Beta-Alanine Supplementation for CrossFit® Performance
Abstract
1. Introduction
2. Methods
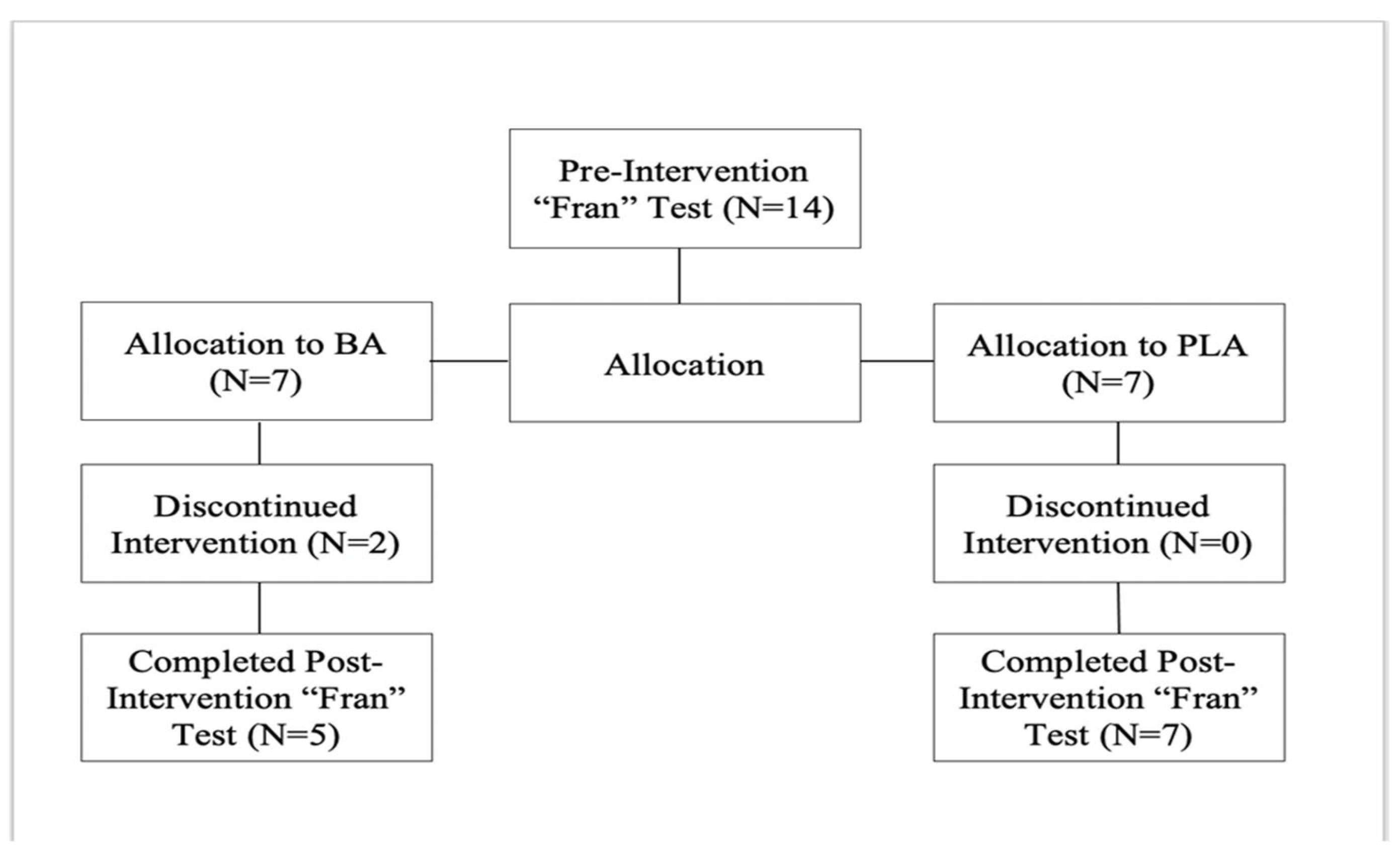
2.1. Participants
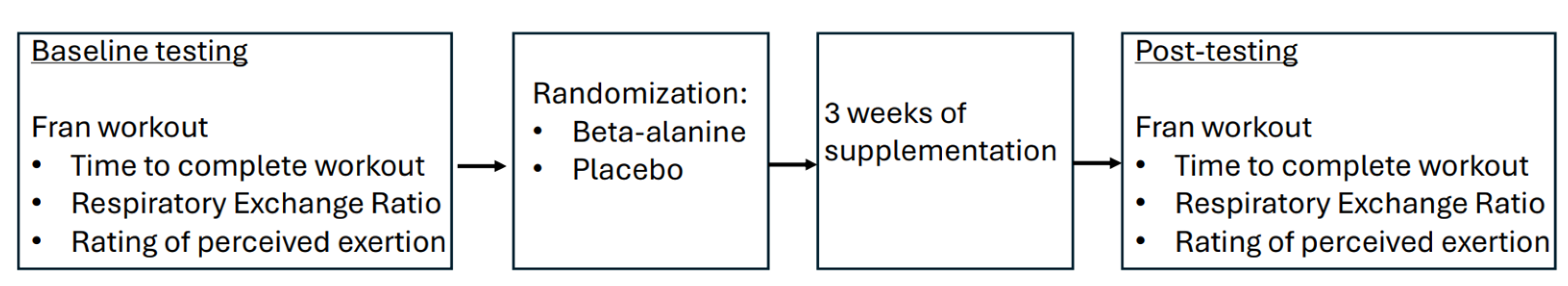
2.2. Protocol
2.3. Statistical Analysis
3. Results (See Data Available as Supplementary Material)
3.1. Performance
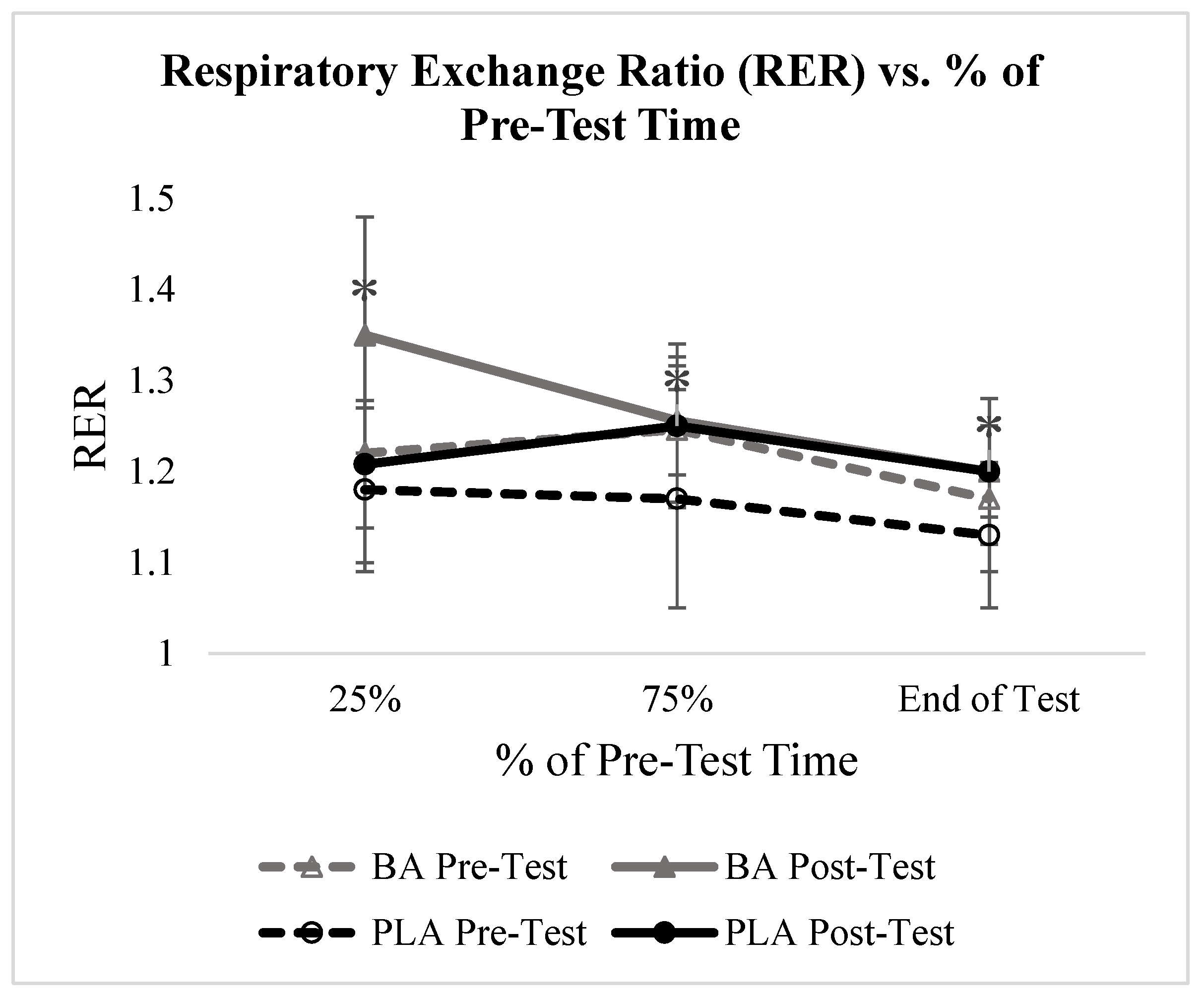
3.2. Respiratory Exchange Ratio
3.3. Rating of Perceived Exertion
3.4. Dietary Variables
4. Discussion
4.1. Performance
4.2. Respiratory Exchange Ratio
4.3. Rating of Perceived Exertion
4.4. Strengths
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butcher, S.J.; Neyedly, T.J.; Horvey, K.J.; Benko, C.R. Do physiological measures predict selected CrossFit(®) benchmark performance? Open Access J. Sports Med. 2015, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Escobar, K.A.; Morales, J.; Vandusseldorp, T.A. Metabolic profile of a high intensity interval training training bout. J. Hum. Sport Exerc. 2017, 12, 1248–1255. [Google Scholar] [CrossRef]
- Tibana, R.A.; De Sousa, N.M.F.; Prestes, J.; Voltarelli, F.A. Lactate, Heart Rate and Rating of Perceived Exertion Responses to Shorter and Longer Duration CrossFit® Training Sessions. J. Funct. Morphol. Kinesiol. 2018, 3, 60. [Google Scholar] [CrossRef] [PubMed]
- Leitão, L.; Dias, M.; Campos, Y.; Vieira, J.G.; Sant’Ana, L.; Telles, L.G.; Tavares, C.; Mazini, M.; Novaes, J.; Vianna, J. Physical and Physiological Predictors of FRAN CrossFit® WOD Athlete’s Performance. Int. J. Environ. Res. Public Health 2021, 18, 4070. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, J.; Solana, R.S.; Moya, D.; Marin, J.M.S.; Ramon, M.M. Acute physiological responses during CrossFit® workouts. Eur. J. Hum. Move 2015, 35, 114–124. [Google Scholar]
- de Souza, R.A.S.; da Silva, A.G.; de Souza, M.F.; Souza, L.K.F.; Roschel, H.; da Silva, S.F.; Saunders, B. A Systematic Review of CrossFit® Workouts and Dietary and Supplementation Interventions to Guide Nutritional Strategies and Future Research in High intensity interval training®. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 187–205. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Hill, C.A.; Harris, R.C.; Kim, H.J.; Harris, B.D.; Sale, C.; Boobis, L.H.; Kim, C.K.; Wise, J.A. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 2007, 32, 225–233. [Google Scholar] [CrossRef]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From exercise performance to health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of acute alkalosis and acidosis on performance: A meta-analysis. Sports Med. 2011, 41, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, P.M.; Minahan, C.L. Metabolic consequences of β-alanine supplementation during exhaustive supramaximal cycling and 4000-m time-trial performance. Appl. Physiol. Nutr. Metab. 2016, 41, 864–871. [Google Scholar] [CrossRef] [PubMed]
- de Salles Painelli, V.; Roschel, H.; Jesus, F.D.; Sale, C.; Harris, R.C.; Solis, M.Y.; Benatti, F.B.; Gualano, B.; Lancha, A.H., Jr.; Artioli, G.G. The ergogenic effect of beta-alanine combined with sodium bicarbonate on high-intensity swimming performance. Appl. Physiol. Nutr. Metab. 2013, 38, 525–532. [Google Scholar] [CrossRef]
- Baguet, A.; Bourgois, J.; Vanhee, L.; Achten, E.; Derave, W. Important role of muscle carnosine in rowing performance. J. Appl. Physiol. 2010, 109, 1096–1101. [Google Scholar] [CrossRef]
- Hobson, R.M.; Harris, R.C.; Martin, D.; Smith, P.; Macklin, B.; Gualano, B.; Sale, C. Effect of beta-alanine, with and without sodium bicarbonate, on 2000-m rowing performance. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 480–487. [Google Scholar] [CrossRef]
- Gilsanz, L.; López-Seoane, J.; Jiménez, S.L.; Pareja-Galeano, H. Effect of β-alanine and sodium bicarbonate co-supplementation on the body’s buffering capacity and sports performance: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5080–5093. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Anwander, H.; Egger, A.; Buehler, T.; Kreis, R.; Decombaz, J.; Boesch, C. Effect of two β-alanine dosing protocols on muscle carnosine synthesis and washout. Amino Acids 2012, 42, 2461–2472. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef]
- Hinckson, E.A.; Hopkins, W.G. Reliability of time to exhaustion analyzed with critical-power and log-log modeling. Med. Sci. Sports Exerc. 2005, 37, 696–701. [Google Scholar] [CrossRef]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.A.; Ramirez, M.; Heinrich, K.M. Acute Caffeine Supplementation Does Not Improve Performance in Trained CrossFit® Athletes. Sports 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jiménez, A.; Hernández-Torres, R.P.; Torres-Durán, P.V.; Romero-Gonzalez, J.; Mascher, D.; Posadas-Romero, C.; Juárez-Oropeza, M.A. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin. Med. Circ. Respirat. Pulm. Med. 2008, 2, CCRPM-S449. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Snarr, R.L.; Esco, M. Metabolic and cardiovascular response to the CrossFit workout ‘Cindy’: A pilot study. J. Sport. Hum. Perform. 2014, 2. [Google Scholar]
- Huerta Ojeda, Á.; Tapia Cerda, C.; Poblete Salvatierra, M.F.; Barahona-Fuentes, G.; Jorquera Aguilera, C. Effects of Beta-Alanine Supplementation on Physical Performance in Aerobic-Anaerobic Transition Zones: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2490. [Google Scholar] [CrossRef]
- Bellinger, P.M.; Minahan, C.L. The effect of β-alanine supplementation on cycling time trials of different length. Eur. J. Sport Sci. 2016, 16, 829–836. [Google Scholar] [CrossRef]
- Brisebois, M.; Kramer, S.; Lindsay, K.G.; Wu, C.T.; Kamla, J. Dietary practices and supplement use among CrossFit® participants. J. Int. Soc. Sports Nutr. 2022, 19, 316–335. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef]
| Variable | Group | |
|---|---|---|
| Beta-Alanine | Placebo | |
| Sex | 2 Female 3 Male | 3 Female 4 Male |
| Age (years) | 30 ± 9.8 | 34 ± 9.2 |
| Weight (kg) | 79.3 ± 14.0 | 73.8 ± 13.4 |
| Height (cm) | 173.0 ± 9.6 | 171.6 ± 7.7 |
| Body mass Index (kg/m2) | 22.8 ± 3.0 | 21.4 ± 3.1 |
| Years of CrossFit®® | 4.3 ± 3.4 | 8.4 ± 3.7 |
| VO2peak (mL/kg/min) (n = 1 female and 3 males from the beta-alanine group and 3 males from the placebo group) | 60 ± 13 | 56 ± 3 |
| Number of Participants with Previous Experience doing the “Fran” Workout of the Day | 4/5 | 7/7 |
| Pre-Intervention | Post-Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) | Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) | |
| Beta-alanine | 1931 ± 324 | 115 ± 39 | 199 ± 63 | 78 ± 11 | 1888 ± 81 | 130 ± 11 | 197 ± 15 | 68 ± 23 |
| Placebo | 1955 ± 322 | 130 ± 51 | 215 ± 27 | 66 ± 9 | 1954 ± 420 | 135 ± 42 | 203 ± 44 | 68 ± 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verity, H.; Candow, D.; Chilibeck, P.D. Beta-Alanine Supplementation for CrossFit® Performance. Nutraceuticals 2024, 4, 673-682. https://doi.org/10.3390/nutraceuticals4040037
Verity H, Candow D, Chilibeck PD. Beta-Alanine Supplementation for CrossFit® Performance. Nutraceuticals. 2024; 4(4):673-682. https://doi.org/10.3390/nutraceuticals4040037
Chicago/Turabian StyleVerity, Hannah, Darren Candow, and Philip D. Chilibeck. 2024. "Beta-Alanine Supplementation for CrossFit® Performance" Nutraceuticals 4, no. 4: 673-682. https://doi.org/10.3390/nutraceuticals4040037
APA StyleVerity, H., Candow, D., & Chilibeck, P. D. (2024). Beta-Alanine Supplementation for CrossFit® Performance. Nutraceuticals, 4(4), 673-682. https://doi.org/10.3390/nutraceuticals4040037








