Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems
Abstract
:1. Introduction
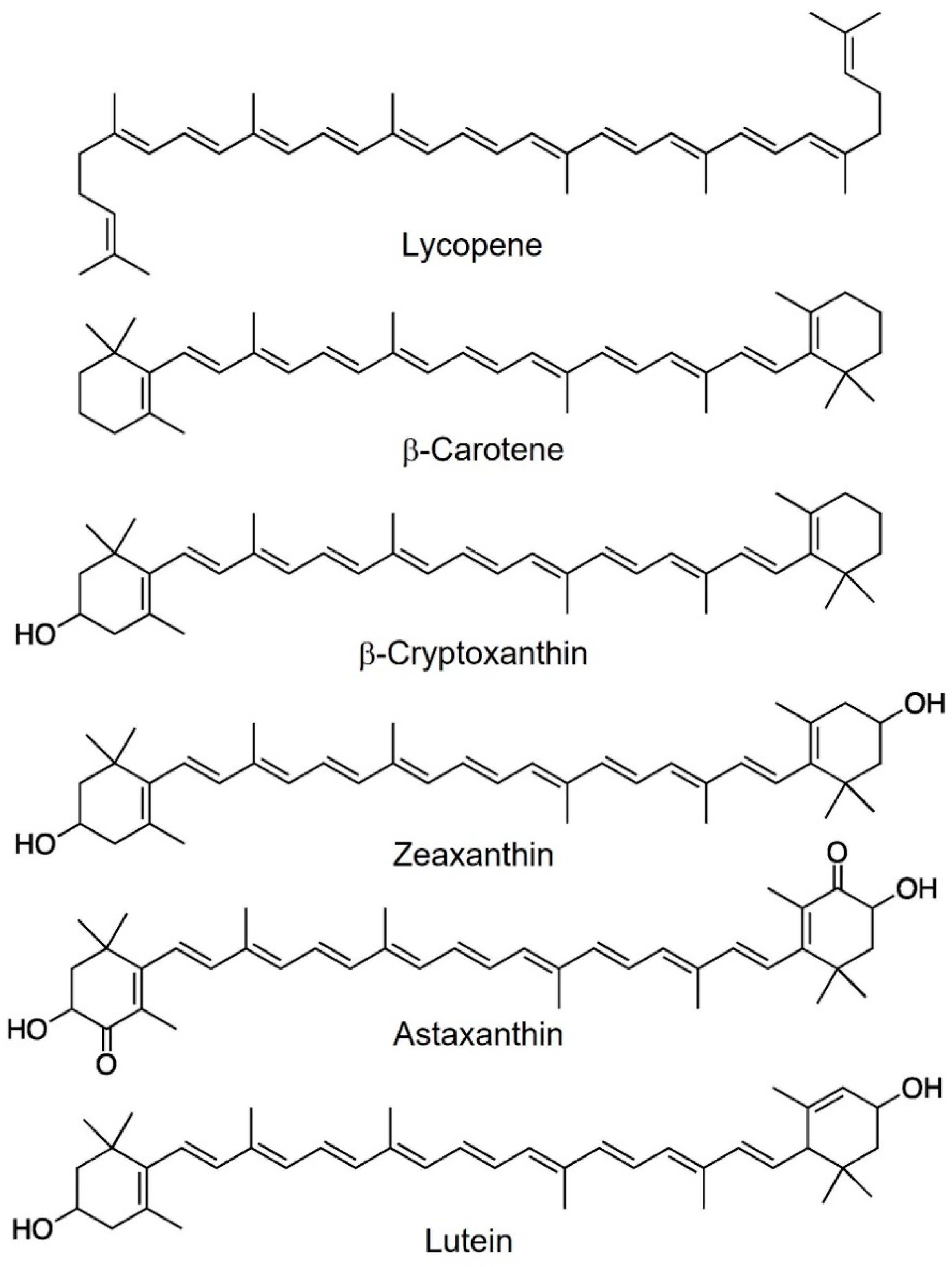
2. Carotenoids
2.1. Physicochemical and Biological Properties
2.2. Dietary Sources and Bioaccessibility/Bioavailability
3. Delivery Systems for Carotenoids
4. Chitosan-Based Delivery Systems for Carotenoids
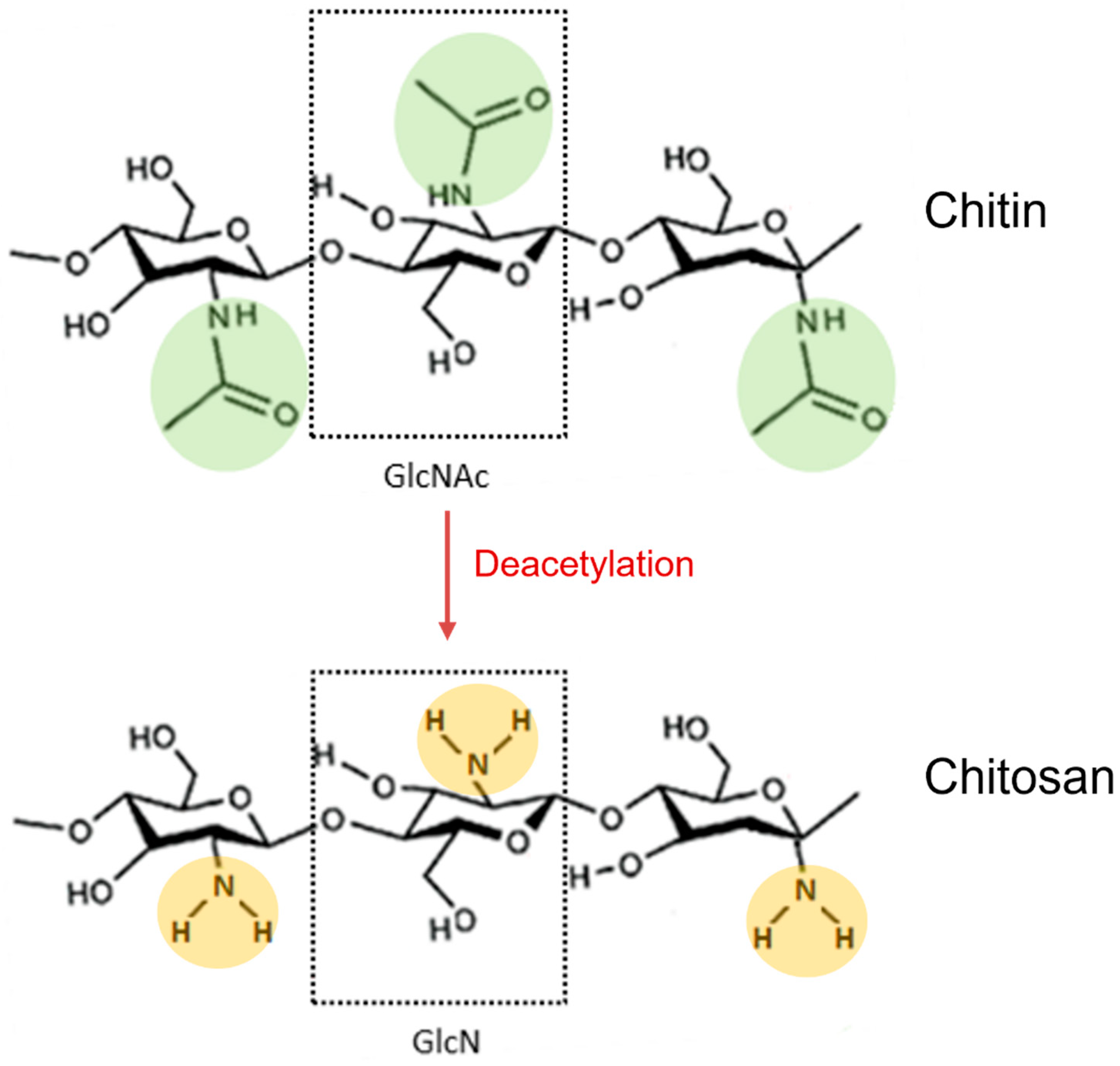
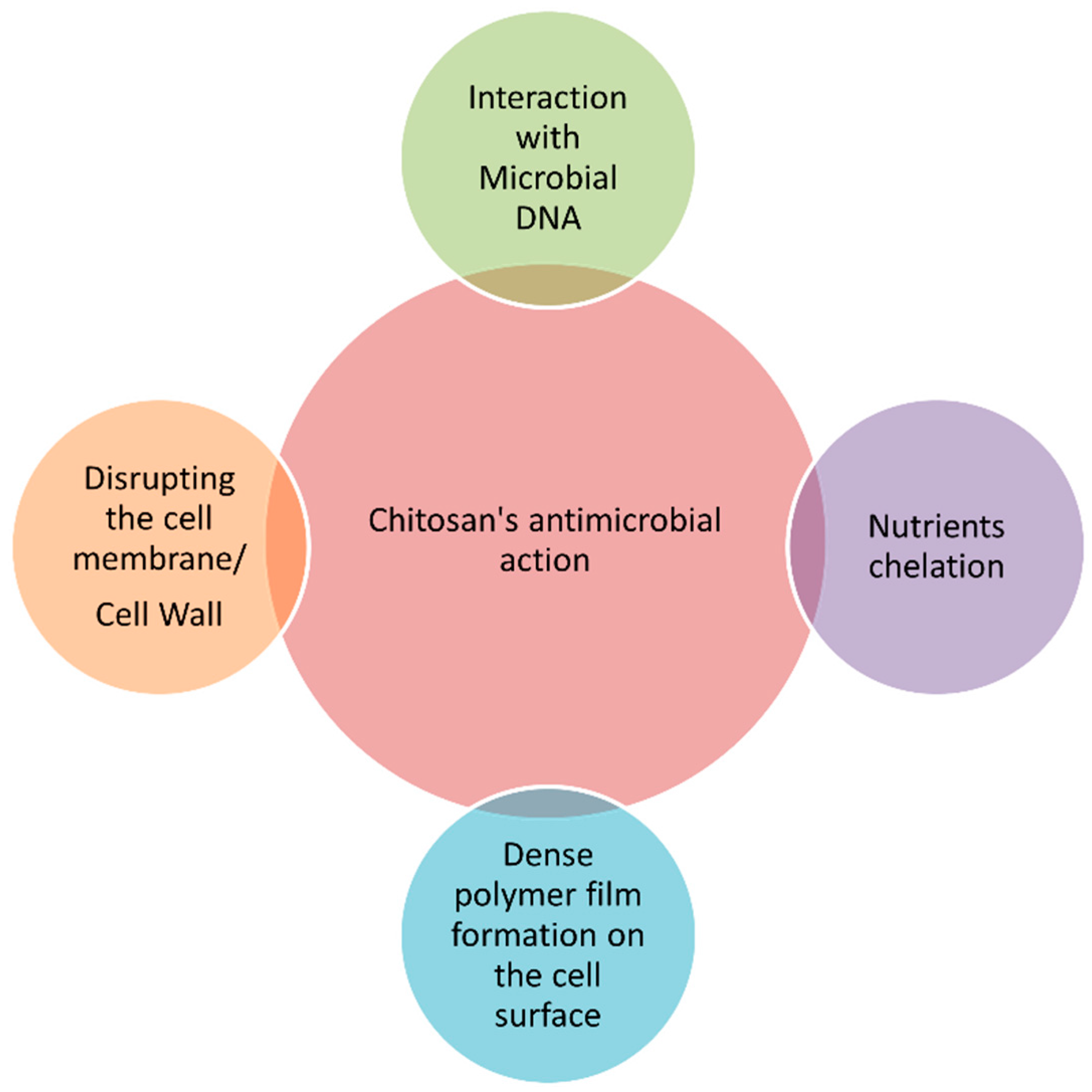
4.1. Chemical Characteristics and Functional Properties of Chitosan
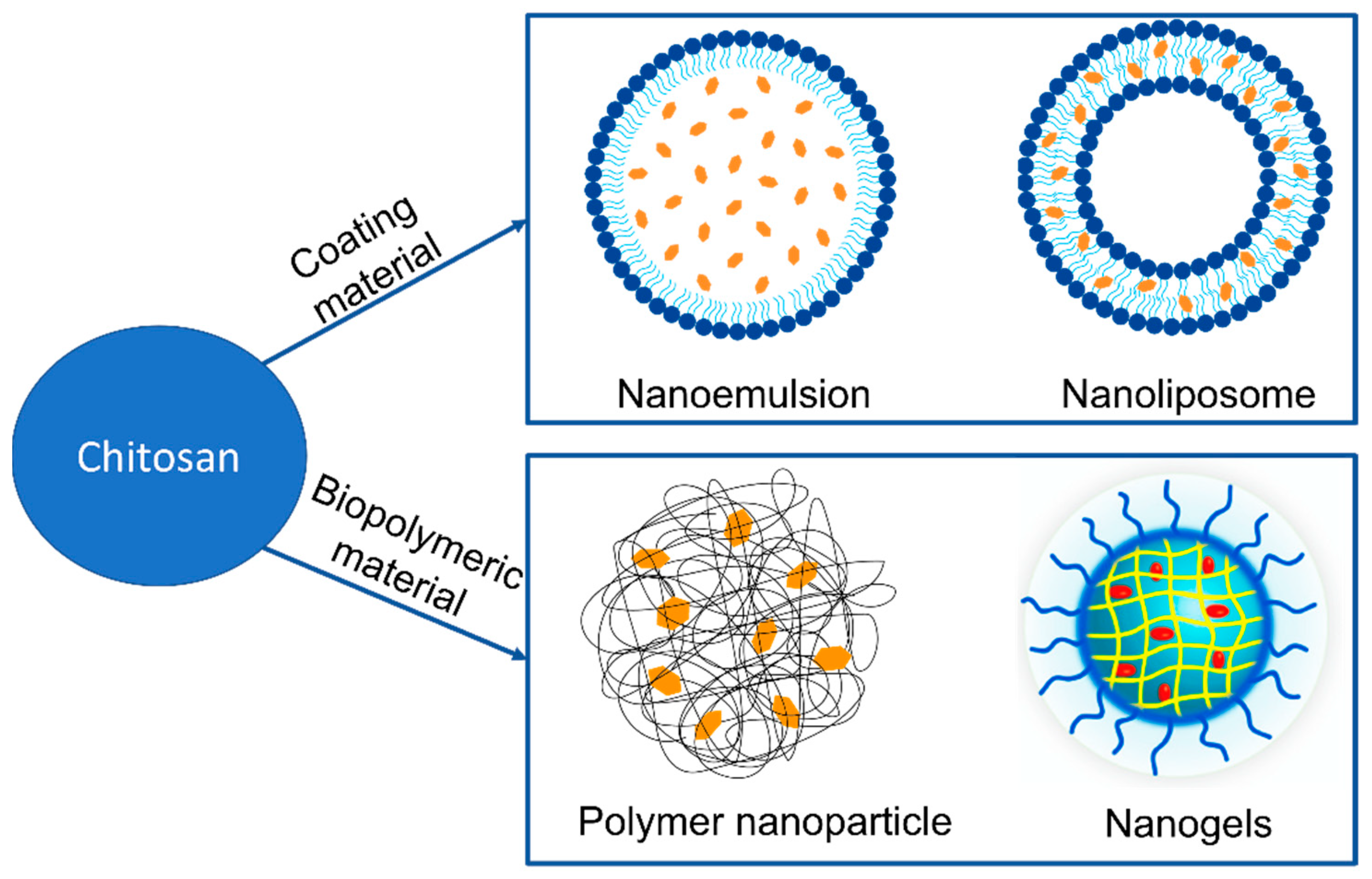
4.2. Chitosan Nanocarriers for Effective Delivery of Carotenoids (as Advanced Delivery Systems)
4.2.1. Chitosan-Coated Nanoemulsions
4.2.2. Chitosan-Coated Nanoliposomes (Chitosomes)
4.2.3. Chitosan-Based Nanocarriers
4.2.4. Chitosan-Based Nanogels
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grainger, E.M.; Webb, M.Z.; Simpson, C.M.; Chitchumroonchokchai, C.; Riedl, K.; Moran, N.E.; Clinton, S.K. Assessment of Dietary Carotenoid Intake and Biologic Measurement of Exposure in Humans. Methods Enzymol. 2022, 674, 255–295. [Google Scholar] [PubMed]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- EFSA. NDA Panel Scientific Opinion on Dietary Reference Values for Vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; ud din Wani, H.M.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mercadante, A.Z. The Bioaccessibility of Carotenoids Impacts the Design of Functional Foods. Curr. Opin. Food Sci. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Roll Zimmer, T.B.; Barboza Mendonça, C.R.; Zambiazi, R.C. Methods of Protection and Application of Carotenoids in Foods—A Bibliographic Review. Food Biosci. 2022, 48, 101829. [Google Scholar] [CrossRef]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan Microspheres as a Potential Carrier for Drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. Encinitas 2016, 15, 33–36. [Google Scholar]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B2 and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B2 and β-Carotene. Foods 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in Nutraceutical Delivery Systems: From Formulation Design for Bioavailability Enhancement to Efficacy and Safety Evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio Molitor as a Source of Interesting Natural Compounds, Their Recovery Processes, Biological Effects, and Safety Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Shukla, R.; Vasdev, N.; Ruwali, M.; Hasnain, M.S.; Beg, S. Chitosan for Delivery of Biomolecules. In Chitosan Drug Delivery; Academic Press: Cambridge, MA, USA, 2022; pp. 433–460. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Eini, M.; Rastegari, A.; Tehrani, M.R. Chitosan as a Machine for Biomolecule Delivery: A Review. Carbohydr. Polym. 2021, 256, 117414. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of Thermal and UV-Light Stability of β-Carotene-Loaded Nanoemulsions by Water-Soluble Chitosan Coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the Thermal Degradation of Astaxanthin through Nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Cofrades, S.; Antoniou, I.; Solas, M.T.; Herrero, A.M.; Jiménez-Colmenero, F. Preparation and Impact of Multiple (Water-in-Oil-in-Water) Emulsions in Meat Systems. Food Chem. 2013, 141, 338–346. [Google Scholar] [CrossRef]
- Damiri, F.; Rojekar, S.; Bachra, Y.; Varma, R.S.; Andra, S.; Balu, S.; Pardeshi, C.V.; Patel, J.; Patel, H.M.; Paiva-Santos, A.C.; et al. Polysaccharide-based nanogels for biomedical applications: A comprehensive review. J. Drug. Deliv. Sci. Technol. 2023, 84, 104447. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Dara, P.K.; Perumcherry Raman, S.; Vijayan, D.K.; Sadasivam, J.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Nanoencapsulation in low-molecular-weight chitosan improves in vivo antioxidant potential of black carrot anthocyanin. J. Sci. Food Agric. 2021, 101, 5264–5271. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–Vis/H2O2) Degradation of Carotenoids: Kinetics and Molecular End Products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef]
- Young, A.; Lowe, G. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein Acts via Multiple Antioxidant Pathways in the Photo-Stressed Retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Mcdonald, K.; Caldarella, S.M.; Chung, H.; Troen, A.M.; Snodderly, D.M. Cognitive Findings of an Exploratory Trial of Docosahexaenoic Acid and Lutein Supplementation in Older Women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Alabdulmunem, M. Antioxidant Effect of Lycopene on Retinal Pigment Epithelial Cell Line. Med. Res. Arch. 2022, 10, 3441. [Google Scholar] [CrossRef]
- Zhang, P.-C.; Wu, C.-R.; Wang, Z.-L.; Wang, L.-Y.; Han, Y.; Sun, S.-L.; Li, Q.-S.; Ma, L. Effect of Lutein Supplementation on Visual Function in Nonproliferative Diabetic Retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef]
- García-Layana, A.; Recalde, S.; Hernandez, M.; Abraldes, M.J.; Nascimento, J.; Hernández-Galilea, E.; Olmedilla-Alonso, B.; Escobar-Barranco, J.J.; Zapata, M.A.; Silva, R.; et al. A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration. Nutrients 2021, 13, 1253. [Google Scholar] [CrossRef]
- Ali, S.S.; Ayuob, N.N.; Al Ansary, A.K.; Soluman, E.R. Antioxidants Protect against Increased Risk of Atherosclerosis Induced by Exposure to Cigarette Smoke: Histological and Biochemical Study. Saudi J. Biol. Sci. 2012, 19, 291–301. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Shi, Z.; Cong, D.; Bai, Y.-S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Wang, X.-D. Mechanistic Understanding of β-Cryptoxanthin and Lycopene in Cancer Prevention in Animal Models. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158652. [Google Scholar] [CrossRef]
- Liao, K.-S.; Wei, C.-L.; Chen, J.-C.; Zheng, H.-Y.; Chen, W.-C.; Wu, C.-H.; Wang, T.-J.; Peng, Y.-S.; Chang, P.-Y.; Lin, Y.-W. Astaxanthin Enhances Pemetrexed-Induced Cytotoxicity by Downregulation of Thymidylate Synthase Expression in Human Lung Cancer Cells. Regul. Toxicol. Pharmacol. 2016, 81, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tan, H.-L.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W.; Moran, N.E.; Clinton, S.K. Dietary Tomato and Lycopene Impact Androgen Signaling- and Carcinogenesis-Related Gene Expression during Early TRAMP Prostate Carcinogenesis. Cancer Prev. Res. 2014, 7, 1228–1239. [Google Scholar] [CrossRef]
- Alhoshani, N.M.; Al-Johani, N.S.; Alkeraishan, N.; Alarifi, S.; Alkahtani, S. Effect of lycopene as an adjuvant therapy with 5-florouracil in human colon cancer. Saudi J. Biol. Sci. 2022, 29, 103392. [Google Scholar] [CrossRef]
- Róvero Costa, M.; Leite Garcia, J.; Cristina Vágula de Almeida Silva, C.; Junio Togneri Ferron, A.; Valentini Francisqueti-Ferron, F.; Kurokawa Hasimoto, F.; Schmitt Gregolin, C.; Henrique Salomé de Campos, D.; Roberto de Andrade, C.; dos Anjos Ferreira, A.L.; et al. Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants 2019, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Raghavendra-Rao Sowmya, P.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Fractionation and Characterization of Lycopene-Oxidation Products by LC-MS/MS (ESI)+: Elucidation of the Chemopreventative Potency of Oxidized Lycopene in Breast-Cancer Cell Lines. J. Agric. Food Chem. 2018, 66, 11362–11371. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Boakye, N.A.; Fears, T.R.; Schleicher, R.L.; Helsel, W.; Anderson, C.; Robinson, J.; Guin, J.D.; Lessin, S.; Ratnasinghe, L.D.; et al. Serum Carotenoids and α-Tocopherol and Risk of Nonmelanoma Skin Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1276–1282. [Google Scholar] [CrossRef]
- Žmitek, K.; Žmitek, J.; Rogl Butina, M.; Hristov, H.; Pogačnik, T.; Pravst, I. Dietary Lutein Supplementation Protects against Ultraviolet-Radiation-Induced Erythema: Results of a Randomized Double-Blind Placebo-Controlled Study. J. Funct. Foods 2020, 75, 104265. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Chu, R.-X.; Liu, H. Vitamin A Intake and Risk of Melanoma: A Meta-Analysis. PLoS ONE 2014, 9, e102527. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kao, C.-J.; Huang, H.-Y.; Huang, S.-Y.; Chen, C.-Y.; Lin, Y.-S.; Wen, Z.-H.; Wang, H.-M.D. Astaxanthin Reduces MMP Expressions, Suppresses Cancer Cell Migrations, and Triggers Apoptotic Caspases of in Vitro and in Vivo Models in Melanoma. J. Funct. Foods 2017, 31, 20–31. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Poston, R.; Ferro, A. The Lipid and Non-Lipid Effects of Statins. Pharmacol. Ther. 2003, 99, 95–112. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of Natural Astaxanthin Increases Serum HDL-Cholesterol and Adiponectin in Subjects with Mild Hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Bechor, S.; Zolberg Relevy, N.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-Cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients 2016, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Meléndez-Martínez, A.J. The Colourless Carotenoids Phytoene and Phytofluene: Sources, Consumption, Bioavailability and Health Effects. Curr. Opin. Food Sci. 2021, 41, 201–209. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The Colorful World of Carotenoids: A Profound Insight on Therapeutics and Recent Trends in Nano Delivery Systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 3658–3697. [Google Scholar] [CrossRef]
- Writing Group for the AREDS2 Research Group; Bonds, D.E.; Harrington, M.; Worrall, B.B.; Bertoni, A.G.; Eaton, C.B.; Hsia, J.; Robinson, J.; Clemons, T.E.; Fine, L.J.; et al. Effect of Long-Chain ω-3 Fatty Acids and Lutein + Zeaxanthin Supplements on Cardiovascular Outcomes: Results of the Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA Intern. Med. 2014, 174, 763–771. [Google Scholar] [CrossRef]
- Han, G.-M.; Liu, P. Higher Serum Lycopene Is Associated with Reduced Prevalence of Hypertension in Overweight or Obese Adults. Eur. J. Integr. Med. 2017, 13, 34–40. [Google Scholar] [CrossRef]
- Jonasson, L.; Wikby, A.; Olsson, A.G. Low Serum Beta-Carotene Reflects Immune Activation in Patients with Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 120–125. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The Secretion from Neural Stem Cells Pretreated with Lycopene Protects against Tert-Butyl Hydroperoxide-Induced Neuron Oxidative Damage. Oxid. Med. Cell Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep Symptoms Associated with Intake of Specific Dietary Nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef]
- Moia, V.M.; Leal Portilho, F.; Almeida Pádua, T.; Barbosa Corrêa, L.; Ricci-Junior, E.; Cruz Rosas, E.; Magalhaes Rebelo Alencar, L.; Savio Mendes Sinfronio, F.; Sampson, A.; Hussain Iram, S.; et al. Lycopene Used as Anti-Inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal Assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef]
- Hammond, B.R.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and Bone: An in Vitro Investigation and a Pilot Prospective Clinical Study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of Brewer’s Spent Grain (BSG) on Larval Growth of Mealworms, Tenebrio Molitor (Coleoptera: Tenebrionidae). Int. J. Ind. Entomol. 2016, 32, 41–48. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High Serum Carotenoids Associated with Lower Risk for Bone Loss and Osteoporosis in Post-Menopausal Japanese Female Subjects: Prospective Cohort Study. PLoS ONE 2012, 7, e52643. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Kozai, Y.; Saito, T.; Takara, T. Effects of Paprika Carotenoid Supplementation on Bone Turnover in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Study. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Giordano, E.; Quadro, L. Lutein, Zeaxanthin and Mammalian Development: Metabolism, Functions and Implications for Health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Polidori, M.C.; Stahl, W.; Griffiths, H.R. Nutritional Cognitive Neuroscience of Aging: Focus on Carotenoids and Cognitive Frailty. Redox Biol. 2021, 44, 101996. [Google Scholar] [CrossRef]
- Arscott, S.A. Food Sources of Carotenoids. In Carotenoids and Human Health; Tanumihardjo, S., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 3–19. [Google Scholar]
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary Fat Composition, Food Matrix and Relative Polarity Modulate the Micellarization and Intestinal Uptake of Carotenoids from Vegetables and Fruits. J. Food Sci. Technol. 2017, 54, 333–341. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Jing, K.; He, S.; Chen, T.; Lu, Y.; Ng, I.-S. Enhancing Beta-Carotene Biosynthesis and Gene Transcriptional Regulation in Blakeslea Trispora with Sodium Acetate. Biochem. Eng. J. 2016, 114, 10–17. [Google Scholar] [CrossRef]
- Luna-Flores, C.H.; Wang, A.; von Hellens, J.; Speight, R.E. Towards Commercial Levels of Astaxanthin Production in Phaffia Rhodozyma. J. Biotechnol. 2022, 350, 42–54. [Google Scholar] [CrossRef]
- Casella, P.; Marino, T.; Iovine, A.; Larocca, V.; Balducchi, R.; Musmarra, D.; Molino, A. Optimization of Lutein Extraction from Scenedesmus Almeriensis Using Pressurized Liquid Extraction. Chem. Eng. Trans. 2021, 87, 475–480. [Google Scholar]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhu, M.J. Recent Developments in Astaxanthin Production from Phaffia Rhodozyma and Its Applications. In Global Perspectives on Astaxanthin; Academic Press: Cambridge, MA, USA, 2021; pp. 225–251. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces Dendrorhous for the Industrial Production of Astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Casella, P.; Sangiorgio, P.; Verardi, A.; Ferraro, A.; Hristoforou, E.; Molino, A.; Musmarra, D. Natural Beta-Carotene: A Microalgae Derivate for Nutraceutical Applications. Chem. Eng. Trans. 2020, 79, 103–108. [Google Scholar] [CrossRef]
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent Progress in Practical Applications of a Potential Carotenoid Astaxanthin in Aquaculture Industry: A Review. Fish. Physiol. Biochem. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of a Feed Additive Consisting of Astaxanthin-rich Phaffia Rhodozyma for Salmon and Trout (Igene Biotechnology, Inc.). EFSA J. 2022, 20, 7161. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Remonatto, D.; Picheli, F.P.; Paula, A.V.; Herculano, R.D.; Santos-Ebinuma, V.C.; Farias, R.L.; Onishi, B.S.D.; Ribeiro, S.J.L.; Pereira, J.F.B.; et al. A Look into Phaffia Rhodozyma Biorefinery: From the Recovery and Fractionation of Carotenoids, Lipids and Proteins to the Sustainable Manufacturing of Biologically Active Bioplastics. Bioresour. Technol. 2022, 362, 127785. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. 65 Food Chemistry, Function and Analysis No. 5 Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, P., Eds.; Royal Society of Chemistry: London, UK, 2018; ISBN 9781788011068. [Google Scholar]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing bioaccessibility and bioavailability of carotenoids using emulsion-based delivery systems. Colloids Surf. B 2022, 209, 112211. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of Carotenoids from Plant and Animal Foods. J. Sci. Food Agric. 2019, 99, 3220–3239. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Carle, R. Carotenoid Deposition in Plant And Animal Foods and Its Impact on Bioavailability. Crit. Rev. Food Sci. Nutr. 2015, 13, 1807–1830. [Google Scholar] [CrossRef]
- Koh, S.; Loh, S. In Vitro Bioaccessibility of Â-Carotene in Pumpkin and Butternut Squash Subjected to Different Cooking Methods. Int. Food Res. J. 2018, 25, 188–195. [Google Scholar]
- Colle, I.J.P.; Van Buggenhout, S.; Lemmens, L.; Van Loey, A.M.; Hendrickx, M.E. The Type and Quantity of Lipids Present during Digestion Influence the in Vitro Bioaccessibility of Lycopene from Raw Tomato Pulp. Food Res. Int. 2012, 45, 250–255. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zhou, H.; Xiao, H.; McClements, D.J. Factors Impacting Lipid Digestion and β-Carotene Bioaccessibility Assessed by Standardized Gastrointestinal Model (INFOGEST): Oil Droplet Concentration. Food Funct. 2020, 11, 7126–7137. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and Bioaccessibility of Food Bioactive Compounds; Overview and Assessment by In Vitro Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a Functional Food Ingredient: Stability and Bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Ornelas-Paz, J.d.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V.; Yahia, E.M.; Gardea-Béjar, A.A. Effects of Pectin on Lipid Digestion and Possible Implications for Carotenoid Bioavailability during Pre-Absorptive Stages: A Review. Food Res. Int. 2017, 99, 917–927. [Google Scholar] [CrossRef]
- Corte-Real, J.; Bohn, T. Interaction of Divalent Minerals with Liposoluble Nutrients and Phytochemicals during Digestion and Influences on Their Bioavailability—A Review. Food Chem. 2018, 252, 285–293. [Google Scholar] [CrossRef]
- Corte-Real, J.; Bertucci, M.; Soukoulis, C.; Desmarchelier, C.; Borel, P.; Richling, E.; Hoffmann, L.; Bohn, T. Negative Effects of Divalent Mineral Cations on the Bioaccessibility of Carotenoids from Plant Food Matrices and Related Physical Properties of Gastro-Intestinal Fluids. Food Funct. 2017, 8, 1008–1019. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J. Antioxidant Components and Their in Vitro Bioaccessibility in Processed and Stored Chick Pea and Amaranth Greens Mix. Croat. J. Food Technol. Biotechnol. Nutr. 2015, 10, 44–49. [Google Scholar]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus Pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, Cellular Uptake, and Transport of Astaxanthin Isomers and Their Antioxidative Effects in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 10223–10232. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Troost, R.; Böger, R.H. Clinical Pharmacokinetics of Antioxidants and Their Impact on Systemic Oxidative Stress. Clin. Pharmacokinet. 2003, 42, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Alves, P.; Tan, Y.; Pan, J.; Zhong, F. Effect of Encapsulation on β-Carotene Absorption and Metabolism in Mice. Food Hydrocoll. 2021, 121, 107009. [Google Scholar] [CrossRef]
- Kamil, A.; Smith, D.E.; Blumberg, J.B.; Astete, C.; Sabliov, C.; Oliver Chen, C.-Y. Bioavailability and Biodistribution of Nanodelivered Lutein. Food Chem. 2016, 192, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Mercke Odeberg, J.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral Bioavailability of the Antioxidant Astaxanthin in Humans Is Enhanced by Incorporation of Lipid Based Formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Singh, G.K.S.; Ismail, M.A.; Zulkefli, N.A.A.; Mohd Affandi, M.M.R.M. Tissue Distribution of Astaxanthin Formulation in Rats. Curr. Nutr. Food Sci. 2018, 14, 329–334. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y. Construction of Lipid-Biomacromolecular Compounds for Loading and Delivery of Carotenoids: Preparation Methods, Structural Properties, and Absorption-Enhancing Mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Emőke Teleky, B.; Ranga, F.; Simon, E.; Lelia Pop, O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian Vodnar, D. Bioaccessibility of Microencapsulated Carotenoids, Recovered from Tomato Processing Industrial by-Products, Using In Vitro Digestion Model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of Carotenoids Extracted from Halophilic Archaea in Oil-in-Water (O/W) Micro- and Nano-Emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, F.; Li, Z.; McClements, D.J.; Xiao, H. Encapsulation of Carotenoids in Emulsion-Based Delivery Systems: Enhancement of β-Carotene Water-Dispersibility and Chemical Stability. Food Hydrocoll. 2017, 69, 49–55. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-Carotene-Loaded Oil Droplets in Caseinate/Alginate Microparticles: Enhancement of Carotenoid Stability and Bioaccessibility. J. Funct. Foods 2018, 40, 527–535. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-Loaded Nanocarriers: A Comprehensive Review. Adv. Colloid. Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Arunkumar, R.; Prashanth, K.V.H.; Baskaran, V. Promising Interaction between Nanoencapsulated Lutein with Low Molecular Weight Chitosan: Characterization and Bioavailability of Lutein In Vitro and In Vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef]
- Wang, D.; Mao, L.; Dai, L.; Yuan, F.; Gao, Y. Characterization of Chitosan-Ferulic Acid Conjugates and Their Application in the Design of β-Carotene Bilayer Emulsions with Propylene Glycol Alginate. Food Hydrocoll. 2018, 80, 281–291. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and Characterization of Carboxymethyl Chitosan and Tea Polyphenols Coating on Zein Nanoparticles to Encapsulate β-Carotene by Anti-Solvent Precipitation Method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles Based on Crocin Loaded Chitosan-Alginate Biopolymers: Antioxidant Activities, Bioavailability and Anticancer Properties. Int. J. Biol. Macromol. 2017, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of Crocin Stability by Biodegradeble Nanoparticles of Chitosan-Alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Lee, J.S.; Lee, H.G. Chitosan/Poly-γ-Glutamic Acid Nanoparticles Improve the Solubility of Lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef]
- Chayasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Crosslinked Chitosan-Dextran Sulfate Nanoparticle for Improved Topical Ocular Drug Delivery. Mol. Vis. 2015, 21, 1224–1234. [Google Scholar]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.S.M.; Mousa, S.A. Self-Assembly of Green Tea Catechin Derivatives in Nanoparticles for Oral Lycopene Delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Malgarim Cordenonsi, L.; Faccendini, A.; Catanzaro, M.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Platcheck Raffin, R.; Scherman Schapoval, E.E.; Lanni, C.; Sandri, G.; et al. The Role of Chitosan as Coating Material for Nanostructured Lipid Carriers for Skin Delivery of Fucoxanthin. Int. J. Pharm. 2019, 567, 118487. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Ghosh, M. In Vitro Study of Anti-Oxidative Effects of β-Carotene and α-Lipoic Acid for Nanocapsulated Lipids. LWT 2012, 49, 131–138. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, Physicochemical Stability and In Vitro Simulated Gastrointestinal Digestion Properties of β-Carotene Loaded Zein-Propylene Glycol Alginate Composite Nanoparticles Fabricated by Emulsification-Evaporation Method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-Carotene in Alginate-Based Hydrogel Beads: Impact on Physicochemical Stability and Bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical Stability and in Vitro Bioaccessibility of β-Carotene Nanoemulsions Stabilized with Whey Protein-Dextran Conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical Properties of β-Carotene and Eugenol Co-Encapsulated Flax Seed Oil Powders Using OSA Starches as Wall Material. Food Hydrocoll. 2017, 73, 274–283. [Google Scholar] [CrossRef]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and Bioaccessibility of β-Carotene in Nanoemulsions Stabilized by Modified Starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating Multilayer Emulsions by Using OSA Starch and Chitosan Suitable for Spray Drying: Application in the Encapsulation of β-Carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical Properties of Oil-in-Water Nanoemulsions Stabilized by OSA-Modified Starch for the Encapsulation of Lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- JIN, H.; XIA, F.; JIANG, C.; ZHAO, Y.; HE, L. Nanoencapsulation of Lutein with Hydroxypropylmethyl Cellulose Phthalate by Supercritical Antisolvent. Chin. J. Chem. Eng. 2009, 17, 672–677. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Sun, R.; Wang, X. Self-Assembly and β-Carotene Loading Capacity of Hydroxyethyl Cellulose-Graft-Linoleic Acid Nanomicelles. Carbohydr. Polym. 2016, 145, 56–63. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-Encapsulation of Saffron Extract through Double-Layered Multiple Emulsions of Pectin and Whey Protein Concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Beicht, J.; Zeeb, B.; Gibis, M.; Fischer, L.; Weiss, J. Influence of Layer Thickness and Composition of Cross-Linked Multilayered Oil-in-Water Emulsions on the Release Behavior of Lutein. Food Funct. 2013, 4, 1457–1467. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey Protein Capsules Obtained through Electrospraying for the Encapsulation of Bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Chu, B.S.; Ichikawa, S.; Kanafusa, S.; Nakajima, M. Preparation and Characterization of β-Carotene Nanodispersions Prepared by Solvent Displacement Technique. J. Agric. Food Chem. 2007, 55, 6754–6760. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.S.L.; Griffin, C.; Roos, Y.H. Stability and Loss Kinetics of Lutein and β-Carotene Encapsulated in Freeze-Dried Emulsions with Layered Interface and Trehalose as Glass Former. Food Res. Int. 2014, 62, 403–409. [Google Scholar] [CrossRef]
- Teo, A.; Lee, S.J.; Goh, K.K.T.; Wolber, F.M. Kinetic Stability and Cellular Uptake of Lutein in WPI-Stabilised Nanoemulsions and Emulsions Prepared by Emulsification and Solvent Evaporation Method. Food Chem. 2017, 221, 1269–1276. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-Carotene Nanoemulsion-Based Delivery Systems Using Dual-Channel Microfluidization: Physical and Chemical Stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of Lutein Encapsulated Whey Protein Nano-Emulsion during Storage. PLoS ONE 2018, 13, 0192511. [Google Scholar] [CrossRef] [PubMed]
- Cornacchia, L.; Roos, Y.H. Stability of β-Carotene in Protein-Stabilized Oil-in-Water Delivery Systems. J. Agric. Food Chem. 2011, 59, 7013–7020. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-Carotene and Zinc in Whey Protein Nanoparticles Using the PH Cycle Method: Evidence of Sustained Release Delivery in Intestinal and Gastric Fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-Carotene Encapsulated in Food Protein Nanoparticles Reduces Peroxyl Radical Oxidation in Caco-2 Cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Controlled Release of β-Carotene in β-Lactoglobulin-Dextran- Conjugated Nanoparticles” in Vitro Digestion and Transport with Caco-2 Monolayers. J. Agric. Food Chem. 2014, 62, 8900–8907. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene Loaded Whey Protein Isolate Nanoparticles: An Innovative Endeavor for Enhanced Bioavailability of Lycopene and Anti-Cancer Activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular Uptake of β-Carotene from Protein Stabilized Solid Lipid Nanoparticles Prepared by Homogenization-Evaporation Method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the Physicochemical Stability of β-Carotene Solid Lipid Nanoparticle (SLNP) Using Whey Protein Isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbet, S.; Marquez, S.A. Complexes of Lutein with Bovine and Caprine Caseins and Their Impact on Lutein Chemical Stability in Emulsion Systems: Effect of Arabinogalactan. J. Dairy Sci. 2018, 101, 18–27. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J.; González-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; González-Navarro, C.J. Thermal Protection of β-Carotene in Re-Assembled Casein Micelles during Different Processing Technologies Applied in Food Industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yao, P.; Jiang, M. Simultaneous Nanoparticle Formation and Encapsulation Driven by Hydrophobic Interaction of Casein-Graft-Dextran and β-Carotene. J. Colloid Interface Sci. 2007, 315, 456–463. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Nakagawa, K.; Adachi, S. Influence of Aggregate Structure of Casein on the Encapsulation Efficiency of β-Carotene Entrapped via Hydrophobic Interaction. Food Struct. 2015, 5, 42–50. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.A.; Sbihi, H.M.; Al-Resayes, S.I.; Malmiri, H.J.; Tan, C.P. Preparation of Astaxanthin Nanodispersions Using Gelatin-Based Stabilizer Systems. Molecules 2014, 19, 14257–14265. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy Glycinin as Food-Grade Pickering Stabilizers: Part. III. Fabrication of Gel-like Emulsions and Their Potential as Sustained-Release Delivery Systems for β-Carotene. Food Hydrocoll. 2016, 56, 434–444. [Google Scholar] [CrossRef]
- Deng, X.X.; Zhang, N.; Tang, C.H. Soy Protein Isolate as a Nanocarrier for Enhanced Water Dispersibility, Stability and Bioaccessibility of β-Carotene. J. Sci. Food Agric. 2016, 97, 2230–2237. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Chen, L. Elaboration and Characterization of Barley Protein Nanoparticles as an Oral Delivery System for Lipophilic Bioactive Compounds. Food Funct. 2014, 5, 92–101. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato Protein- Based Carriers for Enhancing Bioavailability of Astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, Y.; Mao, L.; Zhao, J. Optimisation of Conditions for the Preparation of β-Carotene Nanoemulsions Using Response Surface Methodology. Food Chem. 2008, 107, 1300–1306. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of Mixed Surfactants-Based β-Carotene Nanoemulsions Using Response Surface Methodology: An Ultrasonic Homogenization Approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Zhang, Y.; Zhao, L. Characterization of Catechin-α-Lactalbumin Conjugates and the Improvement in β-Carotene Retention in an Oil-in-Water Nanoemulsion. Food Chem. 2016, 205, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Effect of Degree of Octenyl Succinic Anhydride (OSA) Substitution on the Digestion of Emulsions and the Bioaccessibility of β-Carotene in OSA-Modified-Starch-Stabilized-Emulsions. Food Hydrocoll. 2018, 84, 303–312. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of Mandarin Fiber Addition on Physico-Chemical Properties of Nanoemulsions Containing β-Carotene under Simulated Gastrointestinal Digestion Conditions. LWT 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of Particle Size on Lipid Digestion and β-Carotene Bioaccessibility in Emulsions and Nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Wilson, T.A.; Nicolosi, R.J. Bioavailability of a Nanoemulsion of Lutein Is Greater than a Lutein Supplement. Nano Biomed. Eng. 2009, 1, 38–49. [Google Scholar] [CrossRef]
- Gumus, C.E.; Davidov-Pardo, G.; McClements, D.J. Lutein-Enriched Emulsion-Based Delivery Systems: Impact of Maillard Conjugation on Physicochemical Stability and Gastrointestinal Fate. Food Hydrocoll. 2016, 60, 38–49. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xu, D.; Cao, Y.; Wang, S.; Wang, B.; Sun, B.; Yuan, F.; Gao, Y. Enhancing Physicochemical Properties of Emulsions by Heteroaggregation of Oppositely Charged Lactoferrin Coated Lutein Droplets and Whey Protein Isolate Coated DHA Droplets. Food Chem. 2018, 239, 75–85. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-Enriched Emulsion-Based Delivery Systems: Influence of Emulsifiers and Antioxidants on Physical and Chemical Stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Decker, E.A.; McClements, D.J. Utilisation of Spontaneous Emulsification to Fabricate Lutein-Loaded Nanoemulsion-Based Delivery Systems: Factors Influencing Particle Size and Colour. Int. J. Food Sci. Technol. 2017, 52, 1408–1416. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; Pu, C.; Tang, W.; Sun, Q. Nanoencapsulation of Lutein within Lipid-Based Delivery Systems: Characterization and Comparison of Zein Peptide Stabilized Nano-Emulsion, Solid Lipid Nanoparticle, and Nano-Structured Lipid Carrier. Food Chem. 2021, 358, 129840. [Google Scholar] [CrossRef]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant Activity and Bioaccessibility of Size-Different Nanoemulsions for Lycopene-Enriched Tomato Extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef]
- Dhakane, J.P.; Kar, A.; Singh Patel, A.; Khan, I. Effect of Soy Proteins and Emulsification-Evaporation Process on Physical Stability of Lycopene Emulsions. Int. J. Chem. Stud. 2017, 5, 1354–1358. [Google Scholar]
- Kim, S.O.; Ha, T.V.A.; Choi, Y.J.; Ko, S. Optimization of Homogenization-Evaporation Process for Lycopene Nanoemulsion Production and Its Beverage Applications. J. Food Sci. 2014, 79, N1604–N1610. [Google Scholar] [CrossRef]
- Michelon, M.; Mantovani, R.A.; Sinigaglia-Coimbra, R.; de la Torre, L.G.; Cunha, R.L. Structural Characterization of β-Carotene-Incorporated Nanovesicles Produced with Non-Purified Phospholipids. Food Res. Int. 2016, 79, 95–105. [Google Scholar] [CrossRef]
- de Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid Encapsulation Structures Based on β-Carotene-Loaded Nanoliposomes within Electrospun Fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef]
- Toniazzo, T.; Berbel, I.F.; Cho, S.; Fávaro-Trindade, C.S.; Moraes, I.C.F.; Pinho, S.C. β-Carotene-Loaded Liposome Dispersions Stabilized with Xanthan and Guar Gums: Physico-Chemical Stability and Feasibility of Application in Yogurt. LWT 2014, 59, 1265–1273. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Liu, C.; Chang, Y.; Song, J.; Xiao, Y. Polypeptide-Decorated Nanoliposomes as Novel Delivery Systems for Lutein. RSC Adv. 2018, 8, 31372–31381. [Google Scholar] [CrossRef]
- Xia, F.; Hu, D.; Jin, H.; Zhao, Y.; Liang, J. Preparation of Lutein Proliposomes by Supercritical Anti-Solvent Technique. Food Hydrocoll. 2012, 26, 456–463. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Encapsulation of Lutein in Liposomes Using Supercritical Carbon Dioxide. Food Res. Int. 2017, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, N.; Ilic, S.; Jakovljevic, V.; Stojanovic, N.; Stojnev, S.; Kocic, H.; Stojanovic, M.; Kocic, G. The Encapsulation of Lycopene in Nanoliposomes Enhances Its Protective Potential in Methotrexate-Induced Kidney Injury Model. Oxid. Med. Cell Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Balachandran, B.; Rao, A.V. Time-Dependent Uptake and Antiperoxidative Potential of Lycopene in Multilamellar Liposomes. Food Res. Int. 2003, 36, 611–616. [Google Scholar] [CrossRef]
- Palozza, P.; Muzzalupo, R.; Trombino, S.; Valdannini, A.; Picci, N. Solubilization and Stabilization of β-Carotene in Niosomes: Delivery to Cultured Cells. Chem. Phys. Lipids 2006, 139, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel Encapsulation of Lycopene in Niosomes and Assessment of Its Anticancer Activity. J. Bioequivalence Bioavailab. 2016, 8, 5. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Balasubramaniam, A. Anti-Diabetic Activity of Lycopene Niosomes: Experimental Observation. J. Pharm. Drug Dev. 2017, 4. [Google Scholar] [CrossRef]
- Nik, A.M.; Langmaid, S.; Wright, A.J. Digestibility and β-Carotene Release from Lipid Nanodispersions Depend on Dispersed Phase Crystallinity and Interfacial Properties. Food Funct. 2012, 3, 234–245. [Google Scholar] [CrossRef]
- Triplett, M.D.; Rathman, J.F. Optimization of β-Carotene Loaded Solid Lipid Nanoparticles Preparation Using a High Shear Homogenization Technique. J. Nanoparticle Res. 2009, 11, 601–614. [Google Scholar] [CrossRef]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and Physicochemical Characterization of Lycopene-Loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Thakur, K.; Ghoshal, G.; Singh, B.; Jain, S.; Shivhare, U.S.; Katare, O.P. Fabrication and Functional Attributes of Lipidic Nanoconstructs of Lycopene: An Innovative Endeavour for Enhanced Cytotoxicity in MCF-7 Breast Cancer Cells. Colloids Surf. B Biointerfaces 2017, 152, 482–491. [Google Scholar] [CrossRef]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and Characterization of Nanostructured Lipid Carriers and Solid Lipid Nanoparticles Containing Lycopene for Food Fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and Characterization of Astaxanthin-Loaded Nanostructured Lipid Carriers. Innov. Food Sci. Emerg. Technol. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Pezeshki, A.; Hamishehkar, H.; Ghanbarzadeh, B.; Fathollahy, I.; Keivani Nahr, F.; Khakbaz Heshmati, M.; Mohammadi, M. Nanostructured Lipid Carriers as a Favorable Delivery System for β-Carotene. Food Biosci. 2019, 27, 11–17. [Google Scholar] [CrossRef]
- Hejri, A.; Khosravi, A.; Gharanjig, K.; Hejazi, M. Optimisation of the Formulation of β-Carotene Loaded Nanostructured Lipid Carriers Prepared by Solvent Diffusion Method. Food Chem. 2013, 141, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, A.; Gramdorf, S.; Müller, R.H.; Kurz, T. β-Carotene-Loaded Nanostructured Lipid Carriers. J. Food Sci. 2008, 73, N1–N6. [Google Scholar] [CrossRef]
- Lacatusu, I.; Mitrea, E.; Badea, N.; Stan, R.; Oprea, O.; Meghea, A. Lipid Nanoparticles Based on Omega-3 Fatty Acids as Effective Carriers for Lutein Delivery. Preparation and In Vitro Characterization Studies. J. Funct. Foods 2013, 5, 1260–1269. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid Nanocarriers for Dermal Delivery of Lutein: Preparation, Characterization, Stability and Performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, H.C.; Wu, W.C.; Sahoo, S.L.; Hsu, C.Y. Novel Lutein Loaded Lipid Nanoparticles on Porcine Corneal Distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based Nanoformulation of Lycopene Improves Oral Delivery: Formulation Optimization, Ex Vivo Assessment and Its Efficacy against Breast Cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical Characterization of Lycopene-Loaded Nanostructured Lipid Carrier Formulations for Topical Administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef]
- Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325–344. [Google Scholar] [CrossRef]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Valorisation of Agri-Food Waste and Mealworms Rearing Residues for Improving the Sustainability of Tenebrio molitor Industrial Production. J. Insects Food Feed. 2022, 8, 509–524. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Moliterni, S.; Errico, S.; Spagnoletta, A.; Dimatteo, S. Advanced Technologies for Chitin Recovery from Crustacean Waste. Clean. Technol. Recycl. 2023, 3, 4–43. [Google Scholar] [CrossRef]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Nguyen, H.T.-T.; Tran, T.N.; Ha, A.C.; Huynh, P.D. Impact of Deacetylation Degree on Properties of Chitosan for Formation of Electrosprayed Nanoparticles. J. Nanotechnol. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Li, N.; Xiong, X.; Ha, X.; Wei, X. Comparative Preservation Effect of Water-Soluble and Insoluble Chitosan from Tenebrio Molitor Waste. Int. J. Biol. Macromol. 2019, 133, 165–171. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–123. [Google Scholar]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- And Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Maleki, G.; Milani, J.M. Functional Properties of Chitin and Chitosan-Based Polymer Materials. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–198. [Google Scholar]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Adnan, S.; Ranjha, N.M.; Hanif, M.; Asghar, S. O-Carboxymethylated Chitosan; A Promising Tool with in-Vivo Anti-Inflammatory and Analgesic Properties in Albino Rats. Int. J. Biol. Macromol. 2020, 156, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 457–489. ISBN 9789811502637. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Wong, T.W. Chitosan as Anticancer Compound and Nanoparticulate Matrix for Cancer Therapeutics. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; pp. 1737–1752. [Google Scholar]
- van Huis, A.; Oonincx, D.G.A.B. The Environmental Sustainability of Insects as Food and Feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, J.; Zhang, Z.; Chen, W.; Hu, Q.; Wang, Y. Convenient One-Step Approach Based on Stimuli-Responsive Sol-Gel Transition Properties to Directly Build Chitosan-Alginate Core-Shell Beads. Food Hydrocoll. 2019, 87, 253–259. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of Chitosan Extracted from Mealworm Beetle (Tenebrio Molitor, Zophobas Morio) and Rhinoceros Beetle (Allomyrina Dichotoma) and Their Antibacterial Activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of Carotenoids within Lipid-Based Nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. Chitosomes: Encapsulating Food Bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in Applications of Nanotechnology in Global Food Industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-Coated Liposomes by Electrostatic Adsorption of Chitosan (Chitosomes) as Novel Delivery Systems for Carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Da Rosa, C.G.; Da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Ravi, H.; Baskaran, V. Biodegradable Chitosan-Glycolipid Hybrid Nanogels: A Novel Approach to Encapsulate Fucoxanthin for Improved Stability and Bioavailability. Food Hydrocoll. 2015, 43, 717–725. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome Technology for the Food and Nutraceutical Industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Hamadou, A.H.; Huang, W.-C.; Xue, C.; Mao, X. Comparison of β-Carotene Loaded Marine and Egg Phospholipids Nanoliposomes. J. Food Eng. 2020, 283, 110055. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An Overview of Liposomal Nano-Encapsulation Techniques and Its Applications in Food and Nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the Carotenoid Bioaccessibility through Liposomal Encapsulation. Colloids Surf. B Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef]
- Zamani Ghaleshahi, A.; Rajabzadeh, G. The Influence of Sodium Alginate and Genipin on Physico-Chemical Properties and Stability of WPI Coated Liposomes. Food Res. Int. 2020, 130, 108966. [Google Scholar] [CrossRef]
- dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.d.O. Nanoencapsulation of Carotenoids: A Focus on Different Delivery Systems and Evaluation Parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Nowak, E.; Livney, Y.D.; Niu, Z.; Singh, H. Delivery of Bioactives in Food for Optimal Efficacy: What Inspirations and Insights Can Be Gained from Pharmaceutics? Trends Food Sci. Technol. 2019, 91, 557–573. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide Based Nanogels in the Drug Delivery System: Application as the Carrier of Pharmaceutical Agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ma, G.H.; Su, Z.G. Preparation of Uniform Sized Chitosan Microspheres by Membrane Emulsification Technique and Application as a Carrier of Protein Drug. J. Control. Release 2005, 106, 62–75. [Google Scholar] [CrossRef]
- Ravi, H.; Arunkumar, R.; Baskaran, V. Chitosan-Glycolipid Nanogels Loaded with Anti-Obese Marine Carotenoid Fucoxanthin: Acute and Sub-Acute Toxicity Evaluation in Rodent Model. J. Biomater. Appl. 2015, 30, 420–434. [Google Scholar] [CrossRef]
| Health Effects | Specific Effects | Carotenoids | References |
|---|---|---|---|
| Prevention and mitigation of age-related macular degeneration (AMD) and cataracts | Lutein and Zeaxanthin | [32,33] | |
| Delayed onset and mitigation of diabetic retinopathy | Lutein, Zeaxanthin, Lycopene | [34,35,36] | |
| Antioxidant activities | Diabetes and osteoporosis | Lycopene | [37] |
| Mitigation of multiple sclerosis and atherosclerosis | β-Carotene | [26] | |
| Prevention of age-related disease | Astaxanthin | [38] | |
| Protective effect on the skin | β-Carotene, Lutein | [39,40] | |
| Inhibition of retinal impairment | Lutein and Zeaxanthin | [41] | |
| Cancers | Inhibition of lung cancer | Lutein, Astaxanthin and b-Cryptoxanthin | [42,43,44] |
| Inhibition of risk for prostate cancer | Lycopene | [39,43,45] | |
| Mitigation of risk for colon-rectal cancer | Lycopene | [43,46] | |
| Mitigation of non-alcoholic fatty liver disease (associated with hepatocellular carcinoma) | Lycopene | [47] | |
| Mitigation of risk for breast cancer | Lutein and Zeaxanthin Lycopene | [46,48,49] | |
| Mitigation of risk for non-melanoma cancer | Lutein, β-Carotene, α-Carotene, Lycopene, Zeaxanthin, Astaxanthin, Lutein, Cryptoxanthin, Lycopene, Fucoxanthin | [50,51] | |
| Mitigation of risk for melanoma | β-Carotene | [52,53] | |
| Cardiovascular diseases | Lycopene | [44,46,54] | |
| Astaxanthin and b-Carotene | [55,56] | ||
| Effects on HDL and LDL cholesterol levels | Phytoene and Phytofluene | [57,58] | |
| Reduction in the severity of cardiovascular disease | Lutein and Zeaxanthin | [59] | |
| Antihypertensive and anti-aggregative Effect | Lycopene | [60] | |
| Reduction in immune activation (In patients with cardiovascular disease) | β-Carotene | [61] | |
| Neurological disorders | Regulation of lipid raft formation in neuronal cells (proper membrane fluidity) | Lutein and Zeaxanthin | [62] |
| Protection for neurodegenerative diseases and For neurological, cognitive, and psycho-behavioral disease conditions | Lycopene | [63,64] | |
| Bacterial infections | Inhibition of bacteria cells growth | β-Carotene, b-Cryptoxanthin, Lutein, Violaxanthin, Antheraxanthin, Fucoxanthin, Zeaxanthin | [65] |
| Others | Improvement of sleep quality and duration | Lycopene | [66] |
| Anti-inflammatory properties | Lycopene | [67] | |
| Cognitive performances | β-Carotene and Lutein | [33,68,69] | |
| Bone homeostasis | β-Cryptoxanthin | [70] |
| Carotenoids | Sources | Concentration | References |
|---|---|---|---|
| β-carotene | Sweet potato | 91.8 µg/g FW | [79] |
| Carrot | 88.4 µg/g FW | [79] | |
| Apricots | 66.4 µg/g FW | [79] | |
| Spinach | 55.9 µg/g FW | [79] | |
| Beet greens | 34.4 µg/g FW | [79] | |
| Tomato | 3.4 µg/g FW | [79] | |
| Cassava root, Sweet yellow | 7.27 µg/g FW | [79] | |
| Squash | 3.7 µg/g FW | [79] | |
| Zea mais | 0.17 µg/g DW | [79] | |
| Dunaliella salina (microalgae) | 34,100 µg/g DW | [81] | |
| Blakeslea trispora (fungi) | 59,910 µg/g DW | [82] | |
| Phaffia rhodozyma (yeast) | 42.81 µg/g DW | [83] | |
| Lutein | Spinach | 119.4 µg/g FW | [79] |
| Broccoli | 34.4 µg/g FW | [79] | |
| Tagetes flowers | 2930 µg/g DW | [59] | |
| Scenedesmus almeriensis (microalgae) | 3040 µg/g DW | [84] | |
| Chlorella sp. | 7000 µg/g DW | [85] | |
| Lycopene | Tomato | 30.2 µg/g FW | [79] |
| Grapefruit | 17.51 µg/g FW | [79] | |
| Watermelon | 23.83 µg/g FW | [79] | |
| Astaxanthin | Haematococcus pluvialis (microalgae) | 20,000 µg/g DW | [81] |
| Phaffia rhodozyma (yeast) | 400 µg/g DW | [86] | |
| Xanthophyllomyces dendrorhous (yeast) | 5000 µg/g DW | [87] | |
| Shrimps, prawns, crabs (waste residues) | 57.5 µg/g DW | [88] |
| Nanocarriers | Encapsulation Technique | Encapsulated Carotenoids | Ref. | ||
|---|---|---|---|---|---|
| Biopolymeric NC | Polysaccharide-based NC | Chitosan | Nanocapsules | Lutein | [124] |
| Nanoemulsions | β-carotene | [125] | |||
| Nanoparticles | β-carotene | [126] | |||
| Crocin | [127,128] | ||||
| Lutein | [129,130] | ||||
| Lycopene | [131] | ||||
| Nanostructured lipid carriers | Fucoxanthin | [132] | |||
| Alginates | Nanocapsules | β-carotene | [133] | ||
| Nanoparticles | β-carotene | [134] | |||
| Crocin | [127,128] | ||||
| Nanohydrogel | β-carotene | [135] | |||
| Starches | Nanoemulsions | β-carotene | [136,137,138,139] | ||
| Lycopene | [140] | ||||
| Cellulose | Nanocapsules | Lutein | [141] | ||
| Nanomicelles | β-carotene | [142] | |||
| Pectins | Nanoemulsions | Crocin | [143] | ||
| Lutein | [144] | ||||
| Protein-based NC | Whey proteins | Nanocapsules | β-carotene | [145] | |
| Nanodispersions | β-carotene | [146] | |||
| Nanoemulsions | β-carotene, lutein | [136,144,147,148,149,150,151] | |||
| Crocin | [143] | ||||
| Nanoparticles | β-carotene | [152,153,154] | |||
| Lycopene | [155] | ||||
| Solid lipid nanoparticles | β-carotene | [156,157] | |||
| Caseins | Nanoemulsions | β-carotene | [151] | ||
| Lutein | [158] | ||||
| Nanomicelles | β-carotene | [159] | |||
| Nanoparticles | β-carotene | [160,161] | |||
| Gelatin | Nanodispersions | Astaxanthin | [162] | ||
| Nanoemulsions | Lutein | [144] | |||
| Soy proteins | Nanoemulsions | β-carotene | [163] | ||
| Nanoparticles | β-carotene | [153,164] | |||
| Solid lipid nanoparticles | β-carotene | [156] | |||
| Cereal proteins | Nanoparticles | β-carotene | [165] | ||
| Potato proteins | Nanoparticles | Astaxanthin | [166] | ||
| Lipid-based NC | Nanoemulsions | β-carotene | [167,168,169,170,171,172] | ||
| Lutein | [148,150,158,173,174,175,176,177,178] | ||||
| Lycopene | [140,179,180,181] | ||||
| Nanoliposomes | β-carotene | [182,183,184] | |||
| Lutein | [185,186,187] | ||||
| Lycopene | [188,189] | ||||
| Nanoniosomes | β-carotene | [190] | |||
| Lycopene | [191,192] | ||||
| Solid lipid nanoparticles | β-carotene | [157,193,194] | |||
| Lutein | [178] | ||||
| Lycopene | [195,196,197] | ||||
| Nanostructured lipid carriers | Astaxanthin | [198] | |||
| β-carotene | [199,200,201] | ||||
| Lutein | [178,202,203,204] | ||||
| Lycopene | [197,205,206] | ||||
| Field | Chitosan Applications | Form |
|---|---|---|
| Agriculture | Biofertilizer and biocontrol agent (time release of products) Booster of plant growth and plant production Controlled agrochemical release Frost protection Modify plant-microbial interactions Pesticide formulations Soil conditioner Stimulator of crop yield Stimulator of secondary metabolites to induce plant defenses | Solution Film Powder Spray Coating Gel Powder Nanoparticle |
| Aquaculture | Removal of organic/inorganic compounds Removal of bacteria Removal of ammonia Functional food Micro-carrier for bioactive compounds Probiotics Drugs microencapsulation Drug delivery Oral delivery (vaccination) Antimicrobial and antioxidant | Microsphere Bead Powder |
| Pharmaceutical and medical/biomed | Excipients Gene, drug, and vaccine delivery system Antimicrobial agent (antibacterial, antifungal) Anti-inflammatory, antiulcer, and antihypertensive agent Dermatological products Hydrating agents Nutraceutical ingredient Hemostatic and anticoagulant compound Antitumor agent and tumor inhibition Anti-HIV agent Innate immune cell recruitment and activation agent Treatment of leukemia, diabetes Sutures, surgical threads, bandages, sponges Biocompatible and biodegradable materials for use as implants, blood substitutes, blood vessels or wound dressing material Dental implants Contact lenses Magnetic resonance imaging | Solution Powder Tablet Nanoparticle Nanocomposite Sponge Gel and hydrogel Microsphere Capsule and microcapsule Bead Film Fiber and nanofiber |
| Food and nutrition | Additives for human and animal Antibacterial, antifungal, antioxidants Astringency Diet foods and dietary fibers Edible films Hypolipidemic and hypocholesterolemia activities Infant feed ingredient Prebiotics | Solution Film Blend Coating Bead |
| Cosmetic | Antistatic effect Bacteriostatic Body cleaning products Encapsulating agent Functional additives Hydrating and film-forming agent Products for hair care Products for oral/dental care Products: shampoos, creams, lotions, nail polish, make-up powder, etc. Skin delivery formulations Thickening agent | Solution Film Powder |
| Environmental | Adsorbent/biosorbent Antibacterial material Antifouling agent Coagulant/flocculant Interactions with proteins and amino acids Material for treatment of contaminant water Polymer for ultrafiltration Reduce odors | Adsorbent/biosorbent Coagulant/flocculant Antifouling agent Interactions with proteins and amino acids Reduce odors Polymer for ultrafiltration Material for treatment of contaminant water Antibacterial material |
| Textile | Dye-binder for textiles Impregnated textile materials Binding agent for non-woven Surface modification of textiles Textiles with anti-bacterial properties Textile antimicrobial finishing Sanitary fibrous products Surgical threads Textile preservative and deodorant agent Non-allergenic fibers | Microcapsule Fiber Gel and gelatinous dispersion Coating |
| Paper and pulp | Wet strength agent Reduction in paper water vapor permeability Antibacterial and antimicrobial protective coating for paper packaging Antitermite in papermaking Retention and drainage agents Biodegradable packaging Wrapping and toilet paper Carbonless copy paper Cardboard Chromatography paper Modification of cellulose fibers Photochromic paper Papermaking wastewater treatments | Nanoparticle Powder Coating |
| Biotechnology and chemistry | Adhesive activity between metallic surfaces Analytical reagent Binders for silicon/graphite Biosensors, electronic and electrochemical devices Cell-recovery composite electrodes in lithium-ion batteries Corrosion protection of aluminum Enzyme and cell immobilization Ionic liquids and deep eutectic solvents Matrix for chromatography Membranes for lithium batteries Metabolic Analysis of biological fluids Permeability control Protein separation Reverse osmosis Solvent separation Transport direction of target molecules | Powder Bead Nanoparticle Microsphere Sponge Coating Fiber Solution Ionic liquids Membrane Sensors Composite Blend |
| Delivery System | Carotenoids | Particle Size (nm) | Encapsulation Efficiency (%) | Storage Stability (Days) | Ref. |
|---|---|---|---|---|---|
| Chitosan-coated Nanoemulsion | β-carotene | 218; 143.7 | NA | 21 at 37 °C | [18] |
| Chitosan-coated Nanoliposomes | β-carotene, Lutein, Lycopene, Canthaxanthin | 70 to 100 | 75 | NA | [239] |
| Chitosan (Polysaccharide)-Based Nanocarriers | β-carotene | NA | >95 | NA | [240] |
| Chitosan-based Nanogels | Fucoxanthin | 200 to 500 | 47 to 90 | 6 at 37 °C | [241] |
| Delivery System | Biopolymer | Loaded Compound | Findings | Ref. |
|---|---|---|---|---|
| Polysaccharide-based nanocarrier | Chitosan | Lutein | Bioavailability improved by 27.7%. Postprandial levels in blood plasma (54.5%), liver (53.9%), and eyes (62.8%) in mice higher than control. | [21] |
| Poly (ethylene oxide)-4-methoxycinnamoylphthaloyl-chitosan (PCPLC)/ poly(vinylalcohol-co-vinyl-4-methoxycinnamate) (PB4)/ ethylcellulose (EC) | Astaxanthin | In PCLC: high encapsulation efficiency (98%), loading (40%), and high stability to heat. No positive results with PB4 or EC encapsulation | [20] | |
| Chitosan + sodium tripolyphosphate/ chitosan + carboxymethylcellulose | β-carotene | Chitosan + sodium tripolyphosphate carrier: high β-carotene release in aqueous media and gastric fluid, and adequate release in intestinal fluids. Chitosan + carboxymethylcellulose carrier: efficient release in aqueous media and gastric fluid; small release in intestinal fluid. β-carotene release enhanced in food systems with both carriers. | [240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verardi, A.; Sangiorgio, P.; Lopresto, C.G.; Casella, P.; Errico, S. Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems. Nutraceuticals 2023, 3, 451-480. https://doi.org/10.3390/nutraceuticals3030033
Verardi A, Sangiorgio P, Lopresto CG, Casella P, Errico S. Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems. Nutraceuticals. 2023; 3(3):451-480. https://doi.org/10.3390/nutraceuticals3030033
Chicago/Turabian StyleVerardi, Alessandra, Paola Sangiorgio, Catia Giovanna Lopresto, Patrizia Casella, and Simona Errico. 2023. "Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems" Nutraceuticals 3, no. 3: 451-480. https://doi.org/10.3390/nutraceuticals3030033










