Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broccoli Leaf Processing
2.2. Establishment of the Dose of Interest
2.3. Animals
2.4. Gelatine Ingestion Training
2.5. Administration of Broccoli Leaf Flour
2.6. Collection of Biological Samples
2.7. Histopathology
2.8. Determination of Glucosinolates and Derivatives in Plasma
2.8.1. Pre-Processing Method
2.8.2. Reverse Phase—High-Performance Liquid Chromatography—Diode Array Detector (RP-HPLC-DAD) Analysis
2.9. Determination of Glucosinolates and Derivatives in Urine, Faeces, Kidney, Liver and Adipose Tissue
2.9.1. Pre-Processing Method
2.9.2. Ultra High-Pressure Liquid Chromatography Coupled to Electrospray Ionisation (UHPLC-ESI-QqQ-MS/MS) Analysis
2.10. Statistics
3. Results
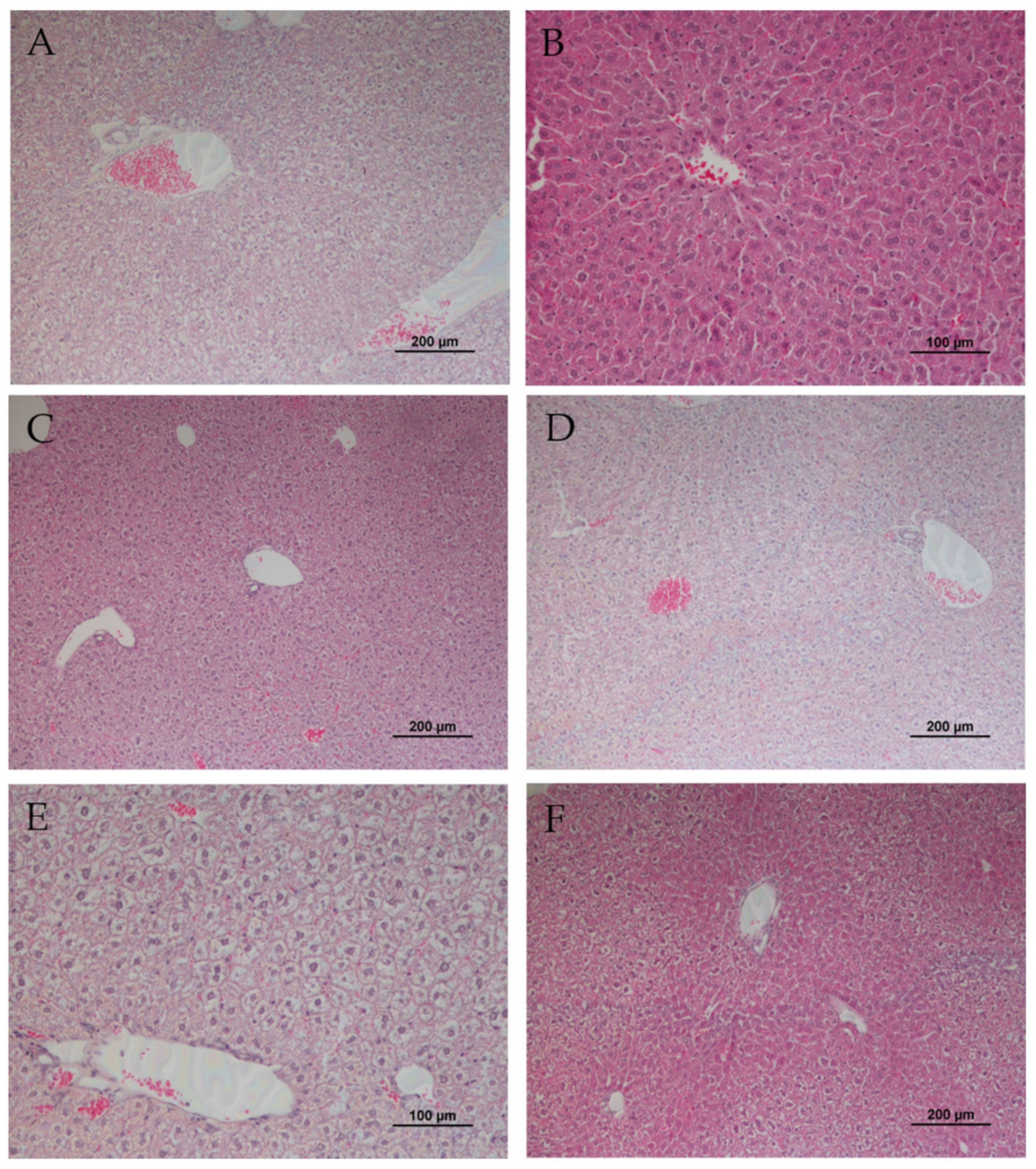
3.1. Liver, Kidney and Lung Histology
3.2. Quantification of Glucosinolates and Isothiocyanates after BLF Ingestion
4. Discussion
4.1. Glucoraphanin (GR), Sulforaphane (SFN) and SFN Conjugates
4.2. Glucoerucin (GE) and Erucin
4.3. Glucobrassicin (GB), Indole-3-carbinol (I3C) and 3,3′-Diindolylmethane (DIM)
4.4. Glucoiberin (GI) and Iberin
4.5. Glucosinolates and Gut Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Lopez, A.D.; Melgar, B.; Conidi, C.; Barros, L.; Ferreira, I.C.F.R.; Cassano, A.; Garcia-Castello, E.M. Food Industry By-Products Valorization and New Ingredients: Cases of Study. In Sustainability of the Food System; Betoret, N., Betoret, E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 71–99. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 March 2023).
- FAO. Food Wastage Footprint: Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- Khedkar, R.; Singh, K. Food Industry Waste: A Panacea or Pollution Hazard? In Paradigms in Pollution Prevention; Jindal, T., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 35–47. [Google Scholar]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food Waste Within Food Supply Chains: Quantification and Potential for Change to 2050. Philos. Trans. R. Soc. B 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Oliveira, P.A.; Pires, M.J.; Neuparth, M.J.; Lanzarin, G.; Félix, L.; Venâncio, C.; Pinto, M.d.L.; Ferreira, J.; Gaivão, I.; et al. Effect of a Sub-Chronic Oral Exposure of Broccoli (Brassica oleracea L. var. italica) By-Products Flour on the Physiological Parameters of FVB/N Mice: A Pilot Study. Foods 2022, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional Values, Beneficial Effects, and Food Applications of Broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Composition and Antioxidant Capacity of a Novel Beverage Produced with Green Tea and Minimally-Processed Byproducts of Broccoli. Innovat. Food Sci. Emerg. Technol. 2011, 12, 361–368. [Google Scholar] [CrossRef]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli By-Products Improve the Nutraceutical Potential of Gluten-Free Mini Sponge Cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Vazquez-Duran, A.; Gallegos-Soto, A.; Bernal-Barragan, H.; Lopez-Perez, M.; Mendez-Albores, A. Physicochemical, Nutritional and Sensory Properties of Deep Fat-Fried Fortified Tortilla Chips with Broccoli (Brassica oleracea L. convar. italica Plenck). Flour. J. Food Nutr. Res. 2014, 53, 313–323. [Google Scholar]
- Zambelli, R.A.; Pontes, B.C.V.; Pontes, E.R.; Silva, M.L.; Junior, E.C.S.; Pinto, L.I.F.; Melo, C.A.L.; Farias, M.M.; da Costa, C.S.; da Silva, A.C. Broccoli and Carrot Industrial Solid Waste Characterization and Application in the Bread Food Matrix. Int. J. Nutr. Food Sci. 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates Among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 305–350. [Google Scholar]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Maldini, M.; Baima, S.; Morelli, G.; Scaccini, C.; Natella, F. A Liquid Chromatography-Mass Spectrometry Approach to Study “Glucosinoloma” in Broccoli Sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Colaco, B.; Venancio, C.; Pires, M.J.; Oliveira, P.A.; Rosa, E.; Antunes, L.M. Potential Effects of Sulforaphane to Fight Obesity. J. Sci. Food Agric. 2018, 98, 2837–2844. [Google Scholar] [CrossRef]
- Mithen, R.; Ho, E. Isothiocyanates for Human Health. Mol. Nutr. Food Res. 2018, 62, e1870079. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, S.; Kanala, S.R.; Khatri, S.; Kutagulla, V.K.; Mallela, V.; Peraman, R. Cardioprotective Potential of Indol-3-Carbinol Against High Salt Induced Myocardial Stress and Hypertrophy in Sprague dawley Rats Besides Molecular Docking on Muscarinic Receptor-2. Nat. Prod. Res. 2022, 36, 2610–2614. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Zeng, Y.; Huang, X.; Chen, H.; Wang, S.; Wu, J.; Li, Q.; Zhu, W.; Li, H.; et al. A Novel Phytochemical, DIM, Inhibits Proliferation, Migration, Invasion and TNF-α Induced Inflammatory Cytokine Production of Synovial Fibroblasts From Rheumatoid Arthritis Patients by Targeting MAPK and AKT/mTOR Signal Pathway. Front. Immunol. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Gong, T.T.; Guo, Q.; Li, X.; Zhang, T.N.; Liu, F.H.; He, X.H.; Lin, B.; Wu, Q.J. Isothiocyanate Iberin Inhibits Cell Proliferation and Induces Cell Apoptosis in the Progression of Ovarian Cancer by Mediating ROS Accumulation and GPX1 Expression. Biomed. Pharmacother. 2021, 142, 111533. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Vidovic, B.; Costa, J.G.; Martins, M.; Ferreira, S.; Oliveira, N.G.; Saraiva, N.; Fernandes, A.S. The Dietary Isothiocyanate Erucin Reduces Kidney Cell Motility by Disturbing Tubulin Polymerization. Mol. Nutr. Food Res. 2023, 67, e2200581. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Shimoyama, M.; Fujii, A.; Sato, J.; Kadena, K.; Ozaki, K.; Hosaka, K. The Anti-Inflammatory Effects of Iberin on TNF-α-Stimulated Human Oral Epithelial Cells: In Vitro Research. Biomedicines 2022, 10, 3155. [Google Scholar] [CrossRef]
- Reyes-Hernández, O.D.; Figueroa-González, G.; Quintas-Granados, L.I.; Gutiérrez-Ruíz, S.C.; Hernández-Parra, H.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chavez, S.A.; Cortés, H.; Peña-Corona, S.I.; et al. 3,3′-Diindolylmethane and Indole-3-Carbinol: Potential Therapeutic Molecules for Cancer Chemoprevention and Treatment via Regulating Cellular Signaling Pathways. Cancer Cell Int. 2023, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Riemschneider, S.; Hoffmann, M.; Slanina, U.; Weber, K.; Hauschildt, S.; Lehmann, J. Indol-3-Carbinol and Quercetin Ameliorate Chronic DSS-Induced Colitis in C57BL/6 Mice by AhR-Mediated Anti-Inflammatory Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 2262. [Google Scholar] [CrossRef] [PubMed]
- Yahiya, Y.I.; Hadi, N.R.; Abu Raghif, A.; Qassam, H.; Al Habooby, N.G.S. Role of Iberin as an Anti-Apoptotic Agent on Renal Ischemia-Reperfusion Injury in Rats. J. Med. Life 2023, 16, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Piberger, A.L.; Budnowski, J.; Herz, C.; Schreiner, M.; Blaut, M.; Hartwig, A.; Lamy, E.; Hanske, L.; Rohn, S. Bioavailability and Biotransformation of Sulforaphane and Erucin Metabolites in Different Biological Matrices Determined by LC-MS-MS. Anal. Bioanal. Chem. 2015, 407, 1819–1829. [Google Scholar] [CrossRef]
- Zhu, W.; Lerno, L.A.; Cremonini, E.; Oteiza, P.I.; Mastaloudis, A.; Bornhorst, G.M.; Mitchell, A.E. Robust UHPLC-(ESI+)-MS/MS Method for Simultaneous Analysis of Glucoraphanin, Sulforaphane, and Sulforaphane Metabolites in Biological Samples. ACS Food Sci. Technol. 2023, 3, 1300–1310. [Google Scholar] [CrossRef]
- Budnowski, J.; Hanske, L.; Schumacher, F.; Glatt, H.; Platz, S.; Rohn, S.; Blaut, M. Glucosinolates Are Mainly Absorbed Intact in Germfree and Human Microbiota-Associated Mice. J. Agric. Food Chem. 2015, 63, 8418–8428. [Google Scholar] [CrossRef]
- Al Janobi, A.A.; Mithen, R.F.; Gasper, A.V.; Shaw, P.N.; Middleton, R.J.; Ortori, C.A.; Barrett, D.A. Quantitative Measurement of Sulforaphane, Iberin and Their Mercapturic Acid Pathway Metabolites in Human Plasma and Urine Using Liquid Chromatography-Tandem Electrospray Ionisation Mass Spectrometry. J. Chromatogr. B 2006, 844, 223–234. [Google Scholar] [CrossRef]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and Metabolism of Isothiocyanates Formed from Broccoli Glucosinolates: Effects of BMI and Daily Consumption in a Randomised Clinical Trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef]
- Maruthanila, V.L.; Poornima, J.; Mirunalini, S. Attenuation of Carcinogenesis and the Mechanism Underlying by the Influence of Indole-3-Carbinol and Its Metabolite 3,3′-Diindolylmethane: A Therapeutic Marvel. Adv. Pharmacol. Sci. 2014, 2014, 832161. [Google Scholar] [CrossRef]
- Amare, D.E.; Bovee, T.F.H.; Mulder, P.P.J.; Hamers, A.; Hoogenboom, R.L.A.P. Acid Condensation Products of Indole-3-Carbinol and Their In-Vitro (Anti)Estrogenic, (Anti)Androgenic and Aryl Hydrocarbon Receptor Activities. Arab. J. Chem. 2020, 13, 7199–7211. [Google Scholar] [CrossRef]
- Orlando, P.; Nartea, A.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Dludla, P.V.; Fiorini, R.; Pacetti, D.; Loizzo, M.R.; Lucci, P.; et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants 2022, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; López-Chillón, M.T.; Zafrilla, P.; Moreno, D.A. Bioavailability of Broccoli Sprouts in Different Human Overweight Populations. J. Funct. Foods 2019, 59, 337–344. [Google Scholar] [CrossRef]
- Nutrition Australia. Australian Dietary Guidelines: Standard Serves. Available online: https://nutritionaustralia.org/fact-sheets/adgs-standard-serves/ (accessed on 10 November 2022).
- Cox, H.M.; Tough, I.R.; Woolston, A.M.; Zhang, L.; Nguyen, A.D.; Sainsbury, A.; Herzog, H. Peptide YY is Critical for Acylethanolamine Receptor Gpr119-Induced Activation of Gastrointestinal Mucosal Responses. Cell Metab. 2010, 11, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.S.; Xia, S.; Tait, D.S.; Bundgaard, C.; Bowman, E.; Brown, V.J. Oral Dosing of Rodents Using a Palatable Tablet. Psychopharmacology 2018, 235, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P.A.; Roughan, J.V.; Stewart, R. Use of Oral Buprenorphine (“Buprenorphine Jello”) for Postoperative Analgesia in Rats—A Clinical Trial. Lab. Anim. 1999, 33, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lee, N.J.; Nguyen, A.D.; Enriquez, R.F.; Riepler, S.J.; Stehrer, B.; Yulyaningsih, E.; Lin, S.; Shi, Y.C.; Baldock, P.A.; et al. Additive Actions of the Cannabinoid and Neuropeptide Y Systems on Adiposity and Lipid Oxidation. Diabetes Obes. Metab. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Kronenberger, J.P.; Médioni, J. Food Neophobia in Wild and Laboratory Mice (Mus musculus domesticus). Behav. Process. 1985, 11, 53–59. [Google Scholar] [CrossRef]
- Martins, T.; Matos, A.F.; Soares, J.; Leite, R.; Pires, M.J.; Ferreira, T.; Medeiros-Fonseca, B.; Rosa, E.; Oliveira, P.A.; Antunes, L.M. Comparison of Gelatin Flavors for Oral Dosing of C57BL/6J and FVB/N Mice. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 89–95. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Medina, S.; Moreno, D.A.; Garcia-Viguera, C.; Ferreres, F.; Gil-Izquierdo, A. A New Ultra-Rapid UHPLC/MS/MS Method for Assessing Glucoraphanin and Sulforaphane Bioavailability in Human Urine. Food Chem. 2014, 143, 132–138. [Google Scholar] [CrossRef]
- Bricker, G.V.; Riedl, K.M.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.M.; Schwartz, S.J. Isothiocyanate Metabolism, Distribution, and Interconversion in Mice Following Consumption of Thermally Processed Broccoli Sprouts or Purified Sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef]
- Susa, S.T.; Hussain, A.; Preuss, C.V. Drug Metabolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. A Critical Review of the Bioavailability of Glucosinolates and Related Compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Webb, D.R.; Peters, J.C. Evaluation of the Potential for Olestra to Affect the Availability of Dietary Phytochemicals. J. Nutr. 1997, 127, 1699S–1709S. [Google Scholar] [CrossRef] [PubMed]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernas, H.; Karlen, A. Correlation of Human Jejunal Permeability (In Vivo) of Drugs with Experimentally and Theoretically Derived Parameters. A Multivariate Data Analysis Approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef]
- Petri, N.; Tannergren, C.; Holst, B.; Mellon, F.A.; Bao, Y.; Plumb, G.W.; Bacon, J.; O’Leary, K.A.; Kroon, P.A.; Knutson, L.; et al. Absorption/Metabolism of Sulforaphane and Quercetin, and Regulation of Phase II Enzymes, in Human Jejunum In Vivo. Drug Metab. Dispos. 2003, 31, 805–813. [Google Scholar] [CrossRef]
- Samiec, P.S.; Dahm, L.J.; Jones, D.P. Glutathione S-Transferase in Mucus of Rat Small Intestine. Toxicol. Sci. 2000, 54, 52–59. [Google Scholar] [CrossRef]
- Bruggeman, I.M.; Temmink, J.H.M.; van Bladeren, P.J. Glutathione- and Cysteine-Mediated Cytotoxicity of Allyl and Benzyl Isothiocyanate. Toxicol. Appl. Pharmacol. 1986, 83, 349–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Kolm, R.H.; Mannervik, B.; Talalay, P. Reversible Conjugation of Isothiocyanates with Glutathione Catalyzed by Human Glutathione Transferases. Biochem. Biophys. Res. Commun. 1995, 206, 748–755. [Google Scholar] [CrossRef]
- Conklin, D.J.; Haberzettl, P.; Lesgards, J.F.; Prough, R.A.; Srivastava, S.; Bhatnagar, A. Increased Sensitivity of Glutathione S-Transferase P-Null Mice to Cyclophosphamide-Induced Urinary Bladder Toxicity. J. Pharmacol. Exp. Ther. 2009, 331, 456–469. [Google Scholar] [CrossRef]
- Hanna, P.E.; Anders, M.W. The Mercapturic Acid Pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Reed, C.J. Xenobiotic Metabolizing Enzymes of the Kidney. Toxicol. Pathol. 1998, 26, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Li, X.; Zou, P.; Schwartz, S.J.; Sun, D. Kinetics of Sulforaphane in Mice After Consumption of Sulforaphane-Enriched Broccoli Sprout Preparation. Mol. Nutr. Food Res. 2013, 57, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Current Potential Health Benefits of Sulforaphane. EXCLI J. 2016, 15, 571–577. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Yadav, K.; Dhankhar, J.; Kundu, P. Isothiocyanates—A Review of their Health Benefits and Potential Food Applications. Curr. Res. Nutr. Food Sci. 2022, 10, 476–502. [Google Scholar] [CrossRef]
- Boddupalli, S.; Mein, J.R.; Lakkanna, S.; James, D.R. Induction of Phase 2 Antioxidant Enzymes by Broccoli Sulforaphane: Perspectives in Maintaining the Antioxidant Activity of Vitamins A, C, and E. Front. Genet. 2012, 3, 7. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular Targets of Isothiocyanates in Cancer: Recent Advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Mondloch, S.; Scholl, C.D.; Arscott, S.A.; Tanumihardjo, S.A. Glucosinolate and Isothiocyanate Contents of Frozen Broccoli, Brussels Sprouts and a Whole-food Cruciferous Supplement Over Time. J. Food Nutr. Sci. 2019, 1, 37–48. [Google Scholar] [CrossRef]
- Kassahun, K.; Davis, M.; Hu, P.; Martin, B.; Baillie, T. Biotransformation of the Naturally Occurring Isothiocyanate Sulforaphane in the Rat: Identification of Phase I Metabolites and Glutathione Conjugates. Chem. Res. Toxicol. 1997, 10, 1228–1233. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H. Biological Profile of Erucin: A New Promising Anticancer Agent from Cruciferous Vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Howie, A.F.; Beckett, G.J.; Mithen, R.; Bao, Y. Sulforaphane, Erucin, and Iberin Up-Regulate Thioredoxin Reductase 1 Expression in Human MCF-7 Cells. J. Agric. Food Chem. 2005, 53, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Riedl, K.M.; Ralston, R.A.; Thomas-Ahner, J.M.; Schwartz, S.J.; Clinton, S.K.; Mortazavi, A. Inhibition of Bladder Cancer by Broccoli Isothiocyanates Sulforaphane and Erucin: Characterization, Metabolism, and Interconversion. Mol. Nutr. Food Res. 2012, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Costa, C.; Traka, M.; Miceli, N.; Mithen, R.; De Pasquale, R.; Trovato, A. Erucin, a New Promising Cancer Chemopreventive Agent from Rocket Salads, Shows Anti-Proliferative Activity on Human Lung Carcinoma A549 Cells. Food Chem. Toxicol. 2009, 47, 1430–1436. [Google Scholar] [CrossRef]
- Bello, I.; Smimmo, M.; d’Emmanuele di Villa Bianca, R.; Bucci, M.; Cirino, G.; Panza, E.; Brancaleone, V. Erucin, an H2S-Releasing Isothiocyanate, Exerts Anticancer Effects in Human Triple-Negative Breast Cancer Cells Triggering Autophagy-Dependent Apoptotic Cell Death. Int. J. Mol. Sci. 2023, 24, 6764. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H. Molecular Targets and Anticancer Potential of Indole-3-Carbinol and Its Derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Ho, E. Metabolic Fate of Dietary Glucosinolates and Their Metabolites: A Role for the Microbiome. Front. Nutr. 2021, 8, 748433. [Google Scholar] [CrossRef]
- Eve, A.A.; Liu, X.; Wang, Y.; Miller, M.J.; Jeffery, E.H.; Madak-Erdogan, Z. Biomarkers of Broccoli Consumption: Implications for Glutathione Metabolism and Liver Health. Nutrients 2020, 12, 2514. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli Consumption Affects the Human Gastrointestinal Microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Kellingray, L.; Tapp, H.S.; Saha, S.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Consumption of a Diet Rich in Brassica Vegetables is Associated with a Reduced Abundance of Sulphate-Reducing Bacteria: A Randomised Crossover Study. Mol. Nutr. Food Res. 2017, 61, 1600992. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.; Beresford, S.A.; Lampe, J.W. Variation of Glucoraphanin Metabolism In Vivo and Ex Vivo by Human Gut Bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Zhu, Y.; Mupunga, J.; Zou, L.; Liu, C.; Liu, S.; Mao, J. Broccoli Ingestion Increases the Glucosinolate Hydrolysis Activity of Microbiota in the Mouse Gut. Int. J. Nutr. Food Sci. 2019, 70, 585–594. [Google Scholar] [CrossRef]
- Zandani, G.; Kaftori-Sandler, N.; Sela, N.; Nyska, A.; Madar, Z. Dietary Broccoli Improves Markers Associated with Glucose and Lipid Metabolism Through Modulation of Gut Microbiota in Mice. Nutrition 2021, 90, 111240. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
| Biological Samples | Time Point | Compounds Detected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GE | GB | SFN | SFN-GSH | SFN-CYS | SFN-NAC | I3C | Iberin | ||
| Plasma (µmol/L) | 0 h | – | - | - | - | - | - | - | - |
| 1 h | - | 0.018 ± 0.004 (n = 3) | 0.012 ± 0.003 (n = 3) | - | - | - | 0.030 ± 0.002 (n = 3) | - | |
| 2 h | 0.018 ± 0.002 (n = 3) | 0.067 ± 0.012 (n = 3) | 0.029 ± 0.003 (n = 3) | - | - | - | 0.103 ± 0.007 (n = 3) | - | |
| 4 h | 0.031 ± 0.001 (n = 3) | 0.094 ± 0.004 (n = 3) | 0.040 ± 0.001 (n = 3) | - | - | - | 0.145 ± 0.005 (n = 3) | - | |
| 8 h | 0.023 ± 0.001 (n = 3) | 0.081 ± 0.001 (n = 3) | 0.035 ± 0.001 (n = 3) | - | - | - | 0.116 ± 0.001 (n = 3) | - | |
| 12 h | - | 0.008 ± 0.001 (n = 3) | 0.005 ± 0.001 (n = 3) | - | - | - | 0.015 ± 0.001 (n = 3) | - | |
| 24 h | - | - | - | - | - | - | - | - | |
| Liver (ng/mg fw) | 0 h | - | - | - | - | - | - | - | - |
| 1 h | - | - | - | - | - | - | 4.107 ± 2.083 (n = 2) $ | - | |
| 2 h | - | - | - | - | 1.195 ± 0.310 (n = 3) | - | 4.040 ± 2.522 (n = 3) | - | |
| 4 h | 0.112 ± 0.153 (n = 2) $ | - | - | - | 0.875 ± 0.282 (n = 2) $ | 0.040 ± 0.001 (n = 2) $ | 9.584 (n = 1) $ | - | |
| 8 h | 0.087 ± 0.009 (n = 2) $ | - | - | - | 0.754 ± 0.208 (n = 3) | 0.035 ± 0.001 (n = 2) $ | 5.017 ± 0.950 (n = 3) | - | |
| 12 h | - | - | - | - | - | - | - | - | |
| 24 h | - | - | - | - | - | - | - | - | |
| Kidney (ng/mg fw) | 0 h | - | - | - | - | - | - | - | - |
| 1 h | - | 1.365 ± 0.775 (n = 3) | - | 6.841 ± 2.749 (n = 3) | - | - | - | - | |
| 2 h | 0.487 ± 0.481 (n = 2) $ | 1.236 ± 0.262 (n = 3) | - | 3.767 ± 0.707 (n = 3) | - | - | - | - | |
| 4 h | 0.691 ± 0.100 (n = 2) $ | 1.796 ± 1.117 (n = 2) $ | - | 4.525 (n = 1) $ | - | - | - | - | |
| 8 h | 0.481 ± 0.1227 (n = 3) | 1.592 ± 0.568 (n = 3) | - | 5.062 ± 1.008 (n = 3) | - | - | - | - | |
| 12 h | - | - | - | - | - | - | - | - | |
| 24 h | - | - | - | - | - | - | - | - | |
| Faeces (ng/mg fw) | 0 h | - | - | - | - | - | - | - | - |
| 1 h | - | - | 0.126 ± 0.142 (n = 3) | 36.010 ± 9.337 (n = 3) | 0.239 ± 0.236 (n = 3) | 0.026 ± 0.020 (n = 2) | - | 0.235 ± 0.308 (n = 3) | |
| 2 h | - | - | 0.112 ± 0.059 (n = 3) | 36.440 ± 8.305 (n = 3) | 0.402 ± 0.336 (n = 3) | 0.045 ± 0.010 (n = 3) | - | 0.037 (n = 1) $ | |
| 4 h | - | - | 0.042 ± 0.016 (n = 2) $ | 37.300 ± 1.663 (n = 2) $ | 0.211 ± 0.113 (n = 2) $ | 0.031 ± 0.013 (n = 2) $ | - | - | |
| 8 h | - | - | 0.025 ± 0.002 (n = 3) | 7.315 (n = 1) $ | 0.291 ± 0.161 (n = 3) | 0.027 ± 0.013 (n = 3) | - | 0.065 ± 0.064 (n = 3) | |
| 12 h | - | - | - | - | - | - | - | - | |
| 24 h | - | - | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, T.; Ferreira, T.; Colaço, B.; Medeiros-Fonseca, B.; Pinto, M.d.L.; Barros, A.N.; Venâncio, C.; Rosa, E.; Antunes, L.M.; Oliveira, P.A.; et al. Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study. Nutraceuticals 2023, 3, 540-555. https://doi.org/10.3390/nutraceuticals3040039
Martins T, Ferreira T, Colaço B, Medeiros-Fonseca B, Pinto MdL, Barros AN, Venâncio C, Rosa E, Antunes LM, Oliveira PA, et al. Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study. Nutraceuticals. 2023; 3(4):540-555. https://doi.org/10.3390/nutraceuticals3040039
Chicago/Turabian StyleMartins, Tânia, Tiago Ferreira, Bruno Colaço, Beatriz Medeiros-Fonseca, Maria de Lurdes Pinto, Ana Novo Barros, Carlos Venâncio, Eduardo Rosa, Luís Miguel Antunes, Paula Alexandra Oliveira, and et al. 2023. "Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study" Nutraceuticals 3, no. 4: 540-555. https://doi.org/10.3390/nutraceuticals3040039
APA StyleMartins, T., Ferreira, T., Colaço, B., Medeiros-Fonseca, B., Pinto, M. d. L., Barros, A. N., Venâncio, C., Rosa, E., Antunes, L. M., Oliveira, P. A., & Pires, M. J. (2023). Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study. Nutraceuticals, 3(4), 540-555. https://doi.org/10.3390/nutraceuticals3040039










