Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biosynthesis of AgNPs and Characterization by UV-Vis Spectroscopy
2.2. Thermogravimetric Analysis (TGA)
2.3. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.4. X-ray Diffraction (XRD) Analysis
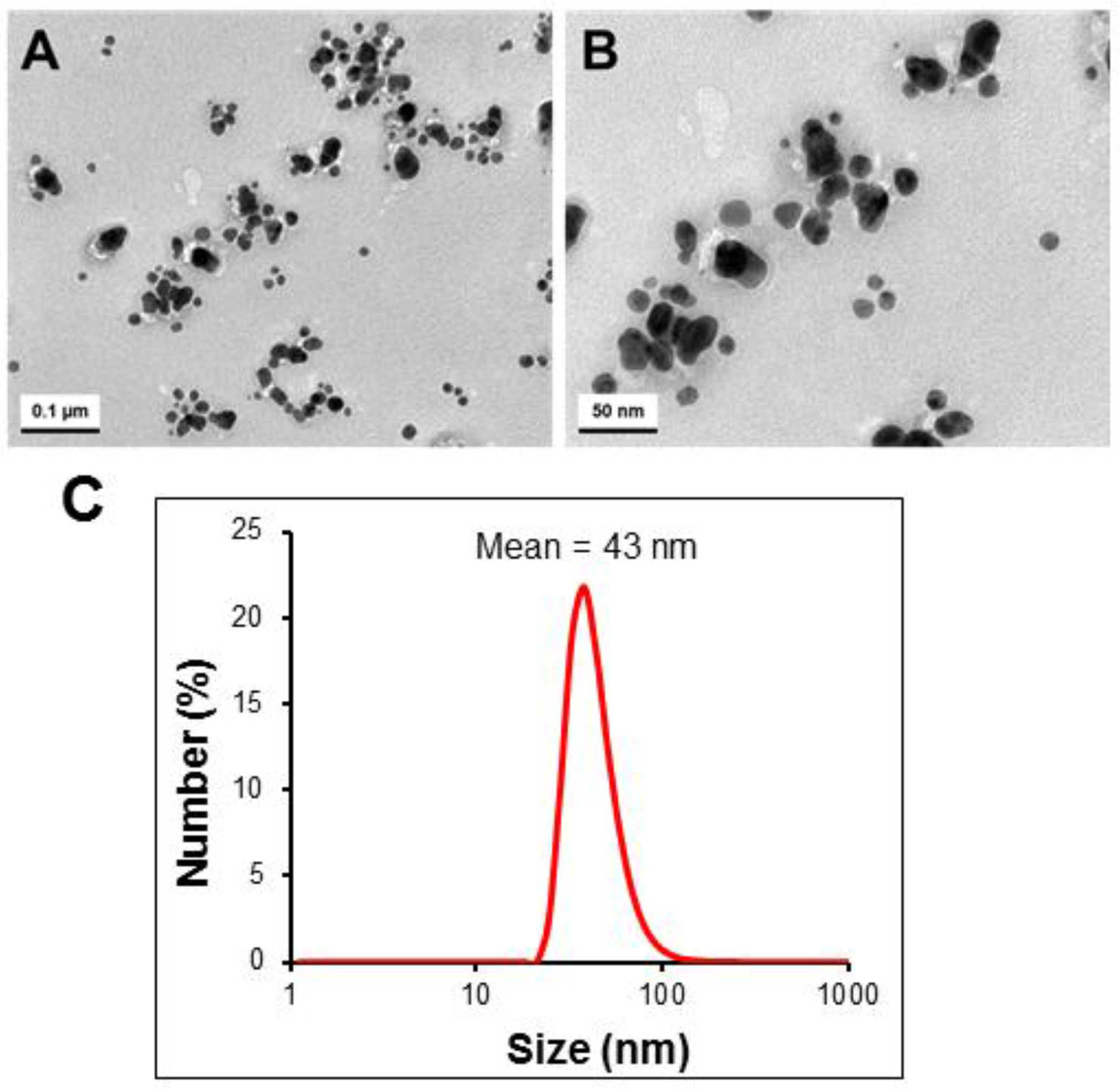
2.5. Size and Morphology Analysis of Biosynthesized AgNPs
2.6. Antimicrobial Activity by Biosynthesized AgNPs
2.7. Antioxidant Activity by Biosynthesized AgNPs
2.8. Anticancer Activity by Biosynthesized AgNPs
2.9. Apoptosis Assay
3. Materials and Methods
3.1. Materials
3.2. Preparation of Aqueous Extract of Ecklonia cava and Biosynthesis of AgNPs
3.3. UV-Visible Spectroscopy
3.4. Thermogravimetric Analysis
3.5. Fourier Transform-Infrared Spectroscopy
3.6. X-ray Diffraction Analysis
3.7. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) Analysis
3.8. Agar Well Diffusion Assay
3.9. DPPH Radical Scavenging Assay
3.10. Cytotoxicity Assay
3.11. Optical Microscopy Analysis
3.12. Annexin V-FITC/Propidium Iodide (PI) Staining
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2016. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E.; Ferdov, S.; Singh, M.P. Silver nanoparticles: Synthesis, properties, and applications. Adv. Colloid Interface Sci. 2015, 2015, 624394. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Gengan, R.; Anand, K.; Phulukdaree, A.; Chuturgoon, A. A549 lung cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids Surf. B 2013, 105, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.-M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B 2016, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, N.; Laskar, R.A.; Sk, I.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids Surf. B 2011, 82, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Yin, B.; Ma, H.; Wang, S.; Chen, S. Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrrolidone). J. Phys. Chem. B 2003, 107, 8898–8904. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Bartels, D.M.; Jonah, C.D.; Takahashi, K.; Rajh, T. Radiolytically induced formation and optical absorption spectra of colloidal silver nanoparticles in supercritical ethane. J. Phys. Chem. B 2001, 105, 954–959. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Roy, N.; Mondal, S.; Laskar, R.A.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Au and Ag nanoparticles by indian propolis and its constituents. Colloids Surf. B 2010, 76, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Roy, N.; Mandal, D.; Begum, N.A. Green chemistry for nanochemistry: Exploring medicinal plants for the biogenic synthesis of metal NPs with fine-tuned properties. RSC Adv. 2013, 3, 11935–11956. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Merin, D.D.; Prakash, S.; Bhimba, B.V. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac. J. Trop. Med. 2010, 3, 797–799. [Google Scholar] [CrossRef]
- Verma, V.C.; Kharwar, R.N.; Gange, A.C. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 2010, 5, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar] [PubMed]
- Kumar, P.; Senthamil Selvi, S.; Lakshmi Prabha, A.; Prem Kumar, K.; Ganeshkumar, R.; Govindaraju, M. Synthesis of silver nanoparticles from Sargassum tenerrimum and screening phytochemicals for its antibacterial activity. Nano Biomed. Eng. 2012, 4, 12–16. [Google Scholar] [CrossRef]
- Rajesh, S.; Raja, D.P.; Rathi, J.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopest. 2012, 5, 119–128. [Google Scholar]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Synthesis and characterization of antimicrobial silver nanoparticles using marine brown seaweed Padina tetrastromatica. Drug Invent. Today 2012, 4, 511–513. [Google Scholar]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int. J. Pharm. Biol. Sci. 2012, 3, 502–510. [Google Scholar]
- Sahayaraj, K.; Rajesh, S.; Rathi, J. Silver nanoparticles biosynthesis using marine algae Padina pavonica (Linn.) and its microbial activity. Dig. J. Nanomater. Biostruct. 2012, 7, 1557–1567. [Google Scholar]
- Dar, M.A.; Ingle, A.; Rai, M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectriasp. evaluated singly and in combination with antibiotics. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- El-Rafie, H.; El-Rafie, M.; Zahran, M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Selvi, S.S.; Govindaraju, M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl. Nanosci. 2013, 3, 495–500. [Google Scholar] [CrossRef]
- Mohandass, C.; Vijayaraj, A.; Rajasabapathy, R.; Satheeshbabu, S.; Rao, S.; Shiva, C.; De-Mello, I. Biosynthesis of silver nanoparticles from marine seaweed Sargassum cinereum and their antibacterial activity. Indian J. Pharm. Sci. 2013, 75, 606–610. [Google Scholar] [PubMed]
- Shiny, P.; Mukherjee, A.; Chandrasekaran, N. Marine algae mediated synthesis of the silver nanoparticles and its antibacterial efficiency. Int. J. Pharm. Pharm. Sci. 2013, 5, 239–241. [Google Scholar]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Angel, K.J.; Govindaraju, K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C.; Paulkumar, K.; Vanaja, M.; Gnanajobitha, G.; Annadurai, G. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int. J. Met. 2014, 2014, 692643. [Google Scholar] [CrossRef]
- Sinha, S.N.; Paul, D.; Halder, N.; Sengupta, D.; Patra, S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015, 5, 703–709. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Rajesh, K.M.; Sreedhara Reddy, P. Sesbania grandiflora leaf extract assisted green synthesis of silver nanoparticles: Antimicrobial activity. Mater. Today Proc. 2016, 3, 1977–1984. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmed, A.S.; Ahmed, F.; Ahmad, E.; Sherwani, A.; Owais, M.; Azam, A. Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS ONE 2013, 8, e59140. [Google Scholar] [CrossRef] [PubMed]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Antimicrobial and cytotoxicity effect of silver nanoparticle synthesized by Croton bonplandianum Baill. leaves. Nanomed. J. 2016, 3, 15–22. [Google Scholar]
- Abdel-Aziz, M.S.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
- Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113–121. [Google Scholar]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arabian J. Chem. 2015. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar]
- Ebrahiminezhad, A.; Bagheri, M.; Taghizadeh, S.-M.; Berenjian, A.; Ghasemi, Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015018. [Google Scholar] [CrossRef]
- Sidjui, L.; Ponnanikajamideen, M.; Malini, M.; Famen, L.; Sindhu, R.; Chandirika, J.U.; Annadurai, G.; Folefoc, G. Lovoa trichilioides root back mediated green synthesis of silver nanoparticles and rating of its antioxidant and antibacterial activity against clinical pathogens. J. Nanosci. Technol. 2015, 2, 32–36. [Google Scholar]
- Gandhi, N.; Sirisha, D.; Sharma, V.C. Microwave-mediated green synthesis of silver nanoparticles using Ficus elastica leaf extract and application in air pollution controlling studies. Int. J. Eng. Res. Appl. 2014, 4, 61–72. [Google Scholar]
- Antony, J.J.; Sithika, M.A.; Joseph, T.A.; Suriyakalaa, U.; Sankarganesh, A.; Siva, D.; Kalaiselvi, S.; Achiraman, S. In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in dal induced mice model. Colloids Surf. B 2013, 108, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Inbathamizh, L.; Ponnu, T.M.; Mary, E.J. In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J. Pharm. Res. 2013, 6, 32–38. [Google Scholar] [CrossRef]
- Kaler, A.; Jain, S.; Banerjee, U.C. Green and rapid synthesis of anticancerous silver nanoparticles by Saccharomyces boulardii and insight into mechanism of nanoparticle synthesis. Biomed. Res. Int. 2013, 2013, 872940. [Google Scholar] [CrossRef] [PubMed]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B 2013, 102, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, T.V.M.; Nagajyothi, P.; Prasad, T.N.V.K.V.; Lee, K. Green synthesis of silver nanoparticles using Citrus reticulata juice and evaluation of their antibacterial activity and cytotoxicity against melanoma-B16/F10 cells. Curr. Nanosci. 2013, 9, 457–462. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2014, 5, 499–504. [Google Scholar] [CrossRef]
- Salari, Z.; Danafar, F.; Dabaghi, S.; Ataei, S.A. Sustainable synthesis of silver nanoparticles using macroalgae spirogyra varians and analysis of their antibacterial activity. J. Saudi Chem. Soc. 2016, 20, 459–464. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J. Agardh. Mater. Lett. 2014, 137, 1–4. [Google Scholar] [CrossRef]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef]
- Haghighi Pak, Z.; Abbaspour, H.; Karimi, N.; Fattahi, A. Eco-friendly synthesis and antimicrobial activity of silver nanoparticles using Dracocephalum moldavica seed extract. Appl. Sci. 2016, 6, 69. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. Peel extract. Nanoscale Res. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kayalvizhi, T.; Ravikumar, S.; Venkatachalam, P. Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J. Environ. Eng. 2016, 142, C4016002. [Google Scholar] [CrossRef]
- Patil, M.P.; Rokade, A.A.; Ngabire, D.; Kim, G.-D. Green synthesis of silver nanoparticles using water extract from galls of Rhus chinensis and its antibacterial activity. J. Clust. Sci. 2016, 27, 1737–1750. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Biorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Lee, H.S.; Shin, H.-C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for alzheimer’s disease therapy from Ecklonia cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Kim, S.-C.; Kim, M.-K.; Boo, H.-J.; Jeon, Y.-J.; Koh, Y.-S.; Yoo, E.-S.; Kang, S.-M.; Kang, H.-K. Effect of dieckol, a component of Ecklonia cava, on the promotion of hair growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Heo, S.-J.; Kim, K.-N.; Lee, S.-H.; Jeon, Y.-J. Isolation and identification of new compound, 2,7″-phloroglucinol-6,6′-bieckol from brown algae, Ecklonia cava and its antioxidant effect. J. Funct. Foods 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.-K.; Byun, H.-G.; Jeon, Y.-J.; Kim, S.-K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-G.; Kang, O.-H.; Brice, O.-O.; Lee, Y.-S.; Chae, H.-S.; Oh, Y.-C.; Sohn, D.-H.; Park, H.; Choi, H.-G.; Kim, S.-G. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart. Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009, 44, 939–943. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Antimicrobial activities of silver nanoparticles synthesized by using water extract of arnicae anthodium. Indian J. Microbiol. 2015, 55, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, M.; Müller, N.; Frasés, S.; Almeida-Paes, R.; Lima, L.M.T.R.; Lemgruber, L.; Farina, M.; de Souza, W.; Sant’Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef]
- Kang, Y.O.; Lee, T.S.; Park, W.H. Green synthesis and antimicrobial activity of silver chloride nanoparticles stabilized with chitosan oligomer. J. Mater. Sci. Mater. Med. 2014, 25, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Wang, B.; Zada, A.; Humayun, M. Biochemical synthesis of Ag/AgCl nanoparticles for visible-light-driven photocatalytic removal of colored dyes. Materials 2015, 8, 2043–2053. [Google Scholar] [CrossRef]
- Kumar, V.A.; Uchida, T.; Mizuki, T.; Nakajima, Y.; Katsube, Y.; Hanajiri, T.; Maekawa, T. Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015002. [Google Scholar] [CrossRef]
- Devi, T.B.; Ahmaruzzaman, M.; Begum, S. A rapid, facile and green synthesis of Ag@AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. New J. Chem. 2016, 40, 1497–1506. [Google Scholar] [CrossRef]
- Durán, N.; Cuevas, R.; Cordi, L.; Rubilar, O.; Diez, M.C. Biogenic silver nanoparticles associated with silver chloride nanoparticles (Ag@AgCl) produced by laccase from Trametes versicolor. SpringerPlus 2014, 3, 645. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.-I.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Kim, S.-K. Handbook of Anticancer Drugs from Marine Origin; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Kim, S.-K. Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B 2013, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R.Br.) Asch. & Magnus mediated silver nanoparticles. J. Photochem. Photobiol. B 2015, 153, 145–152. [Google Scholar] [PubMed]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]










| No. | Species | Reaction Time | Size | Applications | Ref. |
|---|---|---|---|---|---|
| 1 | Trichoderma viride | 48 h | 5–40 nm | Antimicrobial | [13] |
| 2 | Chaetoceros calcitrans | 2 weeks | N.A. | Antimicrobial | [22] |
| 3 | Aspergillus clavatus | N.A. | 10–25 nm | Antifungal | [23] |
| 4 | Porphyra vietnamensis | 15 min | 13 ± 3 nm | Antibacterial | [24] |
| 5 | Gelidiella acerosa | 48 h | 22 nm | Antifungal | [25] |
| 6 | Sargassum tenerrimum | 20 min | 20 nm | Anti-bacterial | [26] |
| 7 | Ulva fasciata | N.A | 28–41 nm | Antimicrobial | [27] |
| 8 | Padina tetrastromatica | 24 h | 14 nm | Antibacterial | [28] |
| 9 | Turbinaria conoides | N.A | 96 nm | Antibacterial | [29] |
| 10 | Padina pavonica | 24 h | 46.8 nm | Antimicrobial | [30] |
| 11 | Cryphonectria | 24 h | 30–70nm | Antimicrobial | [31] |
| 12 | Pterocladia capillacae, Jania rubins, Ulva faciata, Colpmenia sinusa | 3 h | 20 nm | Antimicrobial | [32] |
| 13 | Gracilaria corticata | 20 min | 18–46 nm | Antifungal | [33] |
| 14 | Sargassum cinereum | 3 h | 45–76 nm | Antimicrobial | [34] |
| 15 | Padina gymnospora | N.A. | 25–40 nm | Antimicrobial | [35] |
| 16 | Sargassum plagiophyllum | 24 h | 21–48 nm | Antimicrobial | [36] |
| 17 | Sargassum longifolium | 64 h | N.A. | Antifungal | [37] |
| 18 | Pithophora oedogonia | N.A. | 34.03 nm | Antibacterial | [38] |
| 19 | Sesbania grandiflora | 15 min | 12 nm | Antimicrobial | [39] |
| 20 | Stenotrophomonas maltophilia | N.A. | 93 nm | Antimicrobial | [40] |
| 21 | Croton bonplandianum | N.A. | 32 nm | Antimicrobial and anticancer | [41] |
| 22 | Chenopodium murale | N.A. | 30–50 nm | Antimicrobial | [42] |
| 23 | Alternanthera sessilis | N.A. | 10–30 nm | Anticancer | [43] |
| 24 | Pseudomonas putida | 20 min | 6–16 nm | Antibacterial and anticancer | [44] |
| 25 | Citrullus colocynthis | 24 h | 31 nm | Anticancer | [45] |
| 26 | Chlorella vulgaris | 24 h | 7 nm | Anticancer and antimicrobial | [46] |
| 27 | Lovoa trichilioïdes | 2 h | 37–43 nm | Anti-bacterial | [47] |
| 28 | Ficus elastica | 30 min | 50–60 nm | Air pollution control | [48] |
| 29 | Ficus religiosa | 10 min | 5–35 nm | In vivo antitumor | [49] |
| 30 | Morinda pubescens | N.A. | N.A. | Antioxidant and anticancer | [50] |
| 31 | Saccharomyces boulardii | 4 h | 3–10 nm | Anticancer | [51] |
| 32 | Alternanthera sessilis (Linn.) | N.A. | 20–30 nm | Antimicrobial and antioxidant | [52] |
| 33 | Citrus reticulata juice | N.A. | N.A. | Antimicrobial | [53] |
| 34 | Caulerpa racemosa | 24 h | 5–25 nm | Antibacterial | [54] |
| 35 | Spirogyra varians | N.A. | 17.6 nm | Antibacterial | [55] |
| 36 | Enteromorpha flexuos | N.A. | 2–32 nm | Antimicrobial | [56] |
| 37 | Cyanobacteria and Microalgae | 72 h | 13–31 nm | Antibacterial | [57] |
| 38 | Dracocephalum moldavica | 1 h | 31 ± 6 nm | Antimicrobial | [58] |
| 39 | Dimocarpus longan Lour. | 5 h | 9–32 nm | Anticancer | [59] |
| 40 | Curculigo orchioides rhizome | N.A. | 15–18 nm | Larvicidal and Anticancer | [60] |
| 41 | Rhus chinensis | 12 h | 150 nm. | Antibacterial | [61] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, J.; Kim, S.-K.; Shim, M.S. Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava. Nanomaterials 2016, 6, 235. https://doi.org/10.3390/nano6120235
Venkatesan J, Kim S-K, Shim MS. Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava. Nanomaterials. 2016; 6(12):235. https://doi.org/10.3390/nano6120235
Chicago/Turabian StyleVenkatesan, Jayachandran, Se-Kwon Kim, and Min Suk Shim. 2016. "Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava" Nanomaterials 6, no. 12: 235. https://doi.org/10.3390/nano6120235
APA StyleVenkatesan, J., Kim, S.-K., & Shim, M. S. (2016). Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava. Nanomaterials, 6(12), 235. https://doi.org/10.3390/nano6120235