Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines
Abstract
:1. Introduction
2. Strategy of Searching Articles
3. Role of Nutraceuticals
3.1. Antioxidant Activity
3.2. Methods of Estimation of Total Antioxidant Activity (TAC)
3.3. Metabolomics Analysis
4. Botanical Information, Ethnobotanical Practices, and Bioactive Phytochemicals in Wild Edible Plants
| Scientific Name and Important Morphological Characters | Systematics | Etymology | Distribution and Habitat | Ref. |
|---|---|---|---|---|
| Achillea millefolium L. Perennial herbaceous plant, roots in the rhizome; hairy stem; simple or branched; leafy; ascending; can reach up to 80 cm in height. The hairy leaves have contours, both lanceolate and linear. The flowers are white or pink, whitish achenes. The fruits are achenes. | Domain Eukaryota, Kingdom Plantae, Division Magnolio-phyta, Class Magnoliopsida, Subclass Asteridae, Order Asterales, Family Asteraceae, Subfamily Asteroideae, Tribe Anthemideae, Subtribe Achilleinae, Genus Achillea, Species A. millefolium. | Tradition (Pliny) states that Achilles healed some wounds of his comrades in arms during the siege of Troy, with the plant, hence the name of the genre. The name (milfoil) is due to its deeply indented leaves; in fact, the epithet refers to the numerous foliar laciniae that characterize this plant. | The A. millefolium is native to Europe; it grows in temperate regions all over the world up to 2500 m. It prefers sunny places, meadows, and the edges of paths and railways; it also adapts well to dry, stony, and acidic soils. | [70,71] |
| Borago officinalis L. is an herbaceous plant; it can reach up to 80 cm. It has elliptic oval leaves and petioles, with rough hair and a dark green color, collected in a basal rosette 10–15 cm long, which are then smaller on the stem. The flowers have five petals arranged in a blue-purple star. The fruits are achenes that contain small seeds. | Domain Eukaryota, Kingdom Plantae, Division Magnolio-phyta, Class Magnoliopsida, Order Solanales, Family Boraginaceae, Genus Borago and specie B. officinalis. | The etymology of its name is uncertain. Some suppose that it derives from the Arabic “abou” and “rash”. Others assume that it comes from the Latin “wad” or from the Celtic “barrach”, meaning “brave man”. The Italian name Borage comes from the Latin Borago. | This herb is well adapted to the Mediterranean basin and widespread throughout Italy, where it grows spontaneously up to 1800 m above sea level. It prefers a rich soil, without stagnant water. | [72,73] |
| Foeniculum vulgare Mill is complex and difficult to summarize. It derives from the distinction between the varieties of wild fennel and “sweet” fennel (horticultural production). | Eukaryota Domain, Kingdom Plantae, Division Magnolio-phyta, Class Magnoliopsida, Subclass Rosidae, Order Apiales, Family Apiaceae, Foeniculum Genus and F. vulgare Specie. | The names comes from foenum, meaning hay, due to the subtlety of the leaves and its intense aromatic odor. Vulgare means that the plant is quite widespread (vulgar = common). | Fennel is a typical Mediterranean plant. It is mainly found in southern regions and islands, from sea level up to about 1000 m altitude. It prefers sunny, unspoilt, dry, and pebbled places. | [74,75] |
| Juniperus communis L. is an evergreen shrub or tree with a twisted trunk, of 1–10 m tall, with linear, needle-like, pungent leaves, gathered in verticils of 3. Male flowers are yellowish; female flowers are small greenish cones, which produce berries (called cuddles). | Domain Eukaryota, Kingdom Plantae, Subkingdom Tracheo bionta, Superdivision Sperma tophyta, Division Pinophyta, Class Pinopsida, Order Pinales, Family Cupressaceae, Genus Juniperus, Specie J. Communis. | The term Juniperus derives from iúnix, heifer, and pário, meaning giving birth. This is due to its presumed properties favoring childbirth. Communis epithet obviously means common, banal. | It is widespread, from marine regions to mountainous areas, and is found in dry pastures, as well as on moors or scrubland. It is a very long-lived species in the temperate regions of the northern hemisphere. It is resistant to low temperatures, and tolerates aridity and strong wind. | [76,77] |
| Malva sylvestris L. is an annual herbaceous plant that is biennial or perennial. The stem can grow to 60–80 cm. The leaves of palminervia have 5–7 lobes and an irregularly serrated margin. The flowers are grouped axils of leaves. The fruit is a circular poliachenio. | Eukaryota Domain, Kingdom Plantae, Division Magnolio-phyta, Class Magnoliopsida, Order Malvales, Family Malvaceae Genus Malva and Specie M. sylvestris. | The genus name, the consonant with the greek “Malatto” and “malákhe”, means emollient, benevolent, with reference to the soothing properties of these plants. | The plant is native to Europe and temperate Asia; it can be found in fields and uncultivated places. | [78,79] |
| Gentiana lutea L. is an herbaceous, perennial species with very slow growth. It can reach up to 150 cm, with a single stem that is hollow inside, and green leaves. The flowers are yellow, sometimes punctuated with darker color, star-shaped, and gathered in bundles to the axil of the upper leaves. The fruit is an oblong oval capsule, which opens at maturity in two parts, containing brown oval seeds. | Domain Eukaryota, Kingdom Plantae, Phylum Magnolio-phyta, Class Magnoliopsida, Order Gentianales, Family Gentianaceae, Genus Gentiana, Specie G. lutea | According to Pliny, Gentiana derives from Gentius (in Greek, , Gentios) Genzio, last king of the Illyrians (II century BC), discoverer of the antimalaric properties of the roots of G. lutea. The lutea name derives from lúteus (yellow); that is, the floral color. | Gentian is a plant that grows in meadows and low-humidity pastures, as well as in calcareous soils, rich in organic substances, with heavy sunlight. In Italy, it grows in the central-southern Apennines, at an altitude that varies between 1000 and 2200 m above sea level. | [80,81] |
| Laurus nobilis L. The laurel often appears in shrubs when pruned. In natural conditions, it becomes a tall tree reaching up to 10 m. It is an evergreen plant. The leaves are ovate, dark green, leathery, glossy on the top, and dull underneath. The fruits of the laurel are black and shiny berries with only one seed. | Domain Eukaryota, Kingdom Plantae, Subkingdom Tracheo bionta, Superdivision Sperma tophyta, Division Magnolio phyta, Class Magnoliopsida, Subclass Magnoliidae, Order Laurales, Family Lauraceae, Genus Laurus, Specie L. nobilis L. | The name of this plant comes from the Latin “laus”, meaning praise, to highlight the curative properties of the plant, which have been “praised” from ancient times. “Nobilis” stands for illustrious, important, famous. For others, the vulgar name would be derived from the Celtic root “laur”, meaning green. | L. nobilis is a common species along the northern coastal areas of the Mediterranean basin. In Italy, it grows spontaneously in the central and southern areas along the coast, while in the northern regions it is cultivated. | [82,83] |
| Satureja montana L. is an herbaceous species which grows to 50 cm. The stems are woody at the base, tetragonal, erect, and have short back hairs when pubescent. They are usually widely branched from the bottom to form a small bush. The leaves are bright green, opposite, and subsessile. The fruit is formed by 4 oval achenes dotted with small grains. | Domain Eukaryota, Kingdom Plantae, Subkingdom-Tracheo bionta, Superdivision Sperma tophyta, Division Magnolio phyta, Class Magnoliopsida, Subclass Asteridae, Order Lamiales, Family Lamiaceae, Tribe Mentheae, Genus Satureja, Specie S. montana. | The term Satureja is of uncertain etymology. The specie name “mountain” comes from mons montis, mountain, meaning “of the mountains”, because it grows 1000–1400 m above sea level. | Winter savory is a perennial semi-evergreen species native to the mountainous regions of central–southern and western Europe. Its habitat is that of calcareous, rocky, arid lands, at the edge of mountain roads, at up to 1400 m altitude. | [84] |
| Silybum marianum (L) Gaertn is an herbaceous species with vigorous bearing that can reach up to 150 cm. The plant is completely glabrous and spiny. The scape is robust, streaked, and branched, with erect branches. The plant has hermaphroditic flowers, with a tubular red-purple corolla; these are united in large globular end heads, covered with strong bracts. | Domain Eukaryota, Kingdom Plantae, Subkingdom Tracheo bionta, Superdivision Sperma tophyta, Division Magnolio phyta, Class Magnoliopsida, Subclass Asteridae, Order Asterales, Family Asteraceae, Subfamily Cichorioideae, Tribe Cardueae, Subtribe Carduinae, Genus Silybum, Specie S. marianum. | The term Silybum comes from the Greek sílybon/síllybon, the name which Dioscorides called some edible thistles, which was taken over by Pliny to denote sillybus, a type of thistle. The name “marianum” derives from the Virgin Mary. | Milk thistle is a wild species, widespread in all the Mediterranean regions from sea level to submountain areas. Its habitat is in ruins, along roads, and in uncultivated areas, and it is common in desert and sub-desert areas ranging from the Mediterranean basin to Central Asia. | [85,86] |
| Urtica dioica L. Nettle is a perennial, deciduous herbaceous plant, 30–250 cm tall. It has an erect, densely hairy, striated, and grooved stem. The leaves are large, ovate, and opposite; lanceolate, jagged, and pointed; dark green on the upper side, and lighter and hairier on the lower side. The female flowers are collected in long hanging spikes, while the male flowers are grouped in erect spikes. | Domain Eukaryota, Kingdom Plantae, Division Magnolio phyta, Class Magnoliopsida, Subclass Rosidae, Order Urticales, Family Urticaceae, Genus Urtica, Specie U.dioica. | The name “nettle” probably derives from the Latin “urere” (Urtica), meaning burn, indicating the effect of the irritating substances contained in stinging hairs. | U. dioica is widespread in Europe, most of Asia, North Africa, and North America. In Italy, it is found in all regions: uncultivated land, woods, urbanized areas, roadside, and places in the half-shade of nitrate-rich soil, ranging from the plains to 2300 m above sea level. | [87,88] |
| Scientific Name | Ethnobotanical Uses | Phytochemical Components | Biological Activity | Ref. |
|---|---|---|---|---|
| A. millefolium L. | Tea for gastrointestinal disorders. Essential oils (from flowers) against influenza. Infusions, decoctions, or fresh juices against hemorrhage, hemorrhoidal, menstrual problems, and dysmenorrhea, toothache, headache, diuretic, wounds, and burns (hemostatic). | Rutin; luteolin 7-O-glucoside; apigenin 4′-O-glucoside; apigenin 7-O-glucoside; luteolin 4′-O-glucoside | Anti-inflammatory activity, treatment of gastrointestinal and hepato-biliary disorder and skin inflammation. The in vitro anti-inflammatory activity was established through the inhibition of matrix metalloproteinases (MMP-2 and -9), which are involved in psoriasis and atopic dermatitis and in inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. | [89,90,91,92,93] |
| B. officinalis L. | Diuretic; promotes perspiration; emollient; lenitive; mild laxative; diuretic. Decoction of leaves against rheumatism and as a diuretic. Leaf poultice against tooth abscess. Digestive; depurative. | Flavonoids; phenolic acids; rosmarinic acid; syringic; sinapic; chlorogenic acids. β−sitosterol, oleuropein; lithospermic acid (leaves); tocopherols; sterols; squalene. | Anti-inflammatory properties (HaCaT and BJ cell lines) and anti-ageing properties. Weak anti-inflammatory activity in murine RAW 264.7 macrophage cell. Cytotoxic effects of extracts by MTT assay against human liver (HPG2), prostate (LNCaP) and colon (HT-29) cancer. | [11,94,95,96,97,98,99,100,101,102,103,104] |
| F. vulgare Mill. | Antispasmodic and carminative effects. Promotes intestinal peristalsis. Diuretic action. Cures respiratory diseases as an expectorant. | Cirsiliol, 4-O-caffeoylquinic acid (4-CQA); vanillic acid; O-coumaric acid; rosmarinic acid; kaempferol; resveratrol; rutin; myricetin; catechin; quercetin. | Antioxidant; antimicrobial; anti-inflammatory. Protection against cardiovascular diseases, neurological disorders, and diabetes, and hepatoprotective effects. | [105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] |
| G. lutea L. | In folk medicine, it is known for its digestive and appetite-stimulating effects. Other uses include antipyretic, hepatoprotective, hypoglycemic, antianemic, and cardiotonic activity; for sores and minor wound healing; for stomach ulcers, as an immune stimulant. | Isovitexin, isosaponarin, isoorientin, isoorientin-2′-O-glucoside, and isoorientin-4′-O-glucoside | Anti-inflammatory properties, with the rate of enzyme inhibition increasing with time. | [121,122,123,124,125,126,127] |
| J. communis L. | Urinary antiseptic for acute and chronic cystitis; diuretic; emmenagogue; sudorific; digestive; anti-inflammatory. Used as a stimulant and disinfectant against constipation, chronic Bright’s disease, migraine, dropsy, rheumatic swellings, and infantile tuberculosis. | Quercetin, kaempferol, myricetin, isorhamnetin, and patuletin derivatives in their composition. Quinic acids, 5-O-caffeoylquinic, catechin, epicatechin, luteolin, apigenin, naringenin, amentoflavone, and their derivatives. | Antidiabetic, anti-inflammatory, antihypercholesterolemic, antihyperlipidemic, and hepatoprotective effects. Anticancer properties alleviate cardiovascular disorders. Anticataleptic activity alleviates neuropathologies and improves the mental state of individuals. | [128,129,130,131,132,133,134] |
| L. nobilis L. | In cooking recipes, it is used to provide an aroma and a spicy flavour to meat, fish, broths, and vegetables. It is a component of two typical Italian vegetable infusions: one used as a digestive, called ‘‘canarino’’, and one for the treatment of respiratory aliments, called Ricotto or Ricuotto. It is used in treatments for gastro-intestinal disorders, carminative, diarrhea, hemorrhoids, stomach aches, and kidney diseases. | Isoquercitrin, luteolin, rutin, apigenin derivatives, catechin, cinnamtannin B1, epicatechin hexoside, (+)-catechin, (−)-epicatechin, epigallocatechin, and methyl eugenol. Gallic; vanillic; rosmarinic acid; ferulic acid; coumaric acid. Costunolide; santamarine; reynosine. | Anti-inflammatory: reduction in lung inflammation caused by LPS and in skin lesions and inflammation caused by Propionobacterium acnes. | [135,136,137,138,139,140,141,142] |
| M. sylvestris L. | Used to treat various ailments, such as colds, antiseptic, colic, constipation, cough, cystitis, high fever, migraines, puerperal mastitis, stomachic, wounds, and abscesses. Leaves, flowers, fruits, roots, shoots, and seeds are applied in infusions, decoctions, poultices, liniments, lotions, baths, and gargles. | Gossypetin 3-sulphate-8-O-β-Dglucoside; hypolaetin 3′-sulphate; isoscutellarein 8-O-ß-D-glucuronpyranoside; hypolaetin 8-O-ß-D-glucuronopyranoside; 3-O-β-D-glucopyranosyl-8-O-β-D-glucuronopyranoside; hypolaetin 4′-methyl ether 8-O-β-D-glucuronopyranoside. | Antibacterial, anti-inflammatory, antioxidant, and anti-inflammatory activity on carragenin-induced edema in rats. Antiproliferative activity on cancer cell lines. Reduction in nephrotoxicity induced by gentamicin. | [11,143,144,145,146,147,148,149,150,151,152,153,154,155] |
| S. montana L. | Effective against colds, asthma, antitussive and expectorant, cough, bronchitis, and inflammation of the respiratory tract. | Rosmarinic acid, caffeic acid and its glycoside derivatives. Quercetin, catechin or luteolin derivatives. | High antimicrobial potential, together with antioxidant and anxiolytic capacity. Hepatoprotective effects, protection against cardiovascular ailments, and uses in cancer treatment. | [156,157,158,159,160,161] |
| S. marianum (L) Gaertn | Antihypertensive; stimulates milk production in rats and insect (flies) repellents. Used in the treatment of liver dysfunctions and gallbladder disorders, laxatives, and breast cancer treatment. | Flavonoids. Flavonolignan complex composed of isosilychristin, isosilybin A and isosilybin B, silybin A and silybin B, silychristin, silydianin, and taxifolin mariamide A and mariamide B (seeds). | Antidiabetic agent (α-glucosidase and PTP1B inhibitory activities), used in the treatment of chronic hepatitis, cirrhosis, and hepatic toxic lesions, with choleretic and cholagogue effects. Applied in Italy to treat liver and gastrointestinal disorders and as a laxative, with anti-inflammatory, antioxidant, cardiovascular protective, anti-cancer, and neuroprotective effects. | [11,162,163,164,165,166,167,168] |
| U. dioica L. | In folk medicine, it has been used to treat rheumatism, arthritis, gout, eczema, anemia, urinary tract infections, kidney stones, hay fever, and the early stages of an enlarged prostate. | 3-O-caffeoylquinic acid; 4-O-caffeoylquinic acid; 5-O-caffeoylquinic acid; caffeoylmalic acid; p-coumaroylmalic acid; quercetin O-rutinoside. | Antiviral, antimicrobial, antioxidant, anti-inflammatory, antiaging, and cytotoxic/anticancer effects, as well as benign prostatic, hyperplasia, antidiabetic, antiendometriosis, and nephroprotective effects. | [169,170,171] |
4.1. Ethnobotanical and Ethnomedicinal Relevance

4.2. Bioactive Phytochemicals
4.3. Evaluation of In Vitro Antioxidant Activity of Selected Edible Wild Plants
5. Therapeutic Potential of Selected Edible Wild Plants
5.1. Anti-Inflammatory Activity
5.2. Anti-Microbial Activity
5.3. Protection against Cardiovascular Diseases
5.4. Role of Wild Plants in Cancer Prevention and Treatment
5.5. Neurological Disorders and Wild Plants
5.6. Diabetes and Hepatoprotective Effects of Edible Wild Plants
5.7. Other Biological Activity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Paura, B.; Di Marzio, P.; Salerno, G.; Brugiapaglia, E.; Bufano, A. Design a Database of Italian Vascular Alimurgic Flora (AlimurgITA): Preliminary Results. Plants 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, G. Edible wild plants of Italy. Inf. Bot. Ital. 1987, 19, 17–30. [Google Scholar]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Conti, F.; Bartolucci, F. The Vascular Flora of the National Park of Abruzzo, Lazio and Molise (Central Italy); Springer: Cham, Switzerland, 2015. [Google Scholar]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Fortini, P.; Di Marzio, P.; Guarrera, P.M.; Iorizzi, M. Ethnobotanical study on the medicinal plants in the Mainarde Mountains (central-southern Apennine, Italy). J. Ethnopharmacol. 2016, 184, 208–218. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Sharma, P.; Garg, V.K.; Bansal, M. Role of nutraceuticals in health promotion. Int. J. Pharm. Tech. Res. 2011, 3, 442–448. [Google Scholar]
- Bommakanti, V.; Puthenparambil Ajikumar, A.; Sivi, C.M.; Prakash, G.; Mundanat, A.S.; Ahmad, F.; Haque, S.; Prieto, M.A.; Rana, S.S. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations 2023, 10, 177. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients 2023, 15, 2979. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Nutraceuticals, functional foods and dietary supplements in health and disease. J. Food Drug Anal. 2020, 20, 78. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.D.; Rawat, S.; Rawal, R.S. Antioxidants in Medicinal Plants. In Biotechnology for Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 295–326. [Google Scholar]
- Sdona, E.; Ekström, S.; Andersson, N.; Hallberg, J.; Rautiainen, S.; Håkansson, N.; Wolk, A.; Kull, I.; Melén, E.; Bergström, A. Fruit, vegetable and dietary antioxidant intake in school age, respiratory health up to young adulthood. Clin. Exp. Allergy 2021, 52, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Masullo, M.; Montoro, P.; Mari, A.; Pizza, C.; Piacente, S. Medicinal plants in the treatment of women’s disorders: Analytical strategies to assure quality, safety and efficacy. J. Pharm. Biomed. Anal. 2015, 113, 189–211. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavonoid Compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Saeed ur, R.; Bilal, M.; Huang, D.-f. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Lago, J.; Toledo-Arruda, A.; Mernak, M.; Barrosa, K.; Martins, M.; Tibério, I.; Prado, C. Structure-Activity Association of Flavonoids in Lung Diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Dewangan, R.P.; Kumar, A. Structure-Activity Relationship of Flavonoids: Recent Updates. In The Chemistry inside Spices & Herbs: Research and Development; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 237–259. [Google Scholar]
- Jiang, L.; Yanase, E.; Mori, T.; Kurata, K.; Toyama, M.; Tsuchiya, A.; Yamauchi, K.; Mitsunaga, T.; Iwahashi, H.; Takahashi, J. Relationship between flavonoid structure and reactive oxygen species generation upon ultraviolet and X-ray irradiation. J. Photochem. Photobiol. A Chem. 2019, 384, 112044. [Google Scholar] [CrossRef]
- Schloms, L.; Swart, A. Rooibos Flavonoids Inhibit the Activity of Key Adrenal Steroidogenic Enzymes, Modulating Steroid Hormone Levels in H295R Cells. Molecules 2014, 19, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Singh, P.; Mishra, S.K.; Noel, S.; Sharma, S.; Rath, S.K. Acute Exposure of Apigenin Induces Hepatotoxicity in Swiss Mice. PLoS ONE 2012, 7, e31964. [Google Scholar] [CrossRef]
- Peterson, G.L. Review of the folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Choi, S.-W. Antioxidants in Food. In Advances in Food and Nutrition Research; Academic Press is an imprint of Elsevier: Waltham, MA, USA; Volume 71, pp. 1–53.
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Esin Çelik, S.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, G.F.; Menshov, V.A.; Trofimov, A.V.; Vasil’ev, R.F. Facile chemiluminescence assay for antioxidative properties of vegetable lipids: Fundamentals and illustrative examples. Analyst 2009, 134, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Işıl Berker, K.; Güçlü, K.; Tor, İ.; Demirata, B.; Apak, R. Total Antioxidant Capacity Assay Using Optimized Ferricyanide/Prussian Blue Method. Food Anal. Methods 2009, 3, 154–168. [Google Scholar] [CrossRef]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41–R55. [Google Scholar] [CrossRef] [PubMed]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Rajashekaraiah, V. Ferric reducing ability of plasma: A potential oxidative stress marker in stored plasma. Acta Haematol. Pol. 2021, 52, 61–67. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Redox Regulation of γ-Glutamyl Transpeptidase. Am. J. Respir. Cell Mol. Biol. 2009, 41, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kalinovic, S.; Stamm, P.; Oelze, M.; Daub, S.; Kröller-Schön, S.; Kvandova, M.; Steven, S.; Münzel, T.; Daiber, A. Comparison of three methods for in vivo quantification of glutathione in tissues of hypertensive rats. Free Radic. Res. 2021, 55, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev. 2007, 6, 3–14. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2011, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Valentino, G.; Graziani, V.; D’Abrosca, B.; Pacifico, S.; Fiorentino, A.; Scognamiglio, M. NMR-Based Plant Metabolomics in Nutraceutical Research: An Overview. Molecules 2020, 25, 1444. [Google Scholar] [CrossRef] [PubMed]
- Nagana Gowda, G.A.; Raftery, D. Recent Advances in NMR-Based Metabolomics. Anal. Chem. 2016, 89, 490–510. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiu, S.; Cai, Y.; Wang, Z.; Yang, Q.; Tang, S.; Xie, Y.; Zhang, A. Mass spectrometry-based metabolomics for discovering active ingredients and exploring action mechanism of herbal medicine. Front. Chem. 2023, 11, 1142287. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.A.A.; Tenori, L.; Licari, C.; Luchinat, C. Practical considerations for rapid and quantitative NMR-based metabolomics. J. Magn. Reson. 2023, 352, 107462. [Google Scholar] [CrossRef] [PubMed]
- Saviano, G.; Paris, D.; Iorizzi, M. Editorial: Exploring metabolomic diversity in plant species by NMR-based and mass-based spectrometry. Front. Plant Sci. 2023, 14, 1248781. [Google Scholar] [CrossRef] [PubMed]
- Takis, P.G.; Ghini, V.; Tenori, L.; Turano, P.; Luchinat, C. Uniqueness of the NMR approach to metabolomics. TrAC Trends Anal. Chem. 2019, 120, 115300. [Google Scholar] [CrossRef]
- Wishart, D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D.; Theodoridis, G.; Virgiliou, C. A perspective on the standards describing mass spectrometry-based metabolic phenotyping (metabolomics/metabonomics) studies in publications. J. Chromatogr. B 2021, 1164, 122515. [Google Scholar] [CrossRef] [PubMed]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2013, 10, 361–374. [Google Scholar] [CrossRef]
- Vélez-Gavilán, J. Achillea Millefolium (Yarrow); CABI Compendium: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Available online: https://antropocene.it/en/2022/10/25/achillea-millefolium-en/ (accessed on 1 March 2024).
- Borago Officinalis (Borage); PlantwisePlus Knowledge Bank; CABI Compendium: Wallingford, UK, 2022. [CrossRef]
- Available online: https://antropocene.it/en/2022/10/25/borago-officinalis-en/ (accessed on 1 March 2024).
- Datiles, M.J.; Popay, I. Foeniculum Vulgare (Fennel); CABI Compendium: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Available online: https://antropocene.it/en/2022/10/25/foeniculum-vulgare-en/ (accessed on 1 March 2024).
- Available online: https://www.conifers.org/cu/Juniperus_communis.php (accessed on 1 March 2024).
- Available online: https://antropocene.it/en/2022/11/26/juniperus-communis-2/ (accessed on 1 March 2024).
- Available online: https://antropocene.it/en/2022/10/26/malva-sylvestris-en/ (accessed on 1 March 2024).
- CABI Compendium: Wallingford, UK, 2021. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.115485 (accessed on 1 March 2024).
- Available online: https://antropocene.it/en/2022/12/13/gentiana-lutea-2/ (accessed on 1 March 2024).
- CABI Compendium: Wallingford, UK, 2019. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.114518 (accessed on 1 March 2024).
- Alessi, N.; Wellstein, C.; Spada, F.; Zerbe, S. Population structure of Laurus nobilis L. in Central Italian forests: Evidence for its ongoing expansion. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 365–376. [Google Scholar] [CrossRef]
- Available online: https://antropocene.it/en/2022/10/29/laurus-nobilis-en/ (accessed on 1 March 2024).
- Available online: https://antropocene.it/en/2022/12/20/satureja-montana-2/ (accessed on 1 March 2024).
- Popay, I. Silybum marianum (Variegated Thistle); CABI Compendium: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Available online: https://antropocene.it/en/2022/12/19/silybum-marianum-2/ (accessed on 1 March 2024).
- Halder, S.; Sharma, A. A review on Urtica dioica L. World J. Pharm. Pharm. Sci. 2017, 6, 404–421. [Google Scholar] [CrossRef]
- Available online: https://antropocene.it/en/2022/10/26/urtica-dioica-en/ (accessed on 1 March 2024).
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Shah, R.; Peethambaran, B. Anti-inflammatory and Anti-microbial Properties of Achillea millefolium in Acne Treatment. In Immunity and Inflammation in Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 241–248. [Google Scholar]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, J.; Radulović, N.; Zlatković, B.; Palić, R. Composition of Achillea distans Willd. subsp. distansroot essential oil. Nat. Prod. Res. 2010, 24, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Fierascu, R.C.; Ortan, A.; Soare, L.C.; Paunescu, A. In vitro antioxidant and antifungal properties of Achillea millefolium L. Rom. Biotechnol. Lett. 2015, 20, 10626–10636. [Google Scholar]
- Gihan, F.A.; Alaa, Q.R.; Alaa, O.H.; Fatimah, Y.G.; Yousra, N.; Saeed, A. Potential Analgesic and Anti-Inflammatory Effect of Cuminum Cyminum and Borago officinalis in Rats and Mice. Asian J. Pharm. Clin. Res. 2019, 13, 216–218. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Baena, M.-D.; Tasset, I.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; de Haro-Bailón, A. Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants. Nutrients 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef] [PubMed]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional plant use in the areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Amiri, M.S.; Zibartee, E.; Boghrati, Z.; Ayati, Z.; Sahebkar, A.; Emami, S.A. A Review on the Phytochemistry, Ethnobotanical Uses and Pharmacology of Borago Species. Curr. Pharm. Des. 2020, 26, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab. J. Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- Fabrikov, D.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Rodríguez-García, I.; Gómez-Mercado, F.; Urrestarazu, M.; Lao, M.T.; Rincón-Cervera, M.Á.; Álvaro, J.E.; Lyashenko, S. Borage oil: Tocopherols, sterols and squalene in farmed and endemic-wild Borago species. J. Food Compos. Anal. 2019, 83, 103299. [Google Scholar] [CrossRef]
- Gilani, A.H.; Bashir, S.; Khan, A.-u. Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J. Ethnopharmacol. 2007, 114, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Scholarly vs. Traditional Knowledge: Effects of Sacred Natural Sites on Ethnobotanical Practices in Tuscany, Central Italy. Hum. Ecol. 2019, 47, 653–667. [Google Scholar] [CrossRef]
- Motti, R.; Motti, P. An Ethnobotanical Survey of Useful Plants in the Agro Nocerino Sarnese (Campania, Southern Italy). Hum. Ecol. 2017, 45, 865–878. [Google Scholar] [CrossRef]
- Menale, B.; De Castro, O.; Cascone, C.; Muoio, R. Ethnobotanical investigation on medicinal plants in the Vesuvio National Park (Campania, Southern Italy). J. Ethnopharmacol. 2016, 192, 320–349. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Khammassi, M.; Mighri, H.; Ben Mansour, M.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations growing wild in Tunisia. S. Afr. J. Bot. 2022, 148, 407–414. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Milenković, A.; Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Lalević, D.; Stanojević, J.; Danilović, B.; Cvetković, D. Essential Oil Yield, Composition, Antioxidant and Microbial Activity of Wild Fennel (Foeniculum vulgare Mill.) from Monte Negro Coast. Horticulturae 2022, 8, 1015. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, Antibiofilm, and Antioxidant Properties of Essential Oil of Foeniculum vulgare Mill. Leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. A Comparative UHPLC-Q-Trap-MS/MS-Based Metabolomics Analysis to Distinguish Foeniculum vulgare Cultivars’ Antioxidant Extracts. Molecules 2023, 28, 900. [Google Scholar] [CrossRef]
- Šunić, L.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Stanojević, J.; Kovač, R.; Milenković, A.; Cvetković, D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae 2023, 9, 364. [Google Scholar] [CrossRef]
- Križman, M.; Jakše, J. Chemical and Genetic Variability of Istrian Foeniculum vulgare Wild Populations. Plants 2022, 11, 2239. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Vukosavljević, P.; Đurović, S.; Antić, M.; Gorjanović, S. New herbal bitter liqueur with high antioxidant activity and lower sugar content: Innovative approach to liqueurs formulations. J. Food Sci. Technol. 2019, 56, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Tonutti, I.; Liddle, P. Aromatic plants in alcoholic beverages. A review. Flavour Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; de Falco, B. Wild and cultivated plants used in traditional alcoholic beverages in Italy: An ethnobotanical review. Eur. Food Res. Technol. 2022, 248, 1089–1106. [Google Scholar] [CrossRef]
- Esquivel-Ferriño, P.C.; Favela-Hernández, J.M.J.; Garza-González, E.; Waksman, N.; Ríos, M.Y.; Camacho-Corona, M.d.R. Antimycobacterial Activity of Constituents from Foeniculum vulgare Var. Dulce Grown in Mexico. Molecules 2012, 17, 8471–8482. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Naychov, Z.; Batovska, D.; Kerr, P.; Favre, A.; Dimitrov, V.; Aneva, I.; Hamedi, A.; Kozuharova, E. Some European Gentiana Species Are Used Traditionally to Cure Wounds: Bioactivity and Conservation Issues. Diversity 2023, 15, 467. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E.; Quave, C.L. Cross-Cultural Ethnobiology in the Western Balkans: Medical Ethnobotany and Ethnozoology Among Albanians and Serbs in the Pešter Plateau, Sandžak, South-Western Serbia. Hum. Ecol. 2011, 39, 333–349. [Google Scholar] [CrossRef]
- Marković, M.; Pljevljakušić, D.; Menković, N.; Matejić, J.; Papović, O.; Stankov-Jovanović, V. Traditional knowledge on the medicinal use of plants from genus Gentiana in the Pirot County (Serbia). Lek. Sirovine 2021, 41, 46–53. [Google Scholar] [CrossRef]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, M.; Lela, L.; Moles, M.; Mangieri, C.; Bisaccia, D.; Faraone, I.; Falabella, R.; Milella, L. The healing bitterness of Gentiana lutea L., phytochemistry and biological activities: A systematic review. Phytochemistry 2023, 206, 113518. [Google Scholar] [CrossRef] [PubMed]
- Łukasz Mikołajczak, P.; Kędzia, B.; Ożarowski, M.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Białas, W.; Gryszczyńska, A.; Buchwald, W.; Szulc, M.; et al. Evaluation of anti-inflammatory and analgesic activities of extracts from herb of Chelidonium majus L. Cent. Eur. J. Immunol. 2015, 4, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Taranalli, A.D.; Torgal, S.S. Evaluation of Anti-inflammatory and Wound Healing Activity of Gentiana lutea Rhizome Extracts in Animals. Pharm. Biol. 2008, 42, 8–12. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mihasan, M.; Hritcu, L. Anti-acetylcholinesterase and Antioxidant Activities of Inhaled Juniper Oil on Amyloid Beta (1–42)-Induced Oxidative Stress in the Rat Hippocampus. Neurochem. Res. 2015, 40, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Flores-Félix, J.; Coutinho, P.; Alves, G.; Silva, L. Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends. Int. J. Mol. Sci. 2022, 23, 3197. [Google Scholar] [CrossRef] [PubMed]
- Falasca, A.; Melck, D.; Paris, D.; Saviano, G.; Motta, A.; Iorizzi, M. Seasonal changes in the metabolic fingerprint of Juniperus communis L. berry extracts by 1H NMR-based metabolomics. Metabolomics 2013, 10, 165–174. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-W.; Huang, X.-F.; Yang, T.-P.; Chang, K.-F.; Yeh, L.-W.; Hsieh, M.-C.; Weng, J.-C.; Tsai, N.-M. Juniperus communis suppresses melanoma tumorigenesis by inhibiting tumor growth and inducing apoptosis. Am. J. Chin. Med. 2019, 47, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Falasca, A.; Caprari, C.; De Felice, V.; Fortini, P.; Saviano, G.; Zollo, F.; Iorizzi, M. GC-MS analysis of the essential oils of Juniperus communis L. berries growing wild in the Molise region: Seasonal variability and in vitro antifungal activity. Biochem. Syst. Ecol. 2016, 69, 166–175. [Google Scholar] [CrossRef]
- Anzano, A.; de Falco, B.; Grauso, L.; Motti, R.; Lanzotti, V. Laurel, Laurus nobilis L.: A review of its botany, traditional uses, phytochemistry and pharmacology. Phytochem. Rev. 2022, 21, 565–615. [Google Scholar] [CrossRef]
- Staub, P.O.; Geck, M.S.; Weckerle, C.S.; Casu, L.; Leonti, M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J. Ethnopharmacol. 2015, 174, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.R.; Omer, A.K.; Yener, Z.; Uyar, A.; Ahmed, A.K. Biomedical effects of Laurus nobilis L. leaf extract on vital organs in streptozotocin-induced diabetic rats: Experimental research. Ann. Med. Surg. 2021, 61, 188–197. [Google Scholar] [CrossRef]
- Baydoun, S.A.; Kanj, D.; Raafat, K.; Aboul Ela, M.; Chalak, L.; Arnold-Apostolides, N. Ethnobotanical and Economic Importance of Wild Plant Species of Jabal Moussa Bioreserve, Lebanon. J. Ecosyst. Ecography 2017, 7, 1000245. [Google Scholar] [CrossRef]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis Leaves and Fruits: A Review of Metabolite Composition and Interest in Human Health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- Shukla, R.; Jain, N.; Rai, G.; Pandey, V.; Mourya, P.; Pal, B. Unlocking the potential of bay leaf: Exploring its role as a nutraceutical carrier through ethnomedicinal and pharmacological insights Drug Pharm. Sci. Arch. 2023, 3, 13–23. [Google Scholar]
- Chahal, K.K.; Kaur, M.; Bhardwaj, U.; Singla, N.; Kaur, A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. J. Pharmacogn. Phytochem. 2017, 6, 1153–1161. [Google Scholar]
- De Marino, S.; Borbone, N.; Zollo, F.; Ianaro, A.; Di Meglio, P.; Iorizzi, M. Megastigmane and Phenolic Components from Laurus nobilis L. Leaves and Their Inhibitory Effects on Nitric Oxide Production. J. Agric. Food Chem 2004, 52, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Ghane, M.; Pishkar, L. Phytochemical Composition, Antibacterial, and Antibiofilm Activity of Malva sylvestris Against Human Pathogenic Bacteria. Jundishapur J. Nat. Pharm. Prod. 2021, 17, e114164. [Google Scholar] [CrossRef]
- Billeter, M.; Meier, B.; Sticher, O. 8-hydroxyflavonoid glucuronides from Malva sylvestris. Phytochemistry 1991, 30, 987–990. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, A.; Yang, Z.; Wu, J.; Li, X.; Bao, K.; Wang, M.; Wu, B. Malva sylvestris extract alleviates the astrogliosis and inflammatory stress in LPS-induced depression mice. J. Neuroimmunol. 2019, 336, 577029. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Yarijani, Z.; Najafi, H.; Shackebaei, D.; Madani, S.H.; Modarresi, M.; Jassemi, S.V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed. Pharmacother. 2019, 112, 108635. [Google Scholar] [CrossRef] [PubMed]
- Chiclana, C.F.; Enrique, A.; Consolini, A.E. Actividad antiinflamatoria local de Malva sylvestris L. (Malvaceae) en el edema inducido por carragenina en ratas. Lat. Am. J. Pharm. 2009, 28, 275–278. [Google Scholar]
- Camejo-Rodrigues, J.; Ascensão, L.; Bonet, M.À.; Vallès, J. An ethnobotanical study of medicinal and aromatic plants in the Natural Park of “Serra de São Mamede” (Portugal). J. Ethnopharmacol. 2003, 89, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Lucia, L.M. Ethnobotanical remarks on Central and Southern Italy. J. Ethnobiol. Ethnomed. 2007, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Pieroni, A.; Bennett, B.C. Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy. J. Ethnobiol. Ethnomed. 2008, 4, 5. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2015, 54, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Oalđe Pavlović, M.; Kolarević, S.; Đorđević, J.; Jovanović Marić, J.; Lunić, T.; Mandić, M.; Kračun Kolarević, M.; Živković, J.; Alimpić Aradski, A.; Marin, P.D.; et al. A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species. Plants 2021, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.; Pljevljakušić, D.; Matejić, J.; Nikolić, B.; Smiljić, M.; Ðelić, G.; Papović, O.; Ðokić, M.; Stankov-Jovanović, V. The plants traditionally used for the treatment of respiratory infections in the Balkan Peninsula (Southeast Europe). Lek. Sirovine 2022, 42, 68–88. [Google Scholar] [CrossRef]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009, 32, 328–334. [Google Scholar] [CrossRef]
- Cavalloro, V.; Robustelli della Cuna, F.S.; Quai, E.; Preda, S.; Bracco, F.; Martino, E.; Collina, S. Walking around the Autonomous Province of Trento (Italy): An Ethnobotanical Investigation. Plants 2022, 11, 2246. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Aviello, G.; Capasso, F.; Savino, F.; Izzo, A.A.; Lembo, F.; Borrelli, F. Silymarin BIO-C®, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine 2009, 16, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Csupor, D.; Csorba, A.; Hohmann, J. Recent advances in the analysis of flavonolignans of Silybum marianum. J. Pharm. Biomed. Anal. 2016, 130, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Hanlidou, E.; Karousou, R.; Kleftoyanni, V.; Kokkini, S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 2004, 91, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The food plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and clinical evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- Piluzza, G.; Virdis, S.; Serralutzu, F.; Bullitta, S. Uses of plants, animal and mineral substances in Mediterranean ethno-veterinary practices for the care of small ruminants. J. Ethnopharmacol. 2015, 168, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete Isolation and Characterization of Silybins and Isosilybins from Milk Thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Quave, C.L.; Santoro, R.F. Folk pharmaceutical knowledge in the territory of the Dolomiti Lucane, inland southern Italy. J. Ethnopharmacol. 2004, 95, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Kk, M.; Parsuraman, S. Urtica dioica L., (Urticaceae): A Stinging Nettle. Syst. Rev. Pharm. 2014, 5, 6–8. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica -Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Akram, M. Minireview on Achillea millefolium Linn. J. Membr. Biol. 2013, 246, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Arak, E.; Raal, A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European Countries. Nat. Prod. Res. 2007, 20, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Paura, B.; Cozzolino, A.; de Falco, B. Edible Flowers Used in Some Countries of the Mediterranean Basin: An Ethnobotanical Overview. Plants 2022, 11, 3272. [Google Scholar] [CrossRef]
- Available online: https://www.atlantides.it/gentiana-lutea-subsp.-lutea.html (accessed on 1 March 2024).
- Acta Plantarum, from 2007 on—“Lista Delle Schede Botaniche. Available online: https://www.actaplantarum.org/schede/schede.php (accessed on 1 March 2024).
- Available online: http://www.lucolifloraefauna.it/Flora/Schede/Satureja_montana_montana.htm (accessed on 1 March 2024).
- CABI Compendium: Wallingford, UK, 2013. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.50304 (accessed on 1 March 2024).
- Popay, I. Urtica Dioica (Stinging Nettle); CABI Compendium: Wallingford, UK, 2014. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Khammassi, M.; Loupassaki, S.; Tazarki, H.; Mezni, F.; Slama, A.; Tlili, N.; Zaouali, Y.; Mighri, H.; Jamoussi, B.; Khaldi, A. Variation in essential oil composition and biological activities of Foeniculum vulgare Mill. populations growing widely in Tunisia. J. Food Biochem. 2018, 42, e12532. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The flavour of fruit spirits and fruit liqueurs: A review. Flavour Fragr. J. 2015, 30, 197–207. [Google Scholar] [CrossRef]
- Prakash, O.; Singh, R.; Kumar, S.; Srivastava, S.; Ved, A. Gentiana lutea Linn. (Yellow Gentian): A comprehensive review. J. Ayurvedic Herb. Med. 2017, 3, 175–181. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.L.; Zhang, J.; Li, W.Y.; Wang, Y.Z. Phytochemistry and Pharmacological Activities of the Genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Maggi, F.; Öztürk, N.; Öztürk, Y.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. Chemical and biological analysis of the by-product obtained by processing Gentiana lutea L. and other herbs during production of bitter liqueurs. Ind. Crops Prod. 2016, 80, 131–140. [Google Scholar] [CrossRef]
- Fejér, J.; Gruľová, D.; Eliašová, A.; Kron, I.; De Feo, V. Influence of environmental factors on content and composition of essential oil from common juniper ripe berry cones (Juniperus communis L.). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 1227–1235. [Google Scholar] [CrossRef]
- Verheyen, K.; Adriaenssens, S.; Gruwez, R.; Michalczyk, I.M.; Ward, L.K.; Rosseel, Y.; Van den Broeck, A.; García, D. Juniperus communis: Victim of the combined action of climate warming and nitrogen deposition? Plant Biol. 2009, 11, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Gill, N.S.; Kumar, N. Neuroprotective Effect of Juniperus communis on Chlorpromazine Induced Parkinson Disease in Animal Model. Chin. J. Biol. 2015, 2015, 542542. [Google Scholar] [CrossRef]
- Guedri, M.M.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Chemical composition and antimicrobial and antioxidant activities of Tunisian, France and Austrian Laurus nobilis (Lauraceae) essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca. 2020, 48, 1929–1940. [Google Scholar] [CrossRef]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D.J.H. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Tene, S.T.; Teibo, J.O.; Shaheen, H.M.; Oluwatoba, O.S.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; Papadakis, M. The phytochemical profiling, pharmacological activities, and safety of Malva sylvestris: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 396, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Duggan, K.C.; Hermanson, D.J.; Musee, J.; Prusakiewicz, J.J.; Scheib, J.L.; Carter, B.D.; Banerjee, S.; Oates, J.A.; Marnett, L.J. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 2011, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, R. New Dry Grassland Associations from the Ausoni-Aurunci Mountains (Central Italy)—Syntaxonomical Updating and Discussion on the Higher Rank Syntaxa. Hacquetia 2011, 10, 183–231. [Google Scholar] [CrossRef]
- Caprioli, G.; Lupidi, G.; Maggi, F. Comparison of chemical composition and antioxidant activities of two Winter savory subspecies (Satureja montana subsp. variegata and Satureja montana subsp. montana) cultivated in Northern Italy. Nat. Prod. Res. 2018, 33, 3143–3147. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, V.; Silletti, G.; Forte, L. A new association of Satureja montana L. subsp. montana dominated garrigues in Puglia (SE Italy). Plant Sociol. 2021, 58, 1–14. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Andrés, M.F.; Díaz, C.E.; Burillo, J.; González-Coloma, A. Composition and biocidal properties of essential oil from pre-domesticated Spanish Satureja montana. Ind. Crops Prod. 2020, 145, 111958. [Google Scholar] [CrossRef]
- Chizzola, R. Volatile Oil Composition of Four Populations of Satureja montana L. From Southern France. Acta Hortic. 2003, 598, 143–147. [Google Scholar] [CrossRef]
- Bojović, D.; Šoškić, M.; Tadić, V. Comparative study of chemical composition of the essential oils from Satureja cuneifolia ten. and Satureja montana l., lamiaceae collected at national park Lovćen, Montenegro. Stud. Univ. Babeș-Bolyai Chem. 2018, 63, 167–180. [Google Scholar] [CrossRef]
- Lumpert, M.; Kreft, S. Folk use of medicinal plants in Karst and Gorjanci, Slovenia. J. Ethnobiol. Ethnomed. 2017, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Košir, I.; Končić, M.; Čerenak, A.; Potočnik, T.; Srečec, S.; Randić, M.; Kosalec, I. Antimicrobial and Antioxidant Properties of Satureja montana L. and S. Subspicata Vis. (Lamiaceae). Curr. Drug Targets 2015, 16, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Todosijević, M.; Varga, A.; Kiprovski, B.; Tešević, V.; Čabarkapa, I.; Sikora, V. Bioactivity of essential oils from cultivated winter savory, sage and hyssop. Lek. Sirovine 2019, 39, 11–17. [Google Scholar] [CrossRef]
- ČOpra-JanİĆİJevİĆ, A.; VİDİC, D.; MaksİMovİĆ, M. Chemical composition of the essential oil and headspace of Satureja montana L. Nat. Volatiles Essent. 2020, 7, 22–34. [Google Scholar] [CrossRef]
- Souto-Maior, F.N.; Da Fonsêca, D.V.; Salgado, P.R.R.; de Oliveira Monte, L.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2016, 55, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kustrak, D.; Kuftinec, J.; Blazevic, N.; Maffei, M. Comparison of the Essential Oil Composition of Two Subspecies of Satureja montana. J. Essent. Oil Res. 1996, 8, 7–13. [Google Scholar] [CrossRef]
- Taoudiat, A.; Spigno, G.; Ferhat, Z.; Djenane, D. Bioenrichment using Satureja montana L. essential oil for the prevention against photooxidation of flavored extra virgin olive oil during light display. N. Afr. J. Food Nutr. Res. 2021, 4, 351–359. [Google Scholar] [CrossRef]
- Babajafari, S.; Nikaein, F.; Mazloomi, S.M.; Zibaeenejad, M.J.; Zargaran, A. A Review of the Benefits of Satureja Species on Metabolic Syndrome and Their Possible Mechanisms of Action. J. Evid.-Based Complement. Altern. Med. 2015, 20, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W. The use of community herbal monographs to facilitate registrations and authorisations of herbal medicinal products in the European Union 2004–2012. J. Ethnopharmacol. 2014, 158, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Falconieri, D.; Piras, A.; Porcedda, S.; Marongiu, B.; Gonçalves, M.J.; Cabral, C.; Cavaleiro, C.; Salgueiro, L. Chemical Composition and Biological Activity of the Volatile Extracts of Achillea millefolium. Nat. Prod. Commun. 2011, 6, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Abd-Elraouf, M.; Sulaiman, G.M.; Almahmoud, S.A.; Hamada, F.A.; Khan, R.A.; Hegazy, M.M.; Abd-El-Wahab, M.F.; Kedra, T.A.; Ismail, A. Variability in the volatile constituents and biological activities of Achillea millefolium L. essential oils obtained from different plant parts and by different solvents. Arab. J. Chem. 2023, 16, 105103. [Google Scholar] [CrossRef]
- Chaouche, T.A.; Karim, A.; Mourad, B. Phytochemical screening of Algerian Borago officinalis L. and evaluation of its antioxidant and antimicrobial activities against respiratory pathogens. Int. J. Phytomed. 2014, 6, 369–376. [Google Scholar]
- Samy, M.N.; Hamed, A.N.E.-S.; Sugimoto, S.; Otsuka, H.; Kamel, M.S.; Matsunami, K. Officinalioside, a new lignan glucoside from Borago officinalis L. Nat. Prod. Res. 2015, 30, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. J. Food Meas. Charact. 2017, 12, 826–838. [Google Scholar] [CrossRef]
- Herrmann, M.; Joppe, H.; Schmaus, G. Thesinine-4′-O-β-d-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. Phytochemistry 2002, 60, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Montaner, C.; Zufiaurre, R.; Movila, M.; Mallor, C. Evaluation of Borage (Borago officinalis L.) Genotypes for Nutraceutical Value Based on Leaves Fatty Acids Composition. Foods 2021, 11, 16. [Google Scholar] [CrossRef]
- Bowes, K.M.; Zheljazkov, V.D.J.H. Essential oil yields and quality of fennel grown in Nova Scotia. HortScience 2004, 39, 1640–1643. [Google Scholar] [CrossRef]
- Shehata, A.M.; Elhafez, Z.A.A.; Ahmed, A.F. A Comparative Study of the Response of Fennel (Foeniculum vulgare, Mill) Plants from Egypt and China to Spraying with Benzyladenine (BA). Eur. J. Med. Plants 2022, 33, 1–12. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef] [PubMed]
- Šavikin, K.; Menković, N.; Zdunić, G.; Stević, T.; Radanović, D.; Janković, T. Antimicrobial Activity of Gentiana lutea L. Extracts. Z. Naturforschung C 2009, 64, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Caprioli, G.; Dikmen, M.; Kaya, E.; Maggi, F.; Sagratini, G.; Vittori, S.; Öztürk, Y. Evaluation of neuritogenic activity of cultivated, wild and commercial roots of Gentiana lutea L. J. Funct. Foods 2015, 19, 164–173. [Google Scholar] [CrossRef]
- Radanovic, D.; Antic-Mladenovic, S.; Jakovljevic, M.; Kresovic, M. Content of heavy metals in Gentiana lutea L. roots and galenic forms. J. Serbian Chem. Soc. 2007, 72, 133–138. [Google Scholar] [CrossRef]
- Cafaro, T.; Carnicelli, V.; Caprioli, G.; Maggi, F.; Celenza, G.; Perilli, M.; Bozzi, A.; Amicosante, G.; Brisdelli, F. Anti-apoptotic and anti-inflammatory activity of Gentiana lutea root extract. Adv. Tradit. Med. 2020, 20, 619–630. [Google Scholar] [CrossRef]
- Balijagić, J.; Janković, T.; Zdunić, G.; Bošković, J.; Šavikin, K.; Goćevac, D.; Stanojković, T.; Jovančević, M.; Menković, N. Chemical Profile, Radical Scavenging and Cytotoxic Activity of Yellow Gentian Leaves (Genitaneae Luteae Folium) Grown in Northern Regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.M.; Kijjoa, A. Naturally-Occurring Xanthones: Recent Developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef] [PubMed]
- Citová, I.; Ganzera, M.; Stuppner, H.; Solich, P. Determination of gentisin, isogentisin, and amarogentin in Gentiana lutea L. by capillary electrophoresis. J. Sep. Sci. 2008, 31, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.C.; Gulliya, B. Chemistry and Pharmacology of Flavonoids—A Review. Indian J. Pharm. Educ. Res. 2019, 53, 8–20. [Google Scholar] [CrossRef]
- Živić, N.; Milošević, S.; Dekić, V.; Dekić, B.; Ristić, N.; Ristić, M.; Sretić, L. Phytochemical and antioxidant screening of some extracts of Juniperus communis L. and Juniperus oxycedrus L. Czech J. Food Sci 2019, 37, 351–358. [Google Scholar] [CrossRef]
- Lima Reis, P.M.C.; Mezzomo, N.; Aguiar, G.P.S.; Hotza, D.; Baggio Ribeiro, D.H.; Salvador Ferreira, S.R.; Hense, H. Formation, stability and antimicrobial activity of laurel leaves essential oil (Laurus nobilis L.) particles in suspension obtained by SFEE. J. Supercrit. Fluids 2020, 166, 105032. [Google Scholar] [CrossRef]
- Belasli, A.; Ben Miri, Y.; Aboudaou, M.; Aït Ouahioune, L.; Montañes, L.; Ariño, A.; Djenane, D. Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): Potential use as wheat preservative. Food Sci. Nutr. 2020, 8, 4717–4729. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Nazzaro, F.; Souza, L.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Comparison of Chemical Composition and Biological Properties of Essential Oils Obtained by Hydrodistillation and Steam Distillation of Laurus nobilis L. Plant Foods Hum. Nutr. 2020, 75, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Panza, E.; Tersigni, M.; Iorizzi, M.; Zollo, F.; De Marino, S.; Festa, C.; Napolitano, M.; Castello, G.; Ialenti, A.; Ianaro, A. Lauroside B, a Megastigmane Glycoside from Laurus nobilis (Bay Laurel) Leaves, Induces Apoptosis in Human Melanoma Cell Lines by Inhibiting NF-κB Activation. J. Nat. Prod. 2010, 74, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; El Dein, A.; El Sherbeiny, A.; El Ansari, M.A.; El Sissi, H.I. Two new sulphated flavonol glucosides from leaves of Malva sylvestris. Phytochemistry 1977, 16, 145–146. [Google Scholar] [CrossRef]
- Nawwar, M.A.M.; Buddrus, J. A gossypetin glucuronide sulphate from the leaves of Malva sylvestris. Phytochemistry 1981, 20, 2446–2448. [Google Scholar] [CrossRef]
- Brouillard, R.J.P. The in vivo expression of anthocyanin colour in plants. Phytochemistry 1983, 22, 1311–1323. [Google Scholar] [CrossRef]
- Merlin, J.-C.; Statoua, A.; Brouillard, R. Investigation of the in vivo organization of anthocyanins using resonance raman microspectrometry. Phytochemistry 1985, 24, 1575–1581. [Google Scholar] [CrossRef]
- Farina, A.; Doldo, A.; Cotichini, V.; Rajevic, M.; Quaglia, M.G.; Mulinacci, N.; Vincieri, F.F. HPTLC and reflectance mode densitometry of anthocyanins in Malva Sylvestris L.: A comparison with gradient-elution reversed-phase HPLC. J. Pharm. Biomed. Anal. 1995, 14, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C. Effect of polysaccharides on the colour of anthocyanins. Food Chem. 1995, 54, 315–319. [Google Scholar] [CrossRef]
- Sikorska, M.; Matławska, I.; Fra ski, R. 8-Hydroxyflavonoid glucuronides of Malope trifida. Acta Physiol. Plant. 2004, 26, 291–297. [Google Scholar] [CrossRef]
- Takeda, K.; Enoki, S.; Harborne, J.B.; Eagles, J. Malonated anthocyanins in malvaceae: Malonylmalvin from Malva sylvestris. Phytochemistry 1989, 28, 499–500. [Google Scholar] [CrossRef]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef]
- Emets, T.I.; Steblyuk, M.V.; Klyuev, N.A.; Petrenko, V.V. Some components of the seed oil of Malva sylvestris. Chem. Nat. Compd. 1994, 30, 292–294. [Google Scholar] [CrossRef]
- Mukarram, M.; Ahmad, I.; Ahmad, M. HBr-Reactive acids of Malva sylvestris seed oil. J. Am. Oil Chem. Soc. 1984, 61, 1060. [Google Scholar] [CrossRef]
- Cutillo, F.; Dabrosca, B.; Dellagreca, M.; Fiorentino, A.; Zarrelli, A. Terpenoids and phenol derivatives from Malva sylvestris. Phytochemistry 2006, 67, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Paul, Z.A.; Malla, A.T.; Dar, M.A.; Masoodi, M.H. Phytochemistry and Pharmacological Activity of Malva sylvestris L: A Detailed Insight. Comb. Chem. High Throughput Screen. 2023; 26, Online ahead of print. [Google Scholar] [CrossRef]
- Gladikostić, N.; Ikonić, B.; Teslić, N.; Zeković, Z.; Božović, D.; Putnik, P.; Bursać Kovačević, D.; Pavlić, B. Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants 2023, 12, 745. [Google Scholar] [CrossRef]
- Milojevic, S.; Radosavljevic, D.; Pavicevic, V.; Pejanovic, S.; Veljkovic, V. Modeling the kinetics of essential oil hydrodistillation from plant materials. Hem. Ind. 2013, 67, 843–859. [Google Scholar] [CrossRef]
- Baydar, H.; Sağdiç, O.; Özkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations. Pharmaceutics 2019, 12, 7. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Yankova-Tsvetkova, E.; Zheljazkov, V.D.; Koleva-Valkova, L.H.; Nikolova, R. Reproductive Capacity and Scanning Electron Microscopy (SEM) Analyses of the Micromorphological Surfaces of Three Endemic Satureja Species from Bulgaria. Plants 2023, 12, 2436. [Google Scholar] [CrossRef] [PubMed]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 130454. [Google Scholar] [CrossRef] [PubMed]
- Velicković, J.M.; Kostić, E.J. Comparative analysis of phenolic and mineral composition of traditionally used wild medicinal plants from Southeast Serbia. Bulg. Chem. Commun. 2020, 52, 197–202. [Google Scholar] [CrossRef]
- Sucur, J.; Popovic, A.; Petrovic, M.; Anackov, G.; Bursic, V.; Kiprovski, B.; Prvulovic, D. Allelopathic effects and insecticidal activity of aqueous extracts of Satureja montana L. J. Serbian Chem. Soc. 2015, 80, 475–484. [Google Scholar] [CrossRef]
- Arnone, A.; Merlini, L.; Zanarotti, A. Constituents of Silybum marianum. Structure of isosilybin and stereochemistry of silybin. J. Chem. Soc. Chem. Commun. 1979, 16, 696–697. [Google Scholar] [CrossRef]
- Lucini, L.; Kane, D.; Pellizzoni, M.; Ferrari, A.; Trevisi, E.; Ruzickova, G.; Arslan, D. Phenolic profile and in vitro antioxidant power of different milk thistle [Silybum marianum (L.) Gaertn.] cultivars. Ind. Crops Prod. 2016, 83, 11–16. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Ilies, D.C.; Tudor, I.; Radulescu, V. Chemical composition of the essential oil of Urtica dioica. Chem. Nat. Compd. 2012, 48, 506–507. [Google Scholar] [CrossRef]
- Gül, S.; Demirci, B.; Başer, K.H.C.; Akpulat, H.A.; Aksu, P. Chemical Composition and In Vitro Cytotoxic, Genotoxic Effects of Essential Oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012, 6, 5714–5719. [Google Scholar]
- Semwal, P.; Rauf, A.; Olatunde, A.; Singh, P.; Zaky, M.Y.; Islam, M.M.; Khalil, A.A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Ribaudo, G. The medicinal chemistry of Urtica dioica L.: From preliminary evidence to clinical studies supporting its neuroprotective activity. Nat. Prod. Bioprospect. 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Ghasemi Pirbalouti, A. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crops Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- KapŁAn, M.; Borowy, A. Chemical composition and antioxidant activity of borage (Borago officinalis L.) seeds. Acta Sci. Pol. Hortorum Cultus 2020, 19, 79–90. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Bouhlali, E.d.T.; Kasrati, A.; El Rhaffari, L. The effect of domestication on seed yield, essential oil yield and antioxidant activities of fennel seed (Foeniculum vulgare Mill) grown in Moroccan oasis. J. Assoc. Arab Univ. Basic Appl. Sci. 2018, 24, 107–114. [Google Scholar] [CrossRef]
- Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.; Wink, M. Cytotoxicity of the Essential Oil of Fennel (Foeniculum vulgare) from Tajikistan. Foods 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichini, F. Comparative Chemical Composition and Antioxidant Activities of Wild and Cultivated Laurus nobilis L. Leaves and Foeniculum vulgare subsp. piperitum (Ucria) Coutinho Seeds. Biol. Pharm. Bull. 2006, 29, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating Wild Berries as a Dietary Approach to Reducing the Formation of Advanced Glycation Endproducts: Chemical Correlates of In Vitro Antiglycation Activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.A.; Abedindo, B.F.; Hassanzadeh-Khayyat, M. Antioxidant activity of the essential oils of different parts of Juniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae). Iran. J. Pharm. Res. 2011, 10, 799–810. [Google Scholar]
- Petkova, N.; Popova, A.; Alexieva, I. Antioxidant properties and some phytochemical components of the edible medicinal Malva sylvestris L. J. Med. Plants 2019, 7, 96–99. [Google Scholar]
- DellaGreca, M.; Cutillo, F.; Abrosca, B.D.; Fiorentino, A.; Pacifico, S.; Zarrelli, A. Antioxidant and Radical Scavenging Properties of Malva sylvestris. Nat. Prod. Commun. 2009, 4, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Loggia, R.D. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Nehir El, S.; Karakaya, S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int. J. Food Sci. Nutr. 2009, 55, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A Critical Overview on the Pharmacological and Clinical Aspects of Popular Satureja Species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Ćebović, T.; Vidović, S.; Jokić, S. Evaluation of Anticancer Activity of Satureja montana Supercritical and Spray-Dried Extracts on Ehrlich’s Ascites Carcinoma Bearing Mice. Plants 2020, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus Chimie 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Choi, E.-M.; Hwang, J.-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- De Marino, S.; Gala, F.; Borbone, N.; Zollo, F.; Vitalini, S.; Visioli, F.; Iorizzi, M. Phenolic glycosides from Foeniculum vulgare fruit and evaluation of antioxidative activity. Phytochemistry 2007, 68, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Chini, M.G.; Saviano, G.; Finamore, C.; Festa, C.; Lauro, G.; De Marino, S.; Russo, R.; Avagliano, C.; Casapullo, A.; et al. Biological Profile of Two Gentiana lutea L. Metabolites Using Computational Approaches and In Vitro Tests. Biomolecules 2021, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, N.H.; Daher, C.F. Malva sylvestris water extract: A potential anti-Inflammatory and anti-ulcerogenic remedy. Planta Med. 2009, 75, PH10. [Google Scholar] [CrossRef]
- Bach, H.; Benso, B.; Franchin, M.; Massarioli, A.P.; Paschoal, J.A.R.; Alencar, S.M.; Franco, G.C.N.; Rosalen, P.L. Anti-Inflammatory, Anti-Osteoclastogenic and Antioxidant Effects of Malva sylvestris Extract and Fractions: In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0162728. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kundu, J.K.; Chun, K.-S.; Na, H.-K.; Surh, Y.-J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafeek, K.A.; Osman, A.F.; Mouneir, S.M.; Elhenawy, A.A.; Abdallah, W.E. Phytochemical profile, comparative evaluation of Satureja montana alcoholic extract for antioxidants, anti-inflammatory and molecular docking studies. BMC Complement. Med. Ther. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Wu, S.-C. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Collard, M.; Camenzuli, L.; Saunders, D.; Vallotton, N.; Curtis-Jackson, P. Persistence and Mobility (Defined as Organic-Carbon Partitioning) Do Not Correlate to the Detection of Substances Found in Surface and Groundwater: Criticism of the Regulatory Concept of Persistent and Mobile Substances. Sci. Total Environ. 2023, 865, 161228. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Fatima, A.; Naz, S.; Ragni, M.; Tarricone, S.; Tufarelli, V. Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review. Antibiotics 2022, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vigil, I.G.; Mata-Cárdenas, B.D.; Esquivel-Ferriño, P.C.; Avalos-Alanís, F.G.; Vargas-Villarreal, J.; Camacho-Corona, M.d.R. Antigiardial Activity of Foeniculum vulgare Hexane Extract and Some of Its Constituents. Plants 2022, 11, 2212. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. [Google Scholar] [CrossRef]
- Khaleil, M.M.; Alnoman, M.M.; Elrazik, E.S.A.; Zagloul, H.; Khalil, A.M.A. Essential Oil of Foeniculum vulgare Mill. as a Green Fungicide and Defense-Inducing Agent against Fusarium Root Rot Disease in Vicia faba L. Biology 2021, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M. Intellectual property protection in the natural product drug discovery, traditional herbal medicine and herbal medicinal products. Phytother. Res. 2006, 21, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Makola, D.; Peura, D.A.; Crowe, S.E. Helicobacter pylori Infection and Related Gastrointestinal Diseases. J. Clin. Gastroenterol. 2007, 41, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and Bioactive Characterization of the Essential Oils Obtained from Three Mediterranean Plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.; Francisco, V.; Cavaleiro, C.; Gonçalves, M.J.; Cruz, M.T.; Sales, F.; Batista, M.T.; Salgueiro, L. Essential Oil of Juniperus communis subsp. alpina (Suter) Čelak Needles: Chemical Composition, Antifungal Activity and Cytotoxicity. Phytother. Res. 2012, 26, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Nawade, B.; Shaltiel-Harpaz, L.; Ibdah, M. A Review of the Botany, Volatile Composition, Biochemical and Molecular Aspects, and Traditional Uses of Laurus nobilis. Plants 2022, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Bay Leaf. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–74. [Google Scholar]
- Salima, B.; Yasmina Tlili Ait, K.; Abdelghani, D.; Youcef, H.; Azzedine, C. Antibiotic Activity of the Essential Oil of Laurel (Laurus nobilis L.) on Eight Bacterial Strains. J. Life Sci. 2013, 8, 814. [Google Scholar] [CrossRef]
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Coelho de Souza, G.; Haas, A.P.S.; von Poser, G.L.; Schapoval, E.E.S.; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J. Ethnopharmacol. 2004, 90, 135–143. [Google Scholar] [CrossRef]
- Cogo, L.L.; Monteiro, C.L.B.; Miguel, M.D.; Miguel, O.G.; Cunico, M.M.; Ribeiro, M.L.; de Camargo, E.R.; Kussen, G.M.B.; da Silva Nogueira, K.; Dalla Costa, L.M. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Braz. J. Microbiol. 2010, 41, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crops Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Magro, A.; Carolino, M.; Bastos, M.; Mexia, A. Efficacy of plant extracts against stored products fungi. Rev. Iberoam. Micol. 2006, 23, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Pieracci, Y.; Cagnoli, G.; Bertelloni, F.; Munafò, C.; Nardoni, S.; Pistelli, L.; Mancianti, F. In Vitro Antimicrobial Activity of Thymus vulgaris, Origanum vulgare, Satureja montana and Their Mixture against Clinical Isolates Responsible for Canine Otitis Externa. Vet. Sci. 2023, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum vulgare L. and Satureja montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Gil, R.L.; Cunha, E.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Fernandes-Ferreira, M.; Sousa, R.M.O.F.; Santos, C. Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum. Appl. Nano 2022, 3, 126–142. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.C.F.R.; Henriques, M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- El-Hagrassi, A.M.; Abdallah, W.E.; Osman, A.F.; Abdelshafeek, K.A. Phytochemical Study of Bioactive Constituents from Satureja montana L. Growing in Egypt and Their Antimicrobial and Antioxidant Activities. Asian J. Pharm. Clin. Res. 2018, 11, 142–148. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Gan, C.; Terrado, E.; Sanz, M.A.; Ballestero, D.; Langa, E. Antibiotic properties of Satureja montana L. hydrolate in bacteria and fungus of clinical interest and its impact in non-target environmental microorganisms. Sci. Rep. 2022, 12, 18460. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F.; et al. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef]
- Dunkić, V.; Bezić, N.; Vuko, E.; Cukrov, D. Antiphytoviral Activity of Satureja montana L. ssp. variegata (Host) P. W. Ball Essential Oil and Phenol Compounds on CMV and TMV. Molecules 2010, 15, 6713–6721. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.K.; Park, Y.; Park, S.-C.; Woo, E.-R.; Jeong, H.G.; Hahm, K.-S. Gram-positive bacteria specific properties of silybin derived from Silybum marianum. Arch. Pharmacal Res. 2003, 26, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Rakelly de Oliveira, D.; Relison Tintino, S.; Morais Braga, M.F.B.; Boligon, A.A.; Linde Athayde, M.; Douglas Melo Coutinho, H.; de Menezes, I.R.A.; Fachinetto, R. In Vitro Antimicrobial and Modulatory Activity of the Natural Products Silymarin and Silibinin. BioMed Res. Int. 2015, 2015, 292797. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.G.; Lee, D.G. Silibinin triggers yeast apoptosis related to mitochondrial Ca2+ influx in Candida albicans. The Int. J. Biochem. Cell Biol 2016, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hussain, S.; Verma, R.; Sharma, P. Anti-mycobacterial screening of five Indian medicinal plants and partial purification of active extracts of Cassia sophera and Urtica dioica. Asian Pac. J. Trop. Med. 2013, 6, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zenão, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Amin, R. The vascular action of aqueous extracts of Foeniculum vulgare leaves. J. Ethnopharmacol. 1988, 24, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zaman, G.; Anderson, R.A. Bay Leaves Improve Glucose and Lipid Profile of People with Type 2 Diabetes. J. Clin. Biochem. Nutr. 2009, 44, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer Savory: From the Selection of Traditional Applications to the Novel Effect in Relief, Prevention, and Treatment of a Number of Serious Illnesses such as Diabetes, Cardiovascular Disease, Alzheimer’s Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Chkhikvishvili, I.; Sanikidze, T.; Gogia, N.; McHedlishvili, T.; Enukidze, M.; Machavariani, M.; Vinokur, Y.; Rodov, V. Rosmarinic Acid-Rich Extracts of Summer Savory (Satureja hortensis L.) Protect Jurkat T Cells against Oxidative Stress. Oxidative Med. Cell. Longev. 2013, 2013, 456253. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Bnouham, M.; Bendahou, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Mekhfi, H. Inhibition of Rat Platelet Aggregation by Urtica dioica Leaves Extracts. Phytother. Res. 2006, 20, 568–572. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Jiménez-Becker, S.; de Bélair, G. Fatty acid profiles and sn -2 fatty acid distribution of γ-linolenic acid-rich Borago species. J. Food Compos. Anal. 2018, 66, 74–80. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, X.; Lu, Z. Foeniculum vulgare seed extract induces apoptosis in lung cancer cells partly through the down-regulation of Bcl-2. Biomed. Pharmacother. 2021, 135, 111213. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and Anticarcinogenic Effects of Methanolic Extract and Volatile Oil of Fennel Seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-C.; Huang, R.-L.; Huang, X.-F.; Chang, K.-F.; Lee, C.-J.; Hsiao, C.-Y.; Lee, S.-C.; Tsai, N.-M. Evaluation of anticancer effects of Juniperus communis extract on hepatocellular carcinoma cells in vitro and in vivo. Biosci. Rep. 2021, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.-M.; Chang, K.-F.; Wang, J.-C. Juniperus communis Extract Exerts Antitumor Effects in Human Glioblastomas Through Blood-Brain Barrier. Cell. Physiol. Biochem. 2018, 49, 2443–2462. [Google Scholar] [CrossRef]
- Daniela, A.; Pichichero, E.; Canuti, L.; Cicconi, R.; Karou, D.; D’Arcangelo, G.; Canini, A. Identification of phenolic compounds from medicinal and melliferous plants and their cytotoxic activity in cancer cells. Caryologia 2013, 60, 90–95. [Google Scholar] [CrossRef]
- Jayakumar, S.; Madankumar, A.; Asokkumar, S.; Raghunandhakumar, S.; Gokuladhas, K.; Kamaraj, S.; Josephine Divya, M.G.; Devaki, T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2011, 360, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.-H.; Yan, F.-X.; Zu, X.-Y.; Wu, Y.-H.; Wu, X.-P.; Liao, M.-C.; Deng, S.-W.; Yin, L.-L.; Zhuang, Y.-Z. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 2011, 64, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Durak, I.; Biri, H.; Devrim, E.; Sözen, S.; Avcı, A. Aqueous extract of Urtica dioica makes significant inhibition on adenosine deaminase activity in prostate tissue from patients with prostate cancer. Cancer Biol. Ther. 2014, 3, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Ghadami, E.; Asouri, M.; Motevalizadeh Ardekanid, A.; Akhavan-Niaki, H. Urtica dioica inhibits cell growth and induces apoptosis by targeting Ornithine decarboxylase and Adenosine deaminase as key regulatory enzymes in adenosine and polyamines homeostasis in human breast cancer cell lines. Cell. Mol. Biol. 2018, 64, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Fereidoony, B. Effect of Achillea millefolium on Relief of Primary Dysmenorrhea: A Double-Blind Randomized Clinical Trial. J. Pediatr. Adolesc. Gynecol. 2015, 28, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Basati, G.; Abbaszadeh, S.; Zebardast, A.; Teimouri, H. Analgesic Medicinal Plants in Shahrekord, Southwest of Iran: An Ethnobotanical Study. Galen Med. J. 2019, 8, e1593. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Xiao, L.; Ahmad, W.; Anwar, H.; Rasul, A.; Imran, M.; Aziz, N.; Razzaq, A.; Arshad, M.U.; Shabbir, A.; et al. Foeniculum vulgare (Fennel) promotes functional recovery and ameliorates oxidative stress following a lesion to the sciatic nerve in mouse model. J. Food Biochem. 2019, 43, e12983. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, J.; Anwar, H.; Iqbal, J.; Rasul, A.; Imran, A.; Ahmad Malik, S.; Shabbir, A.; Ijaz, F.; Sajid, F.; Akram, R.; et al. Methanolic extract of Fennel (Foeniculum vulgare) escalates functional restoration following a compression injury to the sciatic nerve in a mouse model. Food Sci. Nutr. 2020, 9, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Asle-Rousta, M.; Rahnema, M. Protective effect of fennel, and its major component trans-anethole against social isolation induced behavioral deficits in rats. Physiol. Int. 2020, 107, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Chiba, K.; Mohri, T.J.B.; Bulletin, P. Neuritogenic effect of natural iridoid compounds on PC12h cells and its possible relation to signaling protein kinases. Biol. Pharm. Bull. 1996, 19, 791–795. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Koppula, S.; Kim, I.-S.; Kumar, H.; Kim, B.-W.; Choi, D.-K. The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth. Molecules 2012, 17, 6728–6753. [Google Scholar] [CrossRef] [PubMed]
- Roszek, K.; Czarnecka, J. Is Ecto-nucleoside Triphosphate Diphosphohydrolase (NTPDase)-based Therapy of Central Nervous System Disorders Possible? Mini-Rev. Med. Chem. 2015, 15, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Boiangiu, R.S.; Hancianu, M.; Cioanca, O.; Erdogan Orhan, I.; Hritcu, L. Bay Leaf (Laurus nobilis L.) Incense Improved Scopolamine-Induced Amnesic Rats by Restoring Cholinergic Dysfunction and Brain Antioxidant Status. Antioxidants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, I.; Kandilarov, I.; Kotetarova, M.; Zlatanova, H.; Kostadinov, I.; Delev, D.; Vilmosh, N. Anxiolytic Effect of Satureja montana Dry Extract and its Active Compounds Rosmarinic Acid and Carvacrol in Acute Stress Experimental Model. J. Integr. Neurosci. 2022, 21, 124. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Martins, A.; Salta, J.; Neng, N.R.; Nogueira, J.M.F.; Mira, D.; Gaspar, N.l.; Justino, J.; Grosso, C.; Urieta, J.S.; et al. Phytochemical Profile and Anticholinesterase and Antimicrobial Activities of Supercritical versus Conventional Extracts of Satureja montana. J. Agric. Food Chem. 2009, 57, 11557–11563. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Magaña, M.P.; Cordero-Pérez, P.; Rivas-Morales, C.; Oranday-Cárdenas, M.A.; Moreno-Peña, D.P.; García-Hernández, D.G.; Leos-Rivas, C. Hypoglycemic Activity of Tilia americana, Borago officinalis, Chenopodium nuttalliae, and Piper sanctumon Wistar Rats. J. Diabetes Res. 2019, 2019, 7836820. [Google Scholar] [CrossRef] [PubMed]
- El-Ouady, F.; Lahrach, N.; Ajebli, M.; Haidani, A.E.; Eddouks, M. Antihyperglycemic Effect of the Aqueous Extract of Foeniculum vulgare in Normal and Streptozotocin-induced Diabetic Rats. Cardiovasc. Hematol. Disord.-Drug Targets 2020, 20, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Samadi-Noshahr, Z.; Hadjzadeh, M.A.R.; Moradi-Marjaneh, R.; Khajavi-Rad, A. The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci. Nutr. 2020, 9, 1121–1131. [Google Scholar] [CrossRef]
- Shahat, A.; Radwan, H.A.; Elkholy, Y.; Ghanem, M.M.; Mahdy, E.-S.; Hassanein, H. Phenolic compounds from Foeniculum vulgare (Subsp. Piperitum) (Apiaceae) herb and evaluation of hepatoprotective antioxidant activity. Pharmacogn. Res. 2012, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Wong, N.-K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, L.; Xiong, K.; Zhang, T.; Wang, Z. Exploration of Hepatoprotective Effect of Gentiopicroside on Alpha-Naphthylisothiocyanate-Induced Cholestatic Liver Injury in Rats by Comprehensive Proteomic and Metabolomic Signatures. Cell. Physiol. Biochem. 2018, 49, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Kandis, H.; Karapolat, S.; Yildirim, U.; Saritas, A.; Gezer, S.; Memisogullari, R. Effects of Urtica dioica on hepatic ischemia-reperfusion injury in rats. Clinics 2010, 65, 1357–1361. [Google Scholar] [CrossRef]
- Ved, A.; Gupta, A.; Rawat, A.K.S. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. leaves. Pharmacogn. Mag. 2017, 13, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mohi-ud-din, R.; Mir, R.H.; Sawhney, G.; Dar, M.A.; Bhat, Z.A. Possible Pathways of Hepatotoxicity Caused by Chemical Agents. Curr. Drug Metab. 2019, 20, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Pashtetsky, V.; Ostapchuk, P.; Usmanova, E.; Zyablitskaya, E.; Makalish, T.; Danilova, I.; Kuevda, T.; Zubochenko, D.; Uppe, V.; Pashtetskaia, A.; et al. Satureja montana L. essential oil various dosages effect on the main rats’ biological features. Potravin. Slovak J. Food Sci. 2021, 15, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mehrabi Nasab, D.; Athari, S.S. Effects of Silymarin and Baicalein on Glycogen Storage in the Hepatocytes of Rat Models of Hepatic Injury. Hepat. Mon. 2021, 21, e113114. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.M.; Najafzadeh, H.; Bahmei, S. Protective role of silymarin and D-penicillamine against lead-induced liver toxicity and oxidative stress. Toxicol. Ind. Health 2017, 33, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Türkdoğan, M.K.; Ozbek, H.; Yener, Z.; Tuncer, I.; Uygan, I.; Ceylan, E. The role of Urtica dioica and Nigella sativa in the prevention of carbon tetrachloride-induced hepatotoxicity in rats. Phytother. Res. 2003, 17, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Hardy, G.; Alany, R.G. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Leech, B.; Schloss, J.; Steel, A. Treatment Interventions for the Management of Intestinal Permeability: A Cross-Sectional Survey of Complementary and Integrative Medicine Practitioners. J. Altern. Complement. Med. 2019, 25, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Tewari, R.; Prakash, A.O. Hormonal properties of ethanolic extract of Juniperus communis Linn. Anc. Sci. Life 1990, 10, 106–113. [Google Scholar] [PubMed]
- Turgut, F.; Bayrak, O.; Catal, F.; Bayrak, R.; Atmaca, A.F.; Koc, A.; Akbas, A.; Akcay, A.; Unal, D. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int. Urol. Nephrol. 2008, 40, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wilasrusmee, C.; Kittur, S.; Shah, G.; Siddiqui, J.; Bruch, D.; Wilasrusmee, S.; Kittur, D.S. Immunostimulatory effect of Silybum marianum (milk thistle) extract. Med. Sci. Monit. 2002, 8, BR439–BR443. [Google Scholar] [PubMed]
- Khayyal, M.; El-Ghazaly, M.; Kenawy, S.; Seif-El-Nasr, M.; Mahran, L.; Kafafi, Y.; Okpanyi, S. Antiulcerogenic Effect of Some Gastrointestinally Acting Plant Extracts and their Combination. Arzneimittelforschung 2011, 51, 545–553. [Google Scholar] [CrossRef] [PubMed]


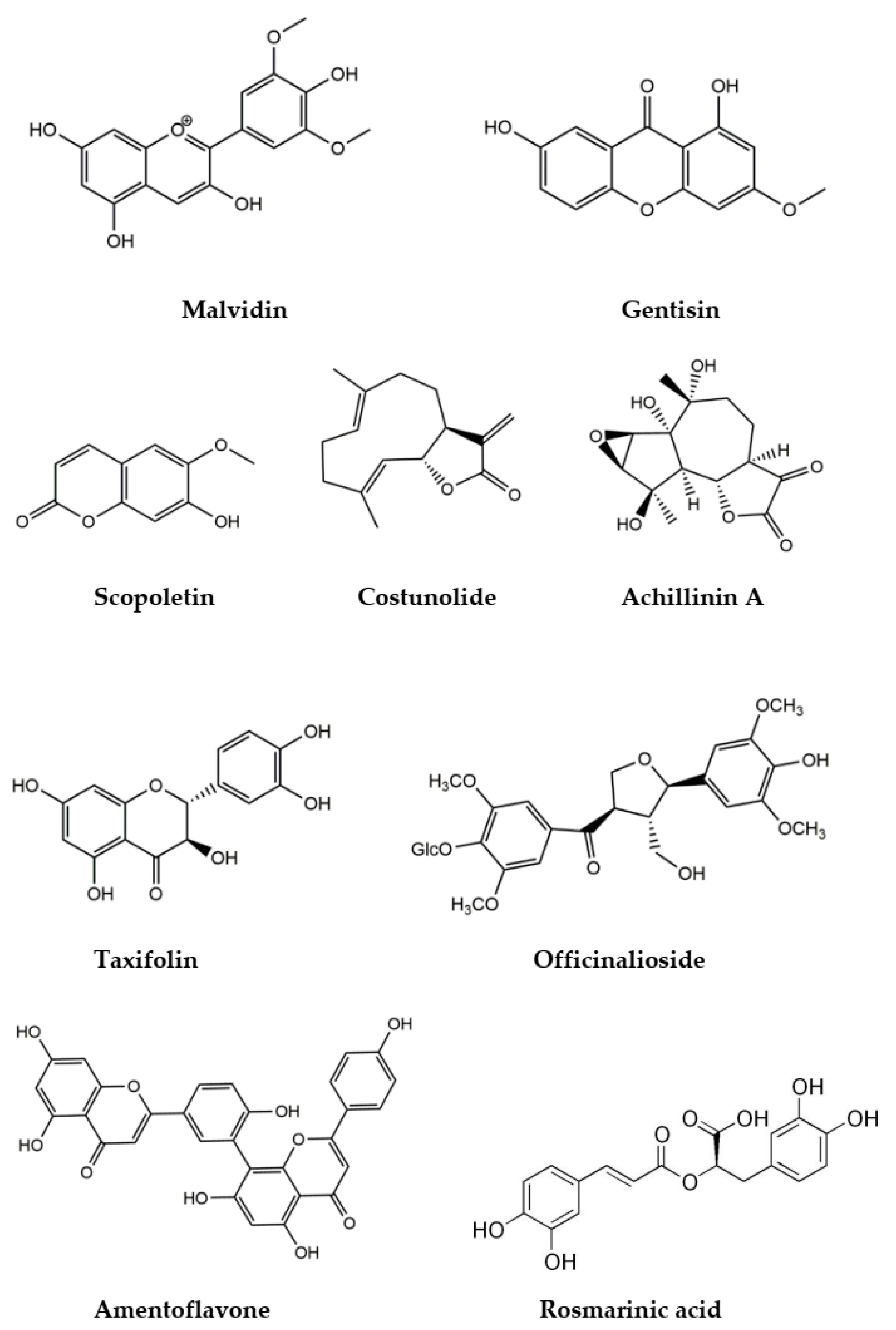
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantasma, F.; Samukha, V.; Saviano, G.; Chini, M.G.; Iorizzi, M.; Caprari, C. Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines. Nutraceuticals 2024, 4, 190-231. https://doi.org/10.3390/nutraceuticals4020013
Fantasma F, Samukha V, Saviano G, Chini MG, Iorizzi M, Caprari C. Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines. Nutraceuticals. 2024; 4(2):190-231. https://doi.org/10.3390/nutraceuticals4020013
Chicago/Turabian StyleFantasma, Francesca, Vadym Samukha, Gabriella Saviano, Maria Giovanna Chini, Maria Iorizzi, and Claudio Caprari. 2024. "Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines" Nutraceuticals 4, no. 2: 190-231. https://doi.org/10.3390/nutraceuticals4020013
APA StyleFantasma, F., Samukha, V., Saviano, G., Chini, M. G., Iorizzi, M., & Caprari, C. (2024). Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines. Nutraceuticals, 4(2), 190-231. https://doi.org/10.3390/nutraceuticals4020013








