Abstract
The major constituent of turmeric, curcumin, is a bioactive phenolic compound that has been studied for its potential health benefits and therapeutic properties. Within this article, the anti-inflammatory, antioxidant and antithrombotic properties and mechanisms of action of curcumin are thoroughly reviewed and the main focus is shifted to its associated health-promoting effects against inflammation-related chronic disorders. An overview of the cardio-protective, anti-tumor, anti-diabetic, anti-obesity, anti-microbial and neuro–protective health-promoting properties of curcumin are thoroughly reviewed, while relative outcomes obtained from clinical trials are also presented. Emphasis is given to the wound-healing properties of curcumin, as presented by several studies and clinical trials, which further promote the application of curcumin as a bioactive ingredient in several functional products, including functional foods, nutraceuticals, cosmetics and drugs. Limitations and future perspectives of such uses of curcumin as a bio-functional ingredient are also discussed.
Keywords:
curcumin; anti-inflammatory; anti-thrombotic; antioxidant; phenolic; turmeric; wound-healing 1. Introduction
Many long-term medical conditions have been related to oxidative stress, thrombotic incidents and inflammatory consequences. The World Health Organization (WHO) reports that chronic inflammatory non-communicable illnesses, including cancer, obesity, diabetes, heart problems, stroke and chronic respiratory diseases account for three out of every five deaths globally. Thus, it has been determined that the biggest risk to human health is chronic inflammatory disease [1]. Such chronic inflammation-related disorders begin and progress as a result of ongoing, inappropriate inflammatory activation, which is followed by endothelial dysfunction and oxidative damage at the inflammatory sites. Several typical platelet agonists, involving collagen and adenosine diphosphate (ADP), thrombo-inflammatory mediators like platelet-activating factor (PAF) and thrombin, as well as reactive oxygen species (ROS) are usually involved in these processes [2,3].
Treatment for inflammatory diseases comprise corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs) and biologic pharmaceuticals, among other medication types. However, these medications exhibit a number of side effects and pose various environmental challenges, while using biologic pharmaceuticals is costly. Therefore, despite their usefulness, the scientific community is looking for alternative solutions. One such alternative approach that has gained the attention of researchers is natural products, which have low toxicity and increased pharmacological activity. Among these products, polysaccharides, flavonoids, polyphenols, alkaloids, terpenes, natural pigments, volatile oils from plants and other pharmacological compounds are listed [4,5,6].
A natural product of great scientific interest and research is turmeric, which has gained international recognition for its medicinal properties and originated in Asia. It belongs to a class of compounds known as polyphenols, which own powerful biological effects. Among a large number of compounds isolated from turmeric, curcumin is the most widely studied compound as evidenced by the vast number of references in the literature. Briefly, it is an unflavored, orange–red, photosensitive powder with a molecular weight equal to 368.39 g/mol (chemical formula: C21H20O6). Although hydrophobic, curcumin dissolves in organic solvents like ethanol and acetone [7,8].
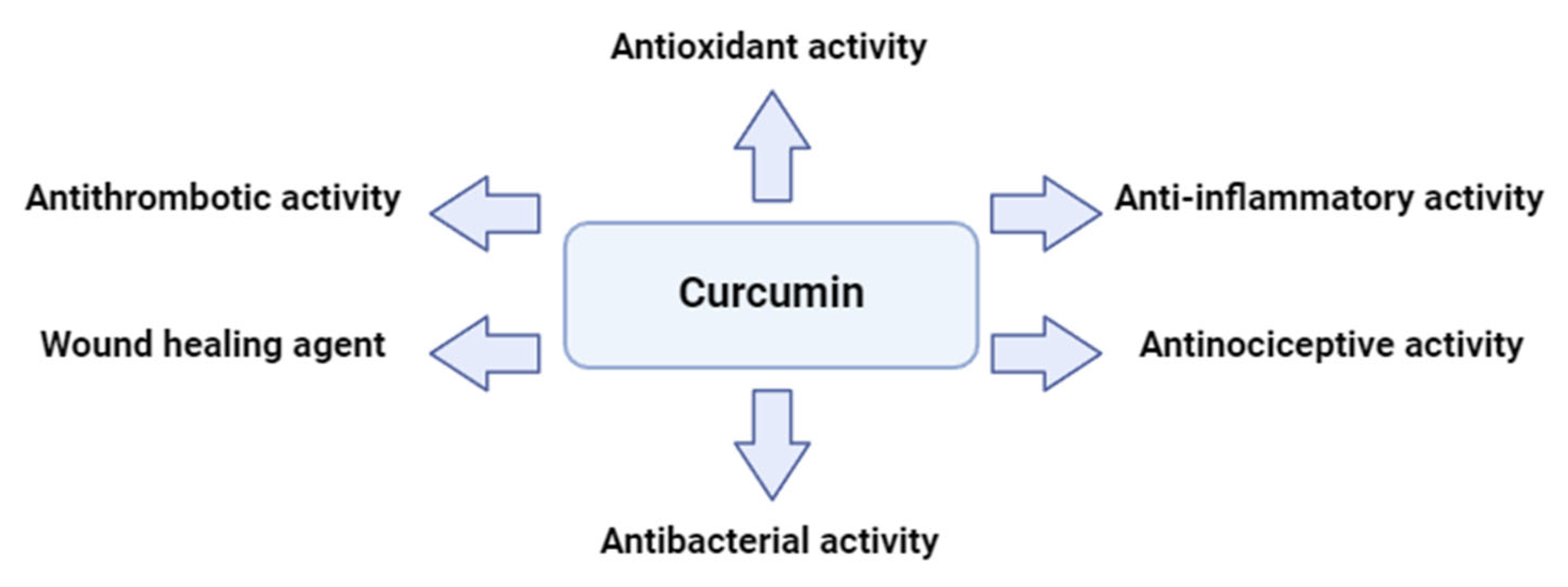
Curcumin is being currently investigated in a number of areas, including medicine, cosmetics and nutrition as a result of its multifaceted biological activity. Targeting several routes and substances, curcumin reduces inflammation. Specifically, it inhibits the nuclear factor kappa B (NF–κB) pathway, a major contributor to inflammation and downregulates the production of inflammatory chemical compounds (interleukins including IL–1β, Il–6 and the tumor necrosis factor α (TNF–α)) and enzymes (inducible nitric oxide synthase (iNOS), cyclooxygenase–2 inhibitor (COX-2) etc.). Additionally, curcumin eliminates free radicals which damage cells in its role as an antioxidant. It also reduces pain perception through antinociceptive actions and promotes tissue regeneration, which aid the healing of wounds. Gram-positive and gram-negative bacterial membranes are highly affected as well by curcumin’s antibacterial activities. It prevents bacteria from adhering to host receptors and limits their growth, virulence factors and biofilm formation. Curcumin prevents platelet aggregation caused by collagen, adrenaline and arachidonic acid (ARA), among other physiological factors. More specifically, platelet aggregation is restrained by curcumin as it inhibits cyclooxygenase (COX), which lowers the production of thromboxane (TX). Furthermore, curcumin effectively suppresses platelet aggregation and TX formation by increasing the lipoxygenase pathway synthesis of 12–hydroxyeicosatetraenoic acid (12-HETE) [9,10,11].
Curcumin has demonstrated potent anticoagulant and antiplatelet aggregation properties, as well as antithrombotic effects. Several studies highlight its beneficial impact on platelets, establishing curcumin as a promising candidate for the treatment of related conditions, given that platelet activation and aggregation are key processes in atherosclerotic thrombosis. The secretion and aggregation of platelets is a complex process, triggered by epinephrine, adenosine diphosphate, PAF, thrombin, collagen and arachidonic acid. Additionally, curcumin is widely used as an anti-inflammatory agent in traditional medicine. In patients with arthritis, it has been found to reduce the production of pro-inflammatory eicosanoids, as well as alleviate edema, morning stiffness, and other symptoms. The oral administration of curcumin also reduced acute inflammation caused by carrageenan in rats. Furthermore, curcumin has been shown to inhibit atherosclerosis and platelet aggregation, while reducing angiogenesis in adipose tissue. In cerebral microcirculation, curcumin significantly decreased platelet and leukocyte adhesion, primarily modulating the endothelium to minimize platelet adhesion. It also improved P-selectin expression and survival rates in mice after cecal ligation and puncture, while altering platelet and leukocyte adhesion and mitigating blood–brain barrier dysfunction [8,12,13]. Curcumin, along with its derivatives (curcuminoids) and more complex curcumin-based compounds, has been shown to inhibit platelet activation and aggregation triggered by PAF-related thrombo-inflammatory signaling. This is achieved by directly blocking the binding of PAF to its receptor (PAFR), as well as through various other mechanisms, such as directly inhibiting the deacetylation of arachidonic acid or indirectly disrupting its interaction with platelet phospholipids. Additionally, curcumin can directly suppress the calcium signaling pathway, antagonize the GP II B/III A receptor, inhibit the cyclooxygenase pathway, and prevent the formation of thromboxane A2, thus blocking platelet aggregation and thrombosis. By regulating multiple processes involved in platelet aggregation, curcuminoids have consistently demonstrated measurable antiplatelet effects. Consequently, curcuminoids could potentially act as therapeutic agents in preventing disorders associated with platelet activation [8,12,14,15]. A summary of curcumin’s great potential and activities is depicted in Figure 1.
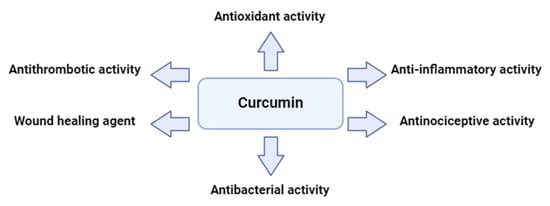
Figure 1.
A glimpse of the multifaceted activities of curcumin.
Curcumin has been the subject of numerous in vitro and in vivo experiments. This compound has attracted attention in scientific research due to its potential health benefits, including its anti-inflammatory, antioxidant and antithrombotic properties [11]. This article’s main purpose is to provide an extensive examination of these studies.
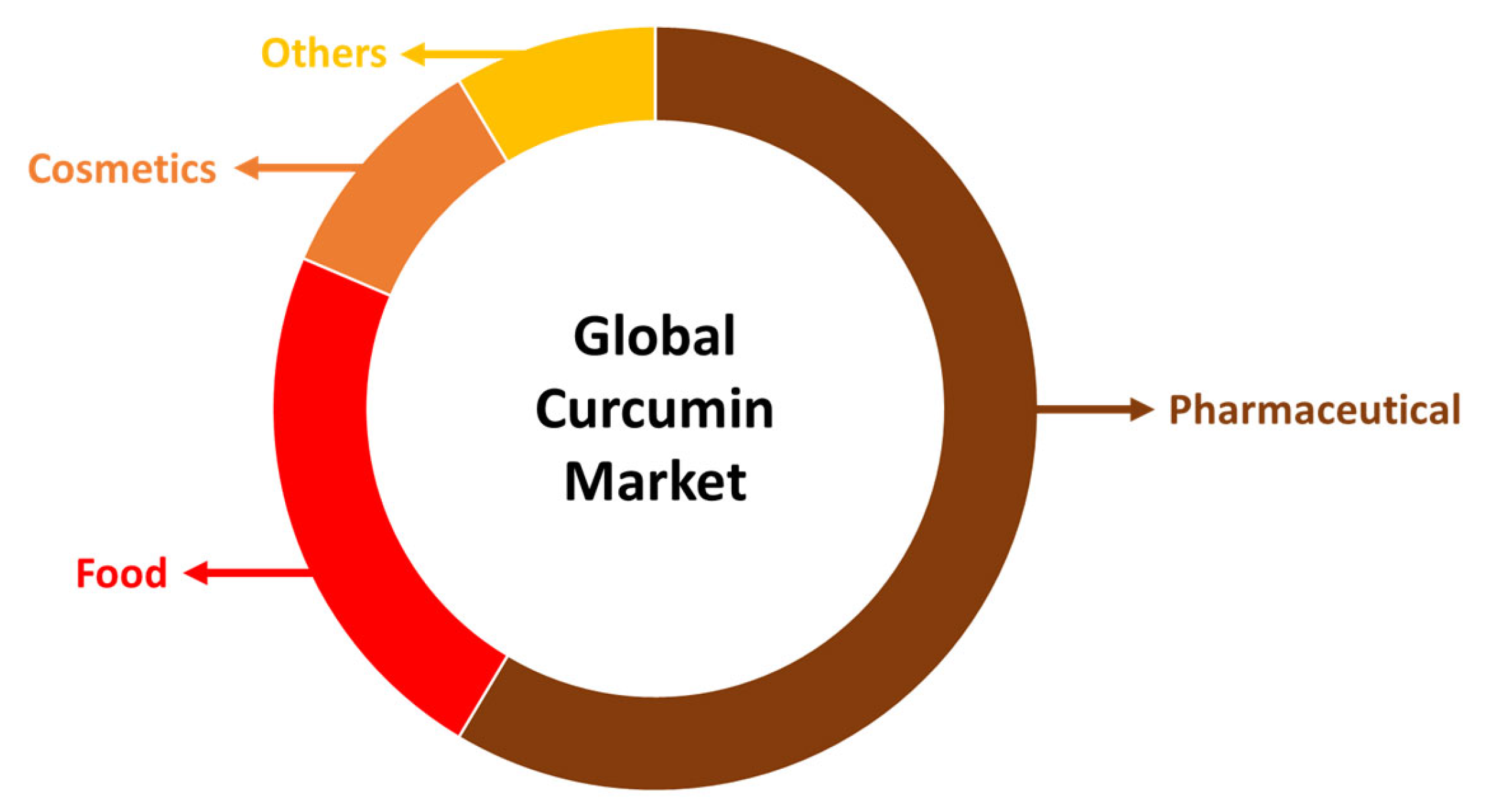
Due to the fact that curcumin has so many beneficial properties, it may be used in a wide range of ways. For instance, it is a major component utilized in the production of functional foods, pharmaceuticals and cosmetics (Figure 2). In terms of functional foods, turmeric content is accountable for an increase in nutritional value and health-promoting attributes. Turmeric functional foods may be applied to cereal-based foods, milk-based products, beverage enrichment, meat-based consumables, films and coatings. Curcumin displays also vast biological action against a number of conditions, such as autoimmune, neurological, cancer and cardiovascular disorders. Additionally, it affects several cellular targets and pathways as it, for example, controls the action of growth factors, transcription factors, cytokines, (pro)inflammatory mediators and enzymes [7].
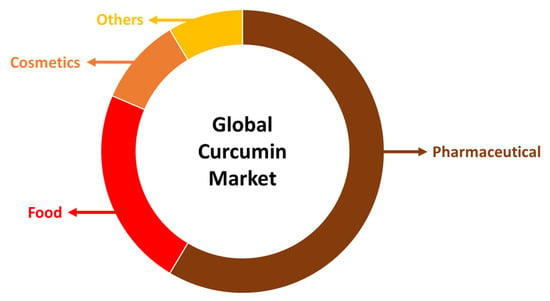
Figure 2.
Global curcumin market by application.
In the pharmaceutical industry, curcumin is the leading component in anticancer drug formulations, comprising more than 50% of the global market. It is closely followed by applications in the food and cosmetics industries. In contemporary cosmetology, there is an increasing incorporation of valuable plant-based ingredients into skincare products, with curcumin’s role becoming more prominent due to its potent antioxidant, anti-inflammatory, and anti-aging properties. This trend is expected to significantly boost the financial growth of the cosmetics market in the near future (Figure 2) [7].
Curcumin targets multiple cellular pathways and thus, curcumin–based pharmaceutical products are being explored for the treatment of various pestering diseases. The considerable potential of curcumin for decreasing photodamage to the skin, minimizing signs of aging and being utilized in skin care products for acne-prone skin, is still far from being fully elucidated [16,17,18,19,20,21].
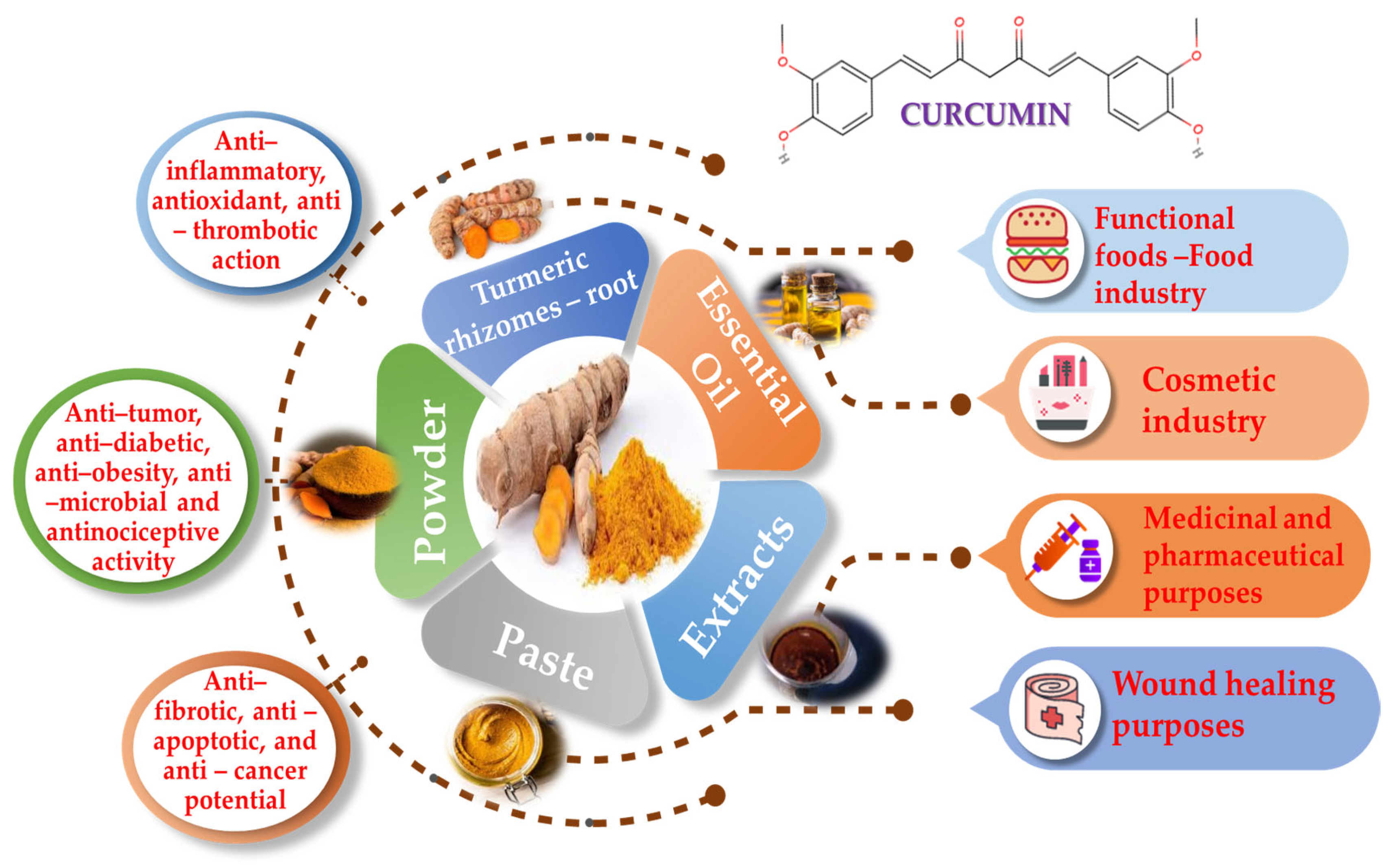
Although curcumin has many advantages with regard to human health, it is important to acknowledge its limits. For example, it confronts difficulties in absorption, distribution, metabolism and excretion (ADME), resulting in a short half-life in the gastrointestinal system. These processes collectively impact the amount of curcumin available for our body to use, limiting its effectiveness. In addition to its low availability, curcumin also has a low water solubility, is unstable in alkaline environments and undergoes rapid metabolism into inactive forms and conjugates, which further reduce its bioactivity. Due to these restrictions, curcumin’s therapeutic potential is hampered, emphasizing the necessity of developing methods to increase its bioavailability, stability and metabolic profile [18,22,23,24]. Numerous tests have demonstrated that curcumin is safe, but because of possible adverse effects, especially regarding larger dosages, prudence is suggested. These complications include the risk of increased oxalate-induced kidney stones, anemia in iron-deficient individuals, liver damage, abnormal cardiac rhythms, drug interaction, allergic reactions and many other side effects [8,11,25]. Within this article, curcumin’s significance for food, cosmetics and pharmaceutical-related applications, as well as its anti-inflammatory, antithrombotic and antioxidant health-promoting properties, will be further evaluated and discussed (Figure 3).
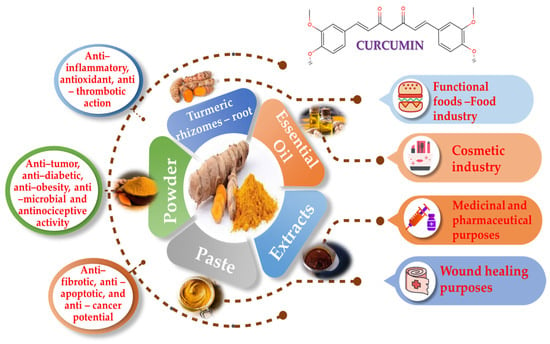
Figure 3.
Curcumin utilized forms, applications and health-promoting properties.
Various extraction procedures, such as Soxhlet extraction, maceration, or contemporary technologies like ultrasonic or microwave extraction, are used to isolate curcumin from turmeric rhizome. Curcumin is extracted, separately using thin-layer chromatography (TLC), and is quantified by high-performance liquid chromatography (HPLC). While modern methods provide advantages like lower temperatures and less solvent usage, Soxhlet extraction is reported to deliver the highest amount of curcumin [9,10].
2. Applications of Curcumin
2.1. Curcumin in Food Industry
The addition of bioactive compounds like curcumin to consumable products has garnered increasing attention, as consumers become more conscious and adopt healthier eating habits. With its appealing bright yellow–orange shade, curcumin has been used as a natural food coloring ingredient. It is frequently preferred in order to enhance the appearance of dishes such as rice, beef, mustards and other food products. Apart from its widespread use as a food coloring, curcumin may also be used in food products as a natural and safe antioxidant and antibacterial agent, replacing artificial, harmful ones [26,27]. Studies on the food uses of curcumin have been widely conducted, many of which are covered in the section that follows and are included in Table 1.

Table 1.
Indicative studies of curcumin in the food industry.
Gao et al. [28] performed an experiment in which curcumin was encapsulated in milk fat beads and protein micelles. The researchers used a simulated digestion model to evaluate how curcumin behaved in milk samples after they mimicked human digestion. Milk loaded with curcumin was compared to a simple mix of curcumin powder and milk. Milk loaded with curcumin reportedly remained stable throughout digestion, while curcumin powder in milk did not. Moreover, milk that was loaded with curcumin also released more curcumin that could potentially be absorbed by the body (higher bio-accessibility), as compared to the mix with curcumin powder. This is likely due to curcumin being hidden within fat or protein parts of the milk, which enhance its protection and its easier release during digestion. Interestingly, whole milk with fat did not necessarily improve curcumin absorption compared to skimmed milk. This finding suggested that other factors besides fat might exist in milk and may aid curcumin’s absorption [28].
Another relevant study investigated the potential use of turmeric root in enhancing the nutritional quality of a street-vended Zobo drink, a popular beverage in Nigeria. The obtained results indicated that adding boiling turmeric to Zobo boosted its nutritional value and antioxidant content, as demonstrated by the higher levels of anthocyanins and vitamin C found [29]. Augustyńska-Prejsnar et al. [30] investigated the effects of adding turmeric (powder or paste) to duck burgers. Turmeric paste and powder efficiently reduced lipid oxidation in the burgers throughout an 18-day refrigerated storage period; the burgers with powdered turmeric showed the lowest level of oxidation. Turmeric additives also influenced pH, water retention and the color of burgers, with turmeric paste resulting in the most desirable sensory attributes, including aroma, taste and juiciness. Overall, the study suggested that turmeric additives enhance the oxidative stability and microbiological safety of duck meat burgers, with turmeric paste yielding particularly favorable sensory qualities [30]. Mancini et al. [31] evaluated the impact of adding ascorbic acid and turmeric powder to rabbit meat burgers during refrigerated storage. According to this study’s results, turmeric powder minimizes cooking loss and promotes meat’s antioxidant capacity to that of ascorbic acid [31].
In a series of studies, researchers inquired into the neuroprotective and hepatoprotective effects of turmeric extract and its components in animal models. Banji et al. [32] studied the effects of turmeric extract on aluminum-induced neurotoxicity in Swiss albino mice. It was then found that treatment with turmeric extract plus essential oil significantly reversed aluminum-induced spatial learning and memory impairment, decreased lipid peroxidation, and improved antioxidant enzyme levels, suggesting its high neuroprotective potential [32]. Lee et al. [33] demonstrated that turmeric extract and curcumin mitigated liver damage induced by carbon tetrachloride in rats by reducing serum liver enzyme activities and boosting hepatic glutathione levels [33]. The most important outcomes of each selective indicative study are depicted in Table 1.
2.2. Curcumin in Medicinal Purposes
The rhizome of Curcuma longa L., commonly known as turmeric, has been historically utilized for its medicinal properties across diverse cultural contexts, notably within traditional medical systems including Islamic, Chinese and Ayurvedic traditions. Its therapeutic applications encompass a broad spectrum of maladies ranging from gastrointestinal disturbances to cardiovascular, hepatic, and neurological ailments, as well as inflammatory conditions like arthritis. Central to turmeric’s medicinal efficacy is curcumin, a bioactive compound characterized by its anti-inflammatory, antioxidant and anticancer attributes, along with its capacity to ameliorate metabolic dysregulation, cognitive function and mood disorders [34].
Li et al. [35] sought to evaluate the efficacy of curcumin in mitigating myocardial ischemia-reperfusion (I/R) injury, examining evidence from both animal experimentation and clinical trials. Employing a systematic review and meta-analysis methodology, the investigation encompassed 24 animal studies and four human trials. Results indicated that curcumin exhibited significant reductions in myocardial infarction size, enhancement of cardiac function indices and favorable modulation of various markers indicative of myocardial injury, oxidative stress, apoptosis and inflammation in animal models. Moreover, clinical investigations suggested potential benefits of curcumin in ameliorating cardiac dysfunction, hospital-acquired myocardial infarction and major adverse cardiovascular events (MACE) within short-term observation periods. Overall, all findings underscore the putative cardioprotective attributes of curcumin against myocardial infarction, primarily attributed to its anti-inflammatory and antioxidant properties [35].
In order to determine if curcumin is effective at reducing cardiac ischemia/reperfusion (I/R) injury in animal models, Zeng et al. [36] performed a meta-analysis. A thorough analysis of research published up to January 2023 revealed 37 studies involving 771 animals. Curcumin dramatically decreased the extent of myocardial infarction, enhanced cardiac function and lowered markers of oxidative stress and myocardial injury, according to the analysis. Subgroup analyses revealed dose and treatment condition-related significant differences, but some publication bias was seen. Overall, research in larger animals and human trials are required for the validation of curcumin’s potential cardioprotective properties against myocardial I/R injury in animal models [36].
Wei et al. [37] studied curcumin and its ability to protect cardiomyocytes against hypoxia/reoxygenation (H/R) injury. Their investigation of the protective effects of Cur used a range of analytical methodologies, with a particular emphasis on morphological alterations, cell viability, oxidative stress and apoptosis in H9c2 cardiomyocytes. Under H/R conditions, the researchers saw significant changes in cell shape and decreased viability, coupled with increased apoptosis and elevated levels of malondialdehyde (MDA) and lactate dehydrogenase (LDH), along with decreased superoxide dismutase (SOD) activity. These effects were successfully counteracted by curcumin therapy, which also increased SOD activity, decreased ROS and apoptosis, and restored LDH and MDA levels. Curcumin also reduced the stress on the endoplasmic reticulum (ER) caused by H/R and inhibited the signaling cascade of mitogen-activated protein kinase (MAPK), which includes p38, JNK and ERK1/2. These results underline curcumin’s cardioprotective qualities by showing how it can reduce heart damage via regulating ER stress and MAPK signaling pathways [37].
Dolatabadi et al. [38] investigated the possibility of using curcumin as a medicinal drug to lessen the cognitive and neurological damage brought on by global cerebral ischemia (GCI). In a rat model of GCI, curcumin—which is well known for its anti-inflammatory, antioxidant and neuroprotective qualities—was examined for its impact on neurological deficiencies, memory impairment and spatial neural distribution in the Cornu Ammonis 1 (CA1) area of the hippocampus. Forty-six male Sprague Dawley rats were used in the investigation, and they were divided into four groups at random. The treatment periods for each group were further split into short-term (7 days) and long-term (28 days). At different post-operative days, neurological evaluations were conducted, such as the traction test, passive avoidance task and neurological severity score (NSS). Furthermore, on days 7 and 28 following GCI, the Voronoi tessellation and the novel object identification test were carried out. In comparison to the control and lower dose (50 mg/kg) groups, the results demonstrated that curcumin at a dose of 100 mg/kg dramatically improved neurological scores and decreased memory deficits. In particular, step-through latency times and the novelty preference index showed significant improvements in the high-dose curcumin group, suggesting improved memory performance. Additionally, a 100 mg/kg dose of curcumin maintained neuronal aggregation in the CA1 area at levels similar to those of normal rats. Overall, the study shows that curcumin successfully improves memory and neurological deficits following GCI and restores neuronal distribution in the CA1 region. This is especially true at higher doses and with long-term treatment. These findings suggest that curcumin holds promise as a therapeutic agent for reducing brain complications associated with ischemia [38].
Zhang and Wu [39] aimed to evaluate curcumin’s possible chemoprotective benefits against doxorubicin-induced cardiotoxicity. Doxorubicin administration was linked, in comparison to control groups, to lower cell survival, increased mortality, decreased body weight, heart weight and heart-to-body weight ratio, according to an analysis of in vitro and in vivo investigations. Curcumin co-administration, however, demonstrated a reversal of these effects seen in groups receiving doxorubicin alone. Additionally, doxorubicin caused notable histological and biochemical changes in heart tissue, both of which were substantially lessened by curcumin cotreatment [39].
Sayevand et al. [40] examined how moderate exercise and curcumin supplementation, both known to have protective effects on the heart, might work together to prevent heart damage caused by lack of blood flow (I/R injury). Researchers used rats and assigned them to groups that either received no treatment, exercise alone, curcumin alone, both exercise and curcumin, or just the I/R injury. They looked at genes involved in amyloid plaque formation and markers of heart damage. The obtained results demonstrated that both exercise and curcumin supplementation individually mitigated the mRNA expression of amyloid precursor protein and associated enzymes (β-secretase-1, presenilin-1 and -2), reduced infarct size and elevated neprilysin gene expression in myocardial tissue. Nonetheless, the combined regimen failed to confer additional benefits compared to singular interventions [40].
Neerati et al. [41] studied the potential benefits of curcumin capsules in lowering lipid levels and inhibiting permeability glycoprotein (P-gp), thereby affecting the pharmacokinetics and pharmacodynamics of glyburide, a medication used in type-2 diabetes mellitus management. An open-label, randomized controlled trial involving eight patients with type-2 diabetes on glyburide therapy was conducted over 11 days. Glucose levels decreased, while Area Under first Movement Curve (AUMC) increased, with no instances of hypoglycemia observed. Furthermore, significant reductions in low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL) and triglyceride levels, along with increased high-density lipoprotein (HDL) content, were noted. Co-administration of curcumin capsules with glyburide demonstrated potential benefits for improving glycemic control and exhibited lipid-lowering and antidiabetic properties, suggesting a potential role for curcumin as a future drug molecule [41].
Han et al. [42] aimed to elucidate the mechanism via which curcumin influenced energy metabolism, focusing on its effects on thermogenesis and obesity. By utilizing a mouse model fed a high-fat diet (HFD), researchers found that curcumin supplementation resulted in reduced weight gain and improved cold tolerance, indicative of enhanced adaptive thermogenesis. These effects were contingent upon the presence of uncoupling protein 1 (Ucp1), a pivotal regulator of thermogenesis. Analysis of the gut microbiota (GM) revealed that curcumin induced alterations in microbial composition in HFD-fed mice. Moreover, curcumin influenced bile acid (BA) metabolism, elevating levels of deoxycholic acid (DCA) and lithocholic acid (LCA), as potent ligands for G protein-coupled membrane receptor 5 (TGR5). Mechanistically, curcumin activated the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway in thermogenic adipose tissue through the modulation of the GM and TGR5. These findings underscore the potential of targeting the GM to modulate thermogenesis and combat obesity [42].
In rats that had been overfed early in the neonatal period, Zhu et al. [43] investigated the effects of curcumin (CUR) supplementation on white adipose tissue (WAT) browning and thermogenesis. Metabolic imprinting resulting from early overfeeding has been linked to reduced energy expenditure and increased WAT accumulation over the course of a lifetime. The purpose of the study was to ascertain whether CUR may mitigate these effects by stimulating thermogenic mechanisms and encouraging WAT browning. For 10 weeks, rats with small litters (SL) were fed either a regular diet or a diet supplemented with 1% or 2% CUR. When compared to SL rats that were not treated, the study’s conclusion showed that the rats administered 2% CUR had decreased body weight, decreased WAT growth, and improved blood lipid levels and glucose tolerance. In subcutaneous adipose tissue (SAT), CUR supplementation significantly enhanced heat production and browning-related gene expression; these effects were more noticeable at the 2% dose. Overall, by increasing energy expenditure and SAT browning, CUR supplementation reduced body fat and metabolic diseases [43].
Du et al. [44] explored how curcumin (CUR) affects postnatal overfed rats, a model for early-life overnutrition, which raises the risk of obesity and metabolic diseases. Male rats were divided into normal (NL) and overfed small litters (SL). From weaning, SL rats received either a normal diet or a 2% CUR diet starting at weaning (W3), puberty (W6), or end of puberty (W8) for 10 weeks. Results showed that CUR starting at weaning (SL-CURW13) or early puberty (SL-CURW16) significantly reduced body weight and fat gain, and improved glucose and lipid levels, with SL-CURW13 being the most effective. CUR had no effect and began at the end of puberty (SL-CURW18). The findings emphasize how early CUR intervention prior to puberty can significantly ameliorate the consequences of early-life overnutrition [44].
The effects of supplementing with curcumin throughout pregnancy and lactation on the offspring of obese mice were studied by Santos et al. [45]. Three groups of pregnant and nursing female mice were used: hyperglycemic (high-sugar diet), curcumin (high-sugar diet combined with curcumin) and control (normal diet). The offspring were subsequently given a 12-week high-sugar diet. The results indicated that, compared to those from hyperglycemic dams, the offspring of curcumin-supplemented dams experienced decreased weight increase and food consumption. They also showed decreased insulin, glucose and cholesterol levels, as well as enhanced glucose tolerance. Adipose tissues had higher expression levels of the thermogenesis markers UCP1 and PRDM16, according to molecular studies. According to the study, supplementing with curcumin during pregnancy and lactation may promote thermogenesis and improve metabolic outcomes in children fed an obesogenic diet [45].
A similar investigation [46] aimed to evaluate the inhibitory capacity of curcumin against two pathogenic gram-positive bacteria, Streptococcus mutans and Streptococcus pyogenes, in comparison to the antibiotic ciprofloxacin, by utilizing the well diffusion method. Minimum Inhibitory Concentration (MIC) assays demonstrated that curcumin effectively hindered the growth of both Streptococcus mutans and Streptococcus pyogenes. Curcumin produced inhibition zones of 9.7 mm and 10.2 mm against S. mutans and S. pyogenes, respectively. While these zones were smaller compared to those produced by Ciprofloxacin (15.52 mm and 13.4 mm for S. mutans and S. pyogenes, correspondingly), they provide evidence for curcumin’s potent antibacterial properties. The observed antimicrobial activity of curcumin against these bacteria suggested its potential application in controlling dental biofilms and preventing dental caries [46].
Namgyal et al. [47] explored the potential neuroprotective effects of curcumin against cadmium-induced neurotoxicity. Using Swiss Albino mice previously exposed to cadmium, the study evaluates the impacts of various concentrations of curcumin (20, 40, 80 and 160 mg/kg) on behavior, biochemical parameters, hippocampal proteins (brain-derived neurotrophic factor (BDNF), cyclic amp–response element binding protein (CREB), doublecortin (DCX), Synapsin II and histological alterations. The findings reveal that cadmium (Cd) exposure elicits behavioral deficits, oxidative stress, diminished levels of hippocampal neurogenesis-associated proteins and neuronal degeneration in the CA3 region and cortex. Nonetheless, treatment with differing concentrations of curcumin effectively mitigates these effects, with the highest dosage (160 mg/kg body weight) exhibiting pronounced efficacy. This finding suggests that curcumin counteracts Cd-induced neurotoxicity and fosters neurogenesis, potentially offering therapeutic advantages against heavy metal-triggered neuronal harm [47]. Below is a summary of several significant and recent pharmacological uses, which are included in Table 2.
2.3. Curcumin in Wound Healing
Curcumin also has considerable potential in the area of wound healing. Curcumin, well known for its anti-inflammatory, antioxidant and antibacterial qualities, provides a variety of benefits to aid the complex process of tissue healing. It helps to create the ideal environment for healing by reducing inflammatory reactions, eliminating damaging free radicals and preventing microbial developments. Its capacity to increase angiogenesis and stimulate collagen production further emphasizes its function in promoting vascularization and tissue regeneration [48].
In this section, the research regarding curcumin in wound healing will be discussed. An in vivo study investigated a curcumin nano-formulation as a wound healing treatment in rats. While wound closure was not significantly faster than in the control treatment, the curcumin formulation appeared to improve other healing aspects. Specifically, the curcumin nano-formulation may reduce inflammation as effectively as the control medication, while both treatments exhibited similar progress in blood vessel formation and skin cell growth. Importantly, the curcumin formulation significantly increased collagen production compared to the control. These observations pointed to the curcumin nano-formulation’s potential as a wound healing agent by suggesting that it may improve wound repair via lowering inflammation, encouraging angiogenesis, fibroblast proliferation and collagen formation [49]. Also, Cao et al. [50] explored how curcumin helps diabetic foot ulcers (DFU) heal. By delving into cells and rats with DFU, researchers discovered the miR-152-3p molecule that is high in DFU and the FBN1 one that is correspondingly low. Curcumin promoted angiogenesis, proliferation, migration and inhibition of fibroblast apoptosis, all of which sped up the wound healing process [50].

Table 2.
Indicative studies of curcumin in the pharmaceutical industry.
Table 2.
Indicative studies of curcumin in the pharmaceutical industry.
| Aim of the Study | Study Design | Results | Reference |
|---|---|---|---|
| This study sought to validate curcumin’s potential for cardio-protection against myocardial ischemia/reperfusion damage (in vivo and in vitro) |
|
| [35] |
| Evaluation of the efficacy of curcumin in preventing myocardial ischemia/reperfusion (I/R) injury in animal models, through a comprehensive meta-analysis of preclinical studies (in vivo) |
|
| [36] |
| Investigation of the efficacy of curcumin in mitigating hypoxia/reoxygenation (H/R) injury in cardiomyocytes (in vitro). |
|
| [37] |
| Investigation of the potential of curcumin as a therapeutic agent for mitigating neurological and cognitive impairments caused by global cerebral ischemia (GCI) (in vivo and in vitro). |
|
| [38] |
| This study aimed to confirm that co-administration of curcumin alleviated doxorubicin-induced cardiotoxicity via its antioxidant, antiapoptotic and anti-inflammatory properties (in vivo and in vitro). |
|
| [39] |
| Moderate-intensity aerobic exercise and curcumin supplementation individually administered would be able to demonstrate cardioprotective effects against myocardial injury induced by I/R, as proved in this clinical trial (in vivo and in vitro). |
|
| [40] |
| Co-administration of curcumin capsules with glyburide may have positive effects on lipid levels and enhance glycemic control in individuals with type-2 diabetes mellitus, as this study shows (in vivo and in vitro). |
|
| [41] |
| Curcumin mediated the enhancement of thermogenesis and the reduction of obesity in mice, as evaluated in this clinical trial (in vivo) |
|
| [42] |
| Examination of the impact of curcumin (CUR) supplementation on white adipose tissue (WAT) browning and thermogenesis in rats subjected to early postnatal overfeeding (in vivo and in vitro). |
|
| [43] |
| The aim of this study is to explore the impact of curcumin (CUR) supplementation on rats subjected to early postnatal overfeeding, a condition that increases the risk of obesity and metabolic diseases (in vivo). |
|
| [44] |
| This study aimed to investigate the long-term effects of maternal obesity and dietary habits during pregnancy and lactation on the metabolic health of offspring, focusing on the potential benefits of curcumin supplementation (in vivo and in vitro). |
|
| [45] |
| This study evaluated the potential of curcumin towards inhibiting the growth of two pathogenic Gram-positive bacteria commonly found in the oral cavity: Streptococcus mutans and Streptococcus pyogenes (in vitro). |
|
| [46] |
| Curcumin administration may counteract the neurotoxic effects induced by Cd exposure in mice, as this study’s main aim proved (in vivo). |
|
| [47] |
The study was conducted on the effectiveness of curcumin on healing burns in rats, and in which 70 female Sprague Dawley rats were randomly assigned to five groups, each receiving different treatments, including various concentrations of curcumin, silver sulfadiazine ointment, or a control substance. The animals were inspected and the burn wounds were evaluated histologically and visually throughout the course of 7, 14 and 21 days. Findings indicated that curcumin, especially at a concentration of 2%, contributed to reduced inflammation, smaller burn wound sizes and improved re-epithelialization compared to other treatments. Histological examination showed well-organized epidermal layers and aligned fibroblasts in the curcumin-treated groups, particularly in the 2% concentration group. Such results suggested that curcumin could be a promising and affordable option for treating burn wounds [51]. Another clinical trial also examined how topical curcumin affects burn wound healing in rats. In this study, Wistar-albino rats were divided into groups based on the time after the burn (4th, 8th, or 12th day), with each group further divided into subgroups, receiving either burn alone or burn plus curcumin treatment. Both microscopic inception and biochemical analysis revealed that these rats exhibited quicker wound healing, which was defined by decreased inflammation, enhanced collagen deposition, blood vessel creation, granulation tissue formation, skin cell regeneration, as well as upregulation of a cell proliferation marker. Overall, the study strongly suggested that topical curcumin application could accelerate burn wound healing in the tested rats [52].
Ranjbar-Mohammadi et al. [53] examined a special wound dressing made of tiny fibers (polycaprolactone/gum tragacanth/curcumin (PCL/GT/Cur) nanofibers), which were loaded with curcumin for diabetic rats. These dressings fought bacteria and were tested in two forms: plain and with added cells. After 15 days, wounds treated with the curcumin nanofibers healed much faster and showed better tissue formation, compared to untreated wounds. This included more collagen deposition, thicker regenerated skin and even signs of sweat glands and hair follicles. Analysis confirmed that the dressing increased blood vessel growth, granulation tissue and the number of healing cells, while also reducing the unhealed wound area. As a whole, the study suggested these curcumin-loaded nanofibers have great promise as a treatment for diabetic wounds [53]. In another similar study, a nanohybrid scaffold containing curcumin-loaded chitosan nanoparticles (CUR-CSNPs) for diabetic wound healing was developed. In vitro tests showed the scaffold’s favorable characteristics, including small particle size, good stability and biocompatibility with fibroblast cells. Moreover, the scaffold exhibited sustained curcumin release. In vivo experiments using diabetic rats, demonstrated improved wound healing outcomes, with faster wound closure, reduced inflammation and enhanced fibroblast activity and collagen deposition compared to control groups [54].
Vinay et al. [55] set out to determine whether topical use of curcumin might be used to accelerate skin wound healing in rats with diabetes. Experimental rats, rendered diabetic via streptozotocin (STZ) induction, were subjected to open excision skin wounds and subsequently categorized into three groups: control, gel-treated and curcumin-treated. Over a 19-day period, Pluronic F-127 gel (25%) and curcumin (0.3%) within Pluronic gel were applied topically once daily to the respective groups. Results demonstrated that the curcumin application resulted in accelerated wound contraction and decreased expression levels of inflammatory cytokines/enzymes, including TNF–α, IL–1β and matrix metalloproteinase–9 (MMP–9). Additionally, curcumin administration elevated levels of the anti-inflammatory cytokine IL-10 and antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). Histopathological examination revealed enhanced granulation tissue characterized by notable fibroblast proliferation and collagen deposition, as well as thickened regenerated epithelial layers in curcumin-treated wounds. These findings underscored curcumin’s potential as a novel therapeutic modality for managing impaired wound healing in diabetic conditions, owing to its anti-inflammatory and antioxidant attributes [55].
Pandya et al. [56] investigated whether a gel made from Curcuma longa (turmeric) could help with healing and pain after tooth extraction. Over a three-month period, 21 patients that underwent bilateral extractions were enrolled in a split-mouth randomized controlled trial. In comparison to the control group, they discovered that the Curcuma longa (turmeric) gel group recovered more quickly and experienced less discomfort on day 7. Researchers advised dentists to use this gel as a therapy, particularly in cases of difficult tooth extraction [56]. A summary of some notable and current applications for wound healing is shown below; these are also included in Table 3.
2.4. Curcumin in the Cosmetic Industry
Curcumin has long been used in the cosmetics sector due to its anti-inflammatory and antioxidant characteristics. Curcumin has shown great potential for a wide range of cosmetic goods intended for the skin, face and hair because of its beneficial benefits against UV radiation, aging, inflammation and hair loss, and for lip and nail care [27].
The skin is in serious danger from ultraviolent B (UVB) radiation, which may lead to skin cancer in addition to sunburn, redness and early aging. In research by Li et al. [57], hairless mice and human keratino-cytes (HaCaT) were used to test curcumin’s photoprotective properties against UVB-induced acute damage. Results showed that the topical application of curcumin effectively reduced UVB-induced inflammation, collagen damage and lipid peroxidation in mice, while also promoting the accumulation of Nrf2 in the skin. Additionally, curcumin treatment in HaCaT cells reduced the UVB-induced release of lactate dehydrogenase, the generation of reactive oxygen species and DNA damage, while enhancing the expression of detoxifying enzymes and DNA repair activity [57]. Ahmed et al. [58] compared the effectiveness of nano-curcumin and a chemical sunscreen composed of phenylbenzimidazole-5-sulfonic acid (PBSA) for protecting rat skin from ultraviolet (UV) radiation damage. Rats were exposed to UV radiation, with some being treated with nano-curcumin, some with PBSA and some being fully untreated. Untreated skin displayed notable damage under the microscope, while skin treated with nano-curcumin appeared mostly normal. Interestingly, nano-curcumin was able to provide better protection than the conventional sunscreen PBSA [58].
The aging process in humans is a highly detailed biochemical and physiological mechanism influenced by a complex interplay of genetic and environmental variables, as aging triggers significant body changes. The most frequent and crucial ones are alterations in the immune response linked to distinct phases of cell differentiation and the occurrence of inflammaging, which is recognized as low-grade, subclinical inflammation indicated by increased levels of proinflammatory factors. Asada et al. [59,60] investigated whether a hot water extract of Curcuma longa (WEC) could improve skin health. They conducted cell studies where the extract was applied to skin cells exposed to ultraviolet B (UVB) light. The extract calmed inflammation and increased the production of hyaluronan, a molecule crucial for keeping the skin hydrated. To confirm these findings, they ran a clinical trial where participants received daily supplements containing the extract, with or without additional turmeric extract, or a placebo for eight weeks. Results showed that the extract alone markedly improved facial skin hydration compared to the placebo [59,60]. Studies on the cosmetic uses of curcumin have been widely conducted, and some of the most recent ones are included in Table 4.

Table 4.
Indicative studies of curcumin in the cosmetic field.

Table 3.
Indicative studies of curcumin in wound healing procedures.
Table 3.
Indicative studies of curcumin in wound healing procedures.
| Aim of the Study | Study Design | Results | Reference |
|---|---|---|---|
| Researchers looked at how individuals with DFU’s metabolic condition and wound healing responded to curcumin use (in vitro and in vivo). |
|
| [49] |
| The investigation of curcumin’s mechanism in DFU wound healing was evaluated in this clinical trial (in vitro and in vivo) |
|
| [50] |
| An assessment of curcumin’s ability to cure burn burns in rats was the primary study aim (in vivo experimental procedure) |
|
| [51] |
| An analysis of the impact of topical curcumin therapy on rat burn wound healing was also further analyzed in this study (in vivo and in vitro). |
|
| [52] |
| A study was done to evaluate the potential of electrospun PCL/GT nanofibers loaded with curcumin for wound healing in diabetic rats (in vivo and in vitro assay). |
|
| [53] |
| The hypothesis that nanohybrid scaffold of curcumin-loaded chitosan-derived nanoparticles or CUR-CSNPs would enhance diabetic wound healing was analyzed further (in vivo and in vitro) as the aim of the study. |
|
| [54] |
| Curcumin’s ability to facilitate cutaneous wound healing in diabetic rats by reducing inflammation, oxidative stress and promoting tissue regeneration was investigated extensively in this study (in vivo). |
|
| [55] |
| Topical application of Curcuma longa (turmeric) gel was discovered to improve healing of wounds and lessen postoperative pain after dental extraction in this in vivo and in vitro clinical trial. |
|
| [56] |
3. Curcumin’s Antioxidant, Anti-Inflammatory and Anticancer Potential
Curcumin has been exploited for centuries due to its non-toxic, antioxidant, analgesic, anti-septic, anti-inflammatory and anticancer profile. Several in vitro and in vivo studies have pointed out curcumin’s vast potential towards cancer’s treatment as a newly emerged therapeutic enhancer of currently available treatment protocols.
3.1. Curcumin’s Antioxidant and Pro-Oxidant Profile
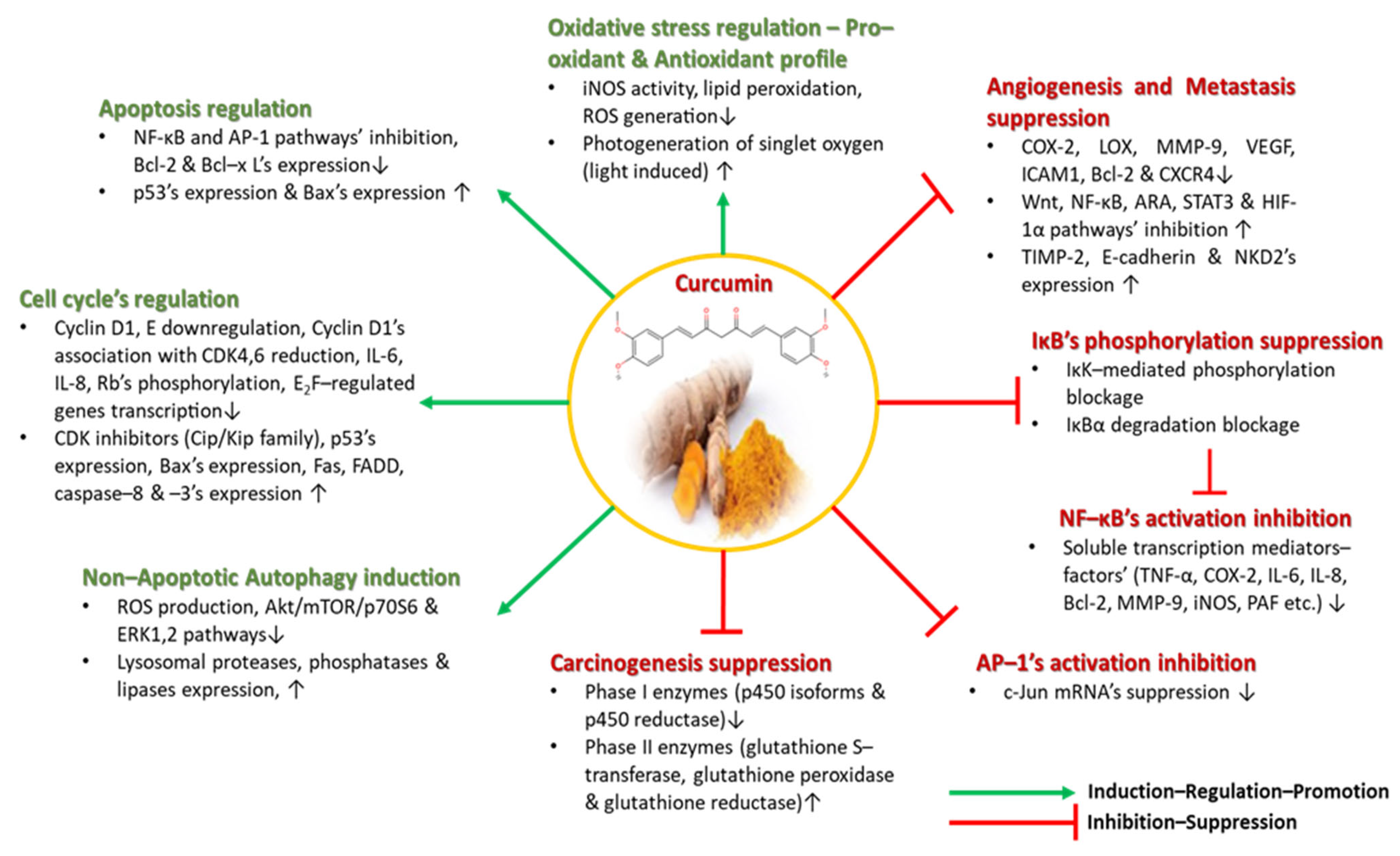
As a lipophilic, water-insoluble polyphenol, curcumin displays a great antioxidant as it is highly protective against ROS and pro-oxidant in character, due to the fact that when induced by light, it is able to initiate the photogeneration of singlet oxygen [61,62] to mobilize endogenous copper ions in order to kill malignant cancer cells, and, thus, results in anticancer, anti-tumor, anti-thrombosis and apoptosis-inducing effects [63,64].
Curcumin has been reportedly able to inhibit both lipid peroxidation and free-radical scavenging. Using oxidized linoleate as a fatty acid radical, curcumin acts as a chain–breaking factor at the 3′ position, and via an intramolecular Diels–Alder reaction and neutralization of the lipid radicals, hence it functions as a lipid peroxidation inhibitor with confirmed efficacy. Moreover, as a free-radical scavenger of various oxygen species generated by differentiated macrophages like superoxide anions, hydrogen peroxide and nitrite radicals, it has been implicated in downregulating iNOS activity by inhibiting macrophages to generate nitric oxide (NO) that reacts with superoxide radicals so as to form the cell-toxic peroxynitrite and by reducing the oxidative stress-responsive ROS formation. As a consequence, many neuroinflammatory diseases related to oxidative stress like Alzheimer’s or Parkinson’s disease and other similar disorders could be efficiently confronted though curcumin’s activity [65,66,67].
3.2. Curcumin’s Anti-Inflammatory Profile
In general, curcumin has been found to be implicated in several anti-inflammatory manifestations, as an effective suppressor of many inflammation-related pathways including the NF-κΒ pathway, which is a transcription regulative factor implicated in inflammation, cellular proliferation and cell survival [62,65,68].
The NF-κΒ pathway is involved in the regulation of pro-inflammatory genes (IL–6, Il–8, TNF–α, iNOS, COX-2, Bcl-2, MMP-9, PAF, etc.) and cellular responses to inflammation-associated stimuli (cytokines, UV radiation, free radicals, hypoxia, several infectious antigens, etc.). The activation of such a potentially malignant pathway is highly related to angiogenesis, cancerous cells’ proliferation, tumor promotion and metastatic procedures’ induction [65,69,70,71]. Curcumin, specifically, may place an inhibitory effect on NF-κΒ pathway’s activation, as it has been reported to block the inhibitor kappa B kinase (IκΚ)-mediated phosphorylation of the inhibitor of NF-κΒ (IκΒ), as well as the NF-κΒ inhibitor’s (IκΒα) degradation, which both result in NF-κΒ’s inability to enter the nucleus and to initiate transcription. All aforementioned pro-inflammatory cytokines, chemokines and enzymes’ production is inhibited by treatment with curcumin or curcuminoid derivatives [65,70,71].
However, curcumin’s suppressive activity is also released upon other inflammatory pathways, including the ARA pathway which is responsible for generating reactive lipid products like prostaglandins, leukotrienes, prostacyclins and thromboxanes [65,72,73]. Curcumin is able to reduce ARA’s metabolism by mainly downregulating lipoxygenase’s (LOX) and COX-2′s activity, by limiting prostaglandin E2’s biosynthesis by directly inhibiting the corresponding enzyme’s action and by reducing oxidative stress factor’s transcription. The anti-inflammatory profile of curcumin has been thoroughly examined in many complications and diseases, such as Alzheimer’s disease [74], cardiovascular diseases [75,76], diabetes [77], inflammatory bowel disease (IBD) [78], renal diseases [79,80], asthma [81], etc.
3.3. Curcumin’s Anticancer Properties
The following of diets rich in curcumin in several Asian countries has been considered as the main reason why several human carcinomas, including melanoma, colon, ovarian, pancreatic, breast, intestinal, prostate, head and neck carcinomas, occur less frequently among these populations. Not only because it inhibits pro-inflammatory transcription factors, signaling proteins and oncogenes, but also because it targets many carcinogenesis stages like DNA mutations initiation, tumorigenesis–angiogenesis, tumor growth, and proliferation and metastasis, does curcumin hold great promise as a chemotherapeutic, cell-growth regulatory agent, for a plethora of cancer-related manifestations [82,83,84,85,86,87,88,89]. As a potent suppressor of carcinogenesis, curcumin, in addition to its antioxidant activity, affects Phase I and Phase II enzymes building the cytochrome p450 enzymatic system, which participate in the oxidation and detoxification of toxic substances [65]. Curcumin inhibits the Phase I enzymes (p450 isoforms and reductase) that are triggered by toxins and produce DNA damaging carcinogenic and mutagenic metabolites, while, on the other hand, it stimulates Phase II enzymes (glutathione S–transferase, glutathione peroxidase and glutathione reductase) that are implicated in the detoxification of harmful metabolites [65].
As previously mentioned, curcumin inhibits NF-κΒ pathway’s activation. The AP-1 pathway, similar to the NF-κΒ pathway, is also activated by pro-inflammatory/inflammation-associated stimuli and is involved through its c-Jun domain in cyclin D1 and p53 expression, p16′s expression downregulation and tumorigenic phenotype induction. Treatment with curcumin reportedly suppressed the c-Jun mRNA of the AP-1 pathway in vivo [90].
Cellular growth and proliferation is a highly regulated procedure summarized in a cell cycle; if the cell cycle is deregulated, the uncontrollable proliferation of normal cells occurs and, thus, tumorigenesis is initiated. Cell cycle’s control is monitored by cyclin-dependent kinases (CDKs) and the tumor suppressor gene p53, while various points of the cycle are regulated by cyclin/CDK complexes (cyclin D (D1)/CDK4,6, cyclin E/CDK2, cyclin A/CDK2 and cyclin B/CDK2). CDK inhibitors including the INK-4 and Cip/Kip families are highly affected by curcumin’s existence. Curcumin has been shown to upregulate Cip/Kip’s family of CDK inhibitors, and, hence, to inhibit cyclin D1′s association with CDK4,6, while decreasing Rb’s phosphorylation, IL-6’s and IL-8′s expression, and suppressing E2F-regulated genes’ transcription. Furthermore, it has been demonstrated to suppress cyclin D1, to initiate mitochondrial apoptosis via increased Bax expression and to upregulate p53, Fas, FADD, caspace–8 and caspase–3′s expression [65,91,92,93].
Autophagy is considered a Type II programmed cell death, where cells break down their own components, are able to dispose of old damaged organelles and proteins, upregulate lysosomal proteases, phosphatases and lipases, and recycle endogenous biosynthetic substrates like amino acids [65]. Curcumin partakes in autophagic cell death in cancer-associated diseases like malignant glioma cells, where it inhibits ROS production and oxidative stress induction, as well as inducing G2/M cell cycle arrest and non-apoptotic autophagic death by inhibiting the Akt/mTOR/p70S6 and ERK1,2 inflammatory pathways, as observed by in vivo tumor volume reduction [94,95,96].
At this point, it is important to elucidate curcumin’s effects on angiogenesis and metastasis procedures, as they are vicious cell cycle processes where the rapid proliferation and mitigation of malignant cancerous cells takes place. Curcumin treatment has been associated with the decreased expression of MMP-9, reduced levels of the angiogenic COX-2 and vascular endothelial growth factor (VEGF) biomarkers, and minimized levels of the intercellular adhesion molecule 1 (ICAM1), Bcl-2 and chemokine receptor 4 (CXCR4). Conversely, it increases the expression of anti-metastatic proteins like the tissue inhibitor metalloproteinase 2 (TIMP-2) and E–cadherin, and, thus, its anti-tumorigenic profile was confirmed in a plethora of clinical trials upon several cancer types. Furthermore, curcumin inhibits tumor epithelial–mesenchymal transition by downregulating the Wnt, NF-κΒ, STAT3 and HIF-1α pathways, while upregulating NKD2′s expression in cancer cells and modulation microRNA expression [65,97,98]. All of curcumin’s aforementioned antioxidant, anti-inflammatory and anticancer properties are depicted in Figure 4.
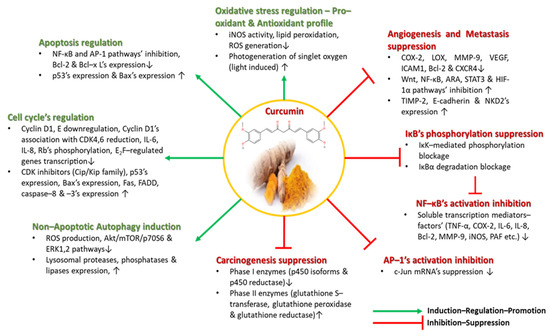
Figure 4.
Curcumin’s antioxidant, anti-inflammatory and anticancer properties.
4. Limitations and Potential Side Effects
Curcumin has attracted substantial attention as a bioactive component in various functional products, such as functional foods, dietary supplements, nutraceuticals and cosmetics. It has demonstrated a broad range of beneficial effects towards human pathological conditions and vast pharmacological activity including anti-inflammatory, antioxidant, antitumor and immune-regulating properties. Curcumin has also shown therapeutic promise in managing neurodegenerative, cardiovascular and cerebrovascular diseases [8]. A relatively low dose of the complex may promote many health benefits for people with undiagnosed health conditions. Curcumin also helps with the management of oxidative stress and inflammation-associated complications, such as metabolic syndrome, arthritis, anxiety, hyper-lipidemia and skin aging. It may also help reduce exercise-induced inflammation and muscle soreness, improving recovery and performance in active individuals. The majority of these advantages are really ascribed to its anti-inflammatory and antioxidant properties [8].
However, despite curcumin’s potential in managing several health conditions, it still faces significant challenges in clinical use. For example, one major issue is its extremely poor water solubility, low absorption in the small intestine and rapid elimination by the liver, leading to its poor bioavailability. Curcumin’s low bioavailability notably limits its clinical applications. Therefore, consuming curcumin alone often does not produce the expected health benefits, while there are many components able to increase its bioavailability. For instance, it has been demonstrated that combining piperine, a significant bioactive component of black pepper, with curcumin increases the resultant bioavailability by 2000%. Other methods involve forming curcumin complexes with metal ions, like Zn2+, Cu2+, Mg2+ and Se2+, or carriers like liposomes, polysaccharides, proteins (e.g., serum albumin), cyclodextrins and nanoparticles, all of which enhance curcumin’s solubility and pharmacological effectiveness [11,15].
The search term “curcumin” on the website “National Institutes of Health Clinical trial.gov” revealed hundreds of clinical studies, though only a few had published results available. While curcumin research remains active, compared to its pre-clinical study, its clinical study is insufficient and suffers from limitations such as small sample sizes, focus on specific diseases (mainly cancers like breast, colorecta or prostate cancer, etc.), variations in dosage and administration, and excessive study endpoints (e.g., survival, safety, tolerance). Consequently, curcumin still has significant hurdles to overcome before being widely applied in clinical practice [8].
Curcumin is generally considered safe, with long-established guidelines from organizations including the Joint United Nations and World Health Organization Expert Committee on Food Additives (JECFA), the European Union Food Science Committee (SCF) and the European Food Safety Authority (EFSA) reports, which set the Allowable Daily Intake (ADI) value of curcumin at 0–3 mg/kg body weight. While curcumin’s safety and effectiveness have been demonstrated in several clinical trials including healthy participants, excessive dosages may cause unintended adverse effects. For instance, consuming too much turmeric may raise oxalate levels in urine, which increases the chance of kidney stones developing in those who are vulnerable. Even though curcumin-induced iron failure may improve the anticancer effects, high dosages of curcumin should be administered cautiously in patients with subclinical iron shortage, chronic anemia and consumptive anemia produced by heavy burdens of malignant tumors. While cases of curcumin-induced liver damage are scarce, those that do exist highlight the significance of curcumin supplementation as a risk factor for drug-induced liver toxicity. Abnormal cardiac conductions influence the pharmacokinetic parameters when curcumin interacts with drugs like tamoxifen, edoxifen and cyclophosphamide, while allergic reactions to curcumin administration and several other risks have also been reported. Reportedly, curcumin may also not be appropriate for those who have a high risk of cancer [8].
A rather recent clinical trial warned the scientific community that several dietary supplements may seriously damage the liver if excessively used. Herbal and dietary supplements (HDSs), including turmeric, as well as green tea, black cohosh, Garcinia cambogia, yeast rice and ashwagandha, when administrated as dietary supplements, may induce liver damage at an excessive dose. Overconsumption of such botanical sources has led to severe hepatotoxicity and hepatocellular damage, while possibly being fatal, leading to urgent liver transplantation or even death, as scientists claimed. Study data from 2017–2021 on nearly 9700 adult patients were obtained from the National Health and Nutrition Examination Survey (NHANES), while baseline weighted characteristics of HDS users and users of the six preferred potentially hepatotoxic botanicals were compared to those of non-HDS users, using a multi-variable analysis for the risk factors associated with HDS use. Surprisingly, among the participants enrolled in this NHANES cohort, a high prevalence of these herbs’ (HDS users) rates were recorded (57.6%), while the most popular goods, notably among older customers with higher educational attainment and a higher likelihood of having arthritis, had botanicals that contained turmeric. In this survey study, as a conclusion, an estimated 15.6 million US adults had consumed at least one of the six listed botanicals with liver liability, with the purpose of improving their health and/or relieving arthritis-derived pain within the past 30 days. The estimated number of patients who used non-steroidal anti-inflammatory medicines (such as simvastatin) and a widely prescribed hypolipidemic medication, where a high risk of unfavorable interactions between HDSs and prescription medication was noted, was comparable to these results. Physicians should be aware of the serious consequences of overindulging in these highly unregulated consumables because of the lack of regulatory monitoring over the production and testing of botanical goods [99].
5. Conclusions and Perspectives
Considering all of the aforementioned points, we may conclude that curcumin exhibits great promise as a medicinal tool. Because curcumin affects a wide range of molecular targets, clinical research on people, both in vivo and in vitro, has demonstrated encouraging results for the prevention and treatment of various disorders. This polyphenolic molecule’s anti-inflammatory, antioxidant, antinociception, antibacterial and anticancer effects have been shown to account for the majority of its beneficial effects. Thus, curcumin, the primary bioactive component of turmeric, has demonstrated a broad variety of uses in the culinary, cosmetic and pharmaceutical industries.
However, as with any natural compound, curcumin is not a panacea, as difficulties such as its poor bioavailability, rapid metabolism, instability and possible toxicity when overconsumed limit its use. These challenges underline the need for further research and development, in order to resolve all existing disadvantages and to fully elucidate curcumin’s great potential. Overcoming these obstacles could unlock the full therapeutic potential of curcumin, ensuring that its benefits are consistently exploited in clinical settings.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; software, all authors; validation, A.T.; investigation, all authors; writing—original draft preparation, E.R., T.A. and A.T.; writing—review and editing, P.E., G.Z.K. and A.T.; visualization, A.T.; supervision, P.E., G.Z.K. and A.T.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the School of Chemistry of the Faculty of Sciences of the Democritus University of Thrace for the continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 4 August 2024).
- Travers, J.B.; Rohan, J.G.; Sahu, R.P. New Insights Into the Pathologic Roles of the Platelet-Activating Factor System. Front. Endocrinol. 2021, 12, 624132. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent Developments in Anti-inflammatory Natural Products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Deng, W.; Du, H.; Liu, D.; Ma, Z. The Role of Natural Products in Chronic Inflammation. Front. Pharmacol. 2022, 13, 901538. [Google Scholar] [CrossRef]
- Tsoupras, A.; Gkika, D.A.; Siadimas, I.; Christodoulopoulos, I.; Efthymiopoulos, P.; Kyzas, G.Z. The Multifaceted Effects of Non-Steroidal and Non-Opioid Anti-Inflammatory and Analgesic Drugs on Platelets: Current Knowledge, Limitations, and Future Perspectives. Pharmaceuticals 2024, 17, 627. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and Its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Hussain, Y.; Abdullah; Khan, F.; Alsharif, K.F.; Alzahrani, K.J.; Saso, L.; Khan, H. Regulatory Effects of Curcumin on Platelets: An Update and Future Directions. Biomedicines 2022, 10, 3180. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet Activation and Prothrombotic Mediators at the Nexus of Inflammation and Atherosclerosis: Potential Role of Antiplatelet Agents. Blood Rev. 2021, 45, 100694. [Google Scholar] [CrossRef]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [Google Scholar] [CrossRef]
- Tsoupras, A.; Pafli, S.; Stylianoudakis, C.; Ladomenou, K.; Demopoulos, C.A.; Philippopoulos, A. Anti-Inflammatory and Antithrombotic Potential of Metal-Based Complexes and Porphyrins. Compounds 2024, 4, 376–400. [Google Scholar] [CrossRef]
- Kumar, H.; Dhalaria, R.; Guleria, S.; Sharma, R.; Cimler, R.; Dhanjal, D.S.; Chopra, C.; Kumar, V.; Manickam, S.; Siddiqui, S.A.; et al. Advances in the Concept of Functional Foods and Feeds: Applications of Cinnamon and Turmeric as Functional Enrichment Ingredients. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Health Benefits, Extraction and Development of Functional Foods with Curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Kryst, J. Cosmetics Containing Turmeric in the Light of the Results of Scientific Research. Aesth. Cosmetol. Med. 2023, 12, 169–174. [Google Scholar] [CrossRef]
- Gopinath, H.; Karthikeyan, K. Turmeric: A Condiment, Cosmetic and Cure. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 16. [Google Scholar] [CrossRef]
- Sri, S.N.; Thiagarajan, R.; Manikandan, R.; Arumugam, M. Curcumin-Based Food Supplements: Challenges and Future Prospects. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Cambridge, MA, USA, 2019; pp. 119–128. [Google Scholar]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic Potential and Limitations of Curcumin as Antimetastatic Agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef] [PubMed]
- Carolina Alves, R.; Perosa Fernandes, R.; Fonseca-Santos, B.; Damiani Victorelli, F.; Chorilli, M. A Critical Review of the Properties and Analytical Methods for the Determination of Curcumin in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2019, 49, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of Curcumin-Loaded Nanocarriers for Food, Drug and Cosmetic Purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, Purification and Applications of Curcumin from Plant Materials-A Comprehensive Review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, C.; Fang, S.; McClements, D.J.; Ma, L.; Chen, X.; Zou, L.; Liang, R.; Liu, W. Study on Curcumin Encapsulated in Whole Nutritional Food Model Milk: Effect of Fat Content, and Partitioning Situation. J. Funct. Foods 2022, 90, 104990. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Toohey, M.J.; Fogliano, V.; Linnemann, A.R. Enriching Street-Vended Zobo (Hibiscus sabdariffa) Drink with Turmeric (Curcuma longa) to Increase Its Health-Supporting Properties. Food Funct. 2021, 12, 761–770. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Topczewska, J.; Ormian, M.; Saletnik, A.; Sokołowicz, Z.; Lechowska, J. The Effect of the Addition Turmeric on Selected Quality Characteristics of Duck Burgers Stored under Refrigeration. Appl. Sci. 2022, 12, 805. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F.; Paci, G. Effect of Turmeric Powder (Curcuma Longa L.) and Ascorbic Acid on Physical Characteristics and Oxidative Status of Fresh and Stored Rabbit Burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.J.F.; Srinivas, K. Neuroprotective Effect of Turmeric Extract in Combination with Its Essential Oil and Enhanced Brain Bioavailability in an Animal Model. Biomed. Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, S.-W.; Lee, G.-H.; Choi, M.-K.; Jung, H.-W.; Kim, Y.-J.; Kwon, H.-J.; Chae, H.-J. Turmeric Extract and Its Active Compound, Curcumin, Protect against Chronic CCl4-Induced Liver Damage by Enhancing Antioxidation. BMC Complement. Altern. Med. 2016, 16, 316. [Google Scholar] [CrossRef]
- Martins-de-Souza, D. Proteomics, Metabolomics, and Protein Interactomics in the Characterization of the Molecular Features of Major Depressive Disorder. Dialogues Clin. Neurosci. 2014, 16, 63–73. [Google Scholar] [CrossRef]
- Li, T.; Jin, J.; Pu, F.; Bai, Y.; Chen, Y.; Li, Y.; Wang, X. Cardioprotective Effects of Curcumin against Myocardial I/R Injury: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Front. Pharmacol. 2023, 14, 1111459. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-F.; Guo, Q.-H.; Wei, X.-Y.; Chen, S.-Y.; Deng, S.; Liu, J.-J.; Yin, N.; Liu, Y.; Zeng, W.-J. Cardioprotective Effect of Curcumin on Myocardial Ischemia/Reperfusion Injury: A Meta-Analysis of Preclinical Animal Studies. Front. Pharmacol. 2023, 14, 1184292. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Peng, J.; Li, J. Curcumin Attenuates Hypoxia/Reoxygenation-induced Myocardial Injury. Mol. Med. Rep. 2019, 20, 4821–4830. [Google Scholar] [CrossRef] [PubMed]
- Kamali Dolatabadi, L.; Emamghoreishi, M.; Namavar, M.R.; Badeli Sarkala, H. Curcumin Effects on Memory Impairment and Restoration of Irregular Neuronal Distribution in the Hippocampal CA1 Region After Global Cerebral Ischemia in Male Rats. Basic Clin. Neurosci. J. 2019, 10, 527–540. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L. In Vitro and In Vivo Cardioprotective Effects of Curcumin against Doxorubicin-Induced Cardiotoxicity: A Systematic Review. J. Oncol. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Sayevand, Z.; Nazem, F.; Nazari, A.; Sheykhlouvand, M.; Forbes, S.C. Cardioprotective Effects of Exercise and Curcumin Supplementation against Myocardial Ischemia–Reperfusion Injury. Sport Sci. Health 2022, 18, 1011–1019. [Google Scholar] [CrossRef]
- Neerati, P.; Devde, R.; Gangi, A.K. Evaluation of the Effect of Curcumin Capsules on Glyburide Therapy in Patients with Type-2 Diabetes Mellitus. Phytother. Res. 2014, 28, 1796–1800. [Google Scholar] [CrossRef]
- Han, Z.; Yao, L.; Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Fan, S.; Zhang, Z.; Gong, S.; Chang, S.; et al. Gut Microbiota Mediates the Effects of Curcumin on Enhancing Ucp1-Dependent Thermogenesis and Improving High-Fat Diet-Induced Obesity. Food Funct. 2021, 12, 6558–6575. [Google Scholar] [CrossRef]
- Zhu, X.; Du, S.; Yan, Q.; Min, C.; Zhou, N.; Zhou, W.; Li, X. Dietary Curcumin Supplementation Promotes Browning and Energy Expenditure in Postnatal Overfed Rats. Nutr. Metab. 2021, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhou, N.; Zheng, W.; Zhu, X.; Ling, R.; Zhou, W.; Li, X. Prepuberty Is a Window Period for Curcumin to Prevent Obesity in Postnatal Overfed Rats. Pediatr Res. 2024, 96, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.C.; Amaro, L.B.R.; Batista Jorge, A.H.; Lelis, S.D.F.; Lelis, D.D.F.; Guimarães, A.L.S.; Santos, S.H.S.; Andrade, J.M.O. Curcumin Improves Metabolic Response and Increases Expression of Thermogenesis-Associated Markers in Adipose Tissue of Male Offspring from Obese Dams. Mol. Cell. Endocrinol. 2023, 563, 111840. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A. Evaluation of Antimicrobial Activity of Curcumin Against Two Oral Bacteria. Autom. Control Intell. Syst. 2015, 3, 21. [Google Scholar] [CrossRef]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The Neuroprotective Effect of Curcumin against Cd-Induced Neurotoxicity and Hippocampal Neurogenesis Promotion through CREB-BDNF Signaling Pathway. Toxicology 2020, 442, 152542. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The Effects of Curcumin Intake on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-blind, Placebo-controlled Trial. Phytother. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef]
- Cao, M.; Duan, Z.; Wang, X.; Gong, P.; Zhang, L.; Ruan, B. Curcumin Promotes Diabetic Foot Ulcer Wound Healing by Inhibiting miR-152-3p and Activating the FBN1/TGF-β Pathway. Mol Biotechnol. 2024, 66, 1266–1278. [Google Scholar] [CrossRef]
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The Healing Effect of Curcumin on Burn Wounds in Rat. World J. Plast. Surg. 2015, 4, 29–35. [Google Scholar] [PubMed] [PubMed Central]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The Effects of Topical Treatment with Curcumin on Burn Wound Healing in Rats. J. Mol. Hist. 2013, 44, 83–90. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.; Abbinayah, D.; Selvakumar, D.; Jayakumar, N. Efficacy of Topical Curcuma Longa in the Healing of Extraction Sockets: A Split-Mouth Clinical Trial. Dent. Res. J. 2023, 20, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Gao, A.; Jiang, N.; Liu, Q.; Liang, B.; Li, R.; Zhang, E.; Li, Z.; Zhu, H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem. Photobiol. 2016, 92, 808–815. [Google Scholar] [CrossRef]
- Mohamad, E.; Rageh, M.; Ahmed, H. Curcumin Provides Skin Protection against UV Radiation. Egypt. J. Chem. 2023, 65, 1341–1343. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Asada, K.; Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Effects of Hot Water Extract of Curcuma Longa on Human Epidermal Keratinocytes in Vitro and Skin Conditions in Healthy Participants: A Randomized, Double-blind, Placebo-controlled Trial. J. Cosmet. Dermatol. 2019, 18, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Rane, G.; Kanchi, M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.; Tan, B.; Kumar, A.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef]
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; Oirdi, M.E.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef]
- Huang, M.-T. Antioxidant and Antitumorigenic Properties of Curcumin. In Food Factors for Cancer Prevention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 249–252. ISBN 978-4-431-67019-3. [Google Scholar]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; Volume 595, pp. 105–125. ISBN 978-0-387-46400-8. [Google Scholar]
- Kosmopoulou, D.; Lafara, M.-P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective Benefits of Rosmarinus Officinalis and Its Bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-κB Signaling Pathway by the Curcumin Analog, 3,5-Bis(2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Cho, S.-S.; Li, Y.; Bae, C.-S.; Park, K.M.; Park, D.-H. Anti-Inflammatory Effect of Curcuma Longa and Allium Hookeri Co-Treatment via NF-κB and COX-2 Pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Hong, J. Modulation of Arachidonic Acid Metabolism by Curcumin and Related -Diketone Derivatives: Effects on Cytosolic Phospholipase A2, Cyclooxygenases and 5-Lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of Curcumin in Diagnosis, Prevention, and Treatment of Alzheimer’s Disease. Neural Regener. Res. 2018, 13, 742–752. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.D.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, H.; Bu, L.; Chen, C.; Ye, X. Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 908077. [Google Scholar] [CrossRef]
- Ghosh, S.; Gehr, T.; Ghosh, S. Curcumin and Chronic Kidney Disease (CKD): Major Mode of Action through Stimulating Endogenous Intestinal Alkaline Phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef]
- Bagherniya, M.; Soleimani, D.; Rouhani, M.H.; Askari, G.; Sathyapalan, T.; Sahebkar, A. The Use of Curcumin for the Treatment of Renal Disorders: A Systematic Review of Randomized Controlled Trials. In Studies on Biomarkers and New Targets in Aging Research in Iran; Guest, P.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1291, pp. 327–343. ISBN 978-3-030-56152-9. [Google Scholar]
- Kim, D.H.; Phillips, J.F.; Lockey, R.F. Oral Curcumin Supplementation in Patients with Atopic Asthma. Allergy Rhinol. 2011, 2, ar-2011. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via miR-222-3p/SOX10/Notch Axis. Dis. Markers 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Zhu, T.; Lu, W.; Fu, Y. Curcumin Enhances the Anti-Cancer Efficacy of Paclitaxel in Ovarian Cancer by Regulating the miR-9-5p/BRCA1 Axis. Front. Pharmacol. 2023, 13, 1014933. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, Y.; Pu, C.; Wang, Z.; Deng, W.; Jin, X. Curcumin Inhibits Pancreatic Cancer Cell Proliferation by Regulating Beclin1 Expression and Inhibiting the Hypoxia-Inducible Factor-1α-Mediated Glycolytic Pathway. J. Gastrointest. Oncol. 2022, 13, 3254–3262. [Google Scholar] [CrossRef]
- Barcelos, K.A.; Mendonça, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165. [Google Scholar] [CrossRef]
- Termini, D.; Den Hartogh, D.J.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.; Petrică-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Diaconu, C.C.; Roman, V. The Effect of Resveratrol or Curcumin on Head and Neck Cancer Cells Sensitivity to the Cytotoxic Effects of Cisplatin. Nutrients 2020, 12, 2596. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.-S.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, X.; Cao, Z.; Ye, L.; Cao, Y.; Pan, J. Curcumin and Analogues against Head and Neck Cancer: From Drug Delivery to Molecular Mechanisms. Phytomedicine 2023, 119, 154986. [Google Scholar] [CrossRef] [PubMed]
- Hesari, A.; Ghasemi, F.; Salarinia, R.; Biglari, H.; Tabar Molla Hassan, A.; Abdoli, V.; Mirzaei, H. Effects of Curcumin on NF-κB, AP-1, and Wnt/Β-catenin Signaling Pathway in Hepatitis B Virus Infection. J. Cell. Biochem. 2018, 119, 7898–7904. [Google Scholar] [CrossRef]
- Ali, M.S.; Smiley, R. Curcumin Induces Apoptosis by Regulating the Cell Cycle Proteins in MDA-MB-231 Breast CancerCells. FASEB J. 2019, 33, 646.1. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular Targets of Curcumin in Breast Cancer (Review). Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of Curcumin-Induced Cell Cycle Arrest and Apoptosis by Cyclin-Dependent Kinase Inhibitor P21/WAF1/CIP1. Cell Cycle 2007, 6, 2953–2961. [Google Scholar] [CrossRef]
- Walker, B.C.; Adhikari, S.; Mittal, S. Therapeutic Potential of Curcumin for the Treatment of Malignant Gliomas. In Gliomas; Debinski, W., Ed.; Exon Publications: Brisbane, Australia, 2021; pp. 139–150. [Google Scholar] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef]
- Luís, Â.; Amaral, L.; Domingues, F.; Pereira, L.; Cascalheira, J.F. Action of Curcumin on Glioblastoma Growth: A Systematic Review with Meta-Analysis of Animal Model Studies. Biomedicines 2024, 12, 268. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Farshadi, M.; Zare, N.; Akhlagh, S.A.; Alipour Nosrani, E.; Mahjoubin-Tehran, M.; Kangari, P.; Sharafi, S.M.; Khan, H.; Aschner, M.; et al. Antimetastatic Effects of Curcumin in Oral and Gastrointestinal Cancers. Front. Pharmacol. 2021, 12, 668567. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Killian, P.H.; Melchart, D. The Role of Curcumin in Prevention and Management of Metastatic Disease. Int. J. Mol. Sci. 2018, 19, 1716. [Google Scholar] [CrossRef] [PubMed]
- Likhitsup, A.; Chen, V.L.; Fontana, R.J. Estimated Exposure to 6 Potentially Hepatotoxic Botanicals in US Adults. JAMA Netw. Open 2024, 7, e2425822. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).