D/H2O US 70 °C | 1056.3 ± 3.63 |
| Season/Extraction Conditions | Mg GAEq/g Chaga |
|---|---|
| A/EtOH | 52.40 ± 0.91 |
| A/EtOH US | 32.65 ± 0.18 |
| A/EtOH 50 °C | 57.46 ± 7.19 |
| A/EtOH US 50 °C | 136.56 ± 0.54 |
| B/EtOH | 34.80 ± 2.40 |
| B/EtOH US | 57.08 ± 1.38 |
| B/EtOH 50 °C | 25.45 ± 0.74 |
| B/EtOH US 50 °C | 34.13 ± 0.98 |
| C/EtOH | 38.93 ± 1.25 |
| C/EtOH US | 38.55 ± 2.23 |
| C/EtOH 50 °C | 52.54 ± 0.58 |
| C/EtOH US 50 °C | 76.72 ± 1.35 |
| D/EtOH | 55.93 ± 1.10 |
| D/EtOH US | 50.80 ± 1.30 |
| D/EtOH 70 °C | 62.02 ± 1.52 |
| D/EtOH US 70 °C | 66.95 ± 2.18 |
| Season/Extraction Conditions | SPF(290–320) | SPF(290–400) | PF-UVA(320–400) |
|---|---|---|---|
| A/H2O | 34.02 ± 1.03 | 23.22 ± 5.23 | 16.70 ± 3.75 |
| A/H2O US | 32.49 ± 0.06 | 17.03 ± 2.84 | 14.97 ± 0.19 |
| A/H2O 70 °C | 33.48 ± 0.57 | 21.13 ± 2.35 | 15.47 ± 0.99 |
| A/H2O US 70 °C | 32.33 ± 0.55 | 16.44 ± 1.83 | 13.95 ± 1.13 |
| B/H2O | 33.47 ± 0.74 | 20.62 ± 3.09 | 17.68 ± 2.54 |
| B/H2O US | 32.95 ± 0.56 | 18.89 ± 2.40 | 16.26 ± 2.62 |
| B/H2O 70 °C | 34.72 ± 0.51 | 27.08 ± 2.81 | 20.63 ± 2.34 |
| B/H2O US 70 °C | 34.97 ± 0.20 | 28.34 ± 0.42 | 22.52 ± 0.29 |
| C/H2O | 35.88 ± 0.32 | 31.97 ± 1.78 | 21.82 ± 1.19 |
| C/H2O US | 34.59 ± 0.54 | 27.93 ± 3.36 | 23.84 ± 3.02 |
| C/H2O 70 °C | 32.97 ± 0.44 | 18.33 ± 1.40 | 15.02 ± 1.05 |
| C/H2O US 70 °C | 34.88 ± 0.42 | 26.00 ± 1.51 | 18.14 ± 0.66 |
| D/H2O | 34.61 ± 0.95 | 26.32 ± 5.03 | 20.14 ± 3.97 |
| D/H2O US | 34.64 ± 0.0.86 | 25.21 ± 3.50 | 18.78 ± 1.48 |
| D/H2O 70 °C | 34.46 ± 1.02 | 24.26 ± 4.35 | 18.06 ± 2.53 |
| D/H2O US 70 °C | 35.33 ± 0.62 | 28.67 ± 2.69 | 19.37 ± 1.31 |
| Season/Extraction Conditions | SPF(290–320) | SPF(290–400) | PF-UVA(320–400) |
|---|---|---|---|
| A/EtOH | 35.21 ± 0.39 | 29.95 ± 3.13 | 22.8 ± 2.71 |
| A/EtOH US | 31.31 ± 0.63 | 13.42 ± 1.73 | 12.50 ± 1.48 |
| A/EtOH 50 °C | 31.27 ± 0.51 | 13.05 ± 1.47 | 11.85 ± 1.30 |
| A/EtOH US 50 °C | 33.79 ± 0.32 | 22.73 ± 1.09 | 19.80 ± 2.99 |
| B/EtOH | 32.21 ± 0.23 | 15.98 ± 0.41 | 14.40 ± 0.49 |
| B/EtOH US | 31.54 ± 0.26 | 13.37 ± 0.61 | 11.4 ± 0.58 |
| B/EtOH 50 °C | 33.50 ± 0.32 | 19.83 ± 1.39 | 15.14 ± 1.10 |
| B/EtOH US 50 °C | 31.74 ± 0.28 | 14.66 ± 0.87 | 14.66 ± 0.56 |
| C/EtOH | 33.08 ± 0.49 | 18.55 ± 1.35 | 15.2 ± 0.40 |
| C/EtOH US | 31.81 ± 0.14 | 14.71 ± 0.37 | 13.4 ± 0.20 |
| C/EtOH 50 °C | 32.67 ± 0.15 | 17.11 ± 0.62 | 14.0 ± 1.11 |
| C/EtOH US 50 °C | 32.64 ± 0.28 | 17.74 ± 0.93 | 15.8 ± 0.50 |
| D/EtOH | 33.91 ± 0.26 | 21.97 ± 0.68 | 17.03 ± 0.35 |
| D/EtOH US | 33.47 ± 0.35 | 21.10 ± 1.06 | 18.27 ± 0.20 |
| D/EtOH 50 °C | 34.66 ± 0.83 | 25.5 ± 3.48 | 18.90 ± 1.04 |
| D/EtOH US 50 °C | 32.27 ± 2.19 | 21.31 ± 1.96 | 19.46 ± 2.17 |
| Source | Potassium (mg/g) a |
|---|---|
| Banana, raw | 3.58 |
| Banana dehydrated or banana powder | 14.91 |
| Potato, skin, baked | 5.73 |
| chaga extracts (H2O, all conditions, four seasons) | 20–35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wontcheu Fotso, Y.A.; Ghazi, S.; Belkaid, A.; Soucy, J.; Tremblay, L.; Lamarre, S.; Clarisse, O.; Touaibia, M. Extraction, Chemical Composition, Antiradical Capacity, and Photoprotective Effect of Inonotus obliquus from Eastern Canada. Nutraceuticals 2023, 3, 380-402. https://doi.org/10.3390/nutraceuticals3030029
Wontcheu Fotso YA, Ghazi S, Belkaid A, Soucy J, Tremblay L, Lamarre S, Clarisse O, Touaibia M. Extraction, Chemical Composition, Antiradical Capacity, and Photoprotective Effect of Inonotus obliquus from Eastern Canada. Nutraceuticals. 2023; 3(3):380-402. https://doi.org/10.3390/nutraceuticals3030029
Chicago/Turabian StyleWontcheu Fotso, Yolande A., Sara Ghazi, Anissa Belkaid, Jason Soucy, Luc Tremblay, Simon Lamarre, Olivier Clarisse, and Mohamed Touaibia. 2023. "Extraction, Chemical Composition, Antiradical Capacity, and Photoprotective Effect of Inonotus obliquus from Eastern Canada" Nutraceuticals 3, no. 3: 380-402. https://doi.org/10.3390/nutraceuticals3030029





