The Migration of Cd in Granitic Residual Soil and Marine Clay: Batch and Column Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Experimental Procedures
2.3. Adsorption Test
2.3.1. Batch Equilibrium Test
2.3.2. Column Infiltration Test
3. Results and Discussions
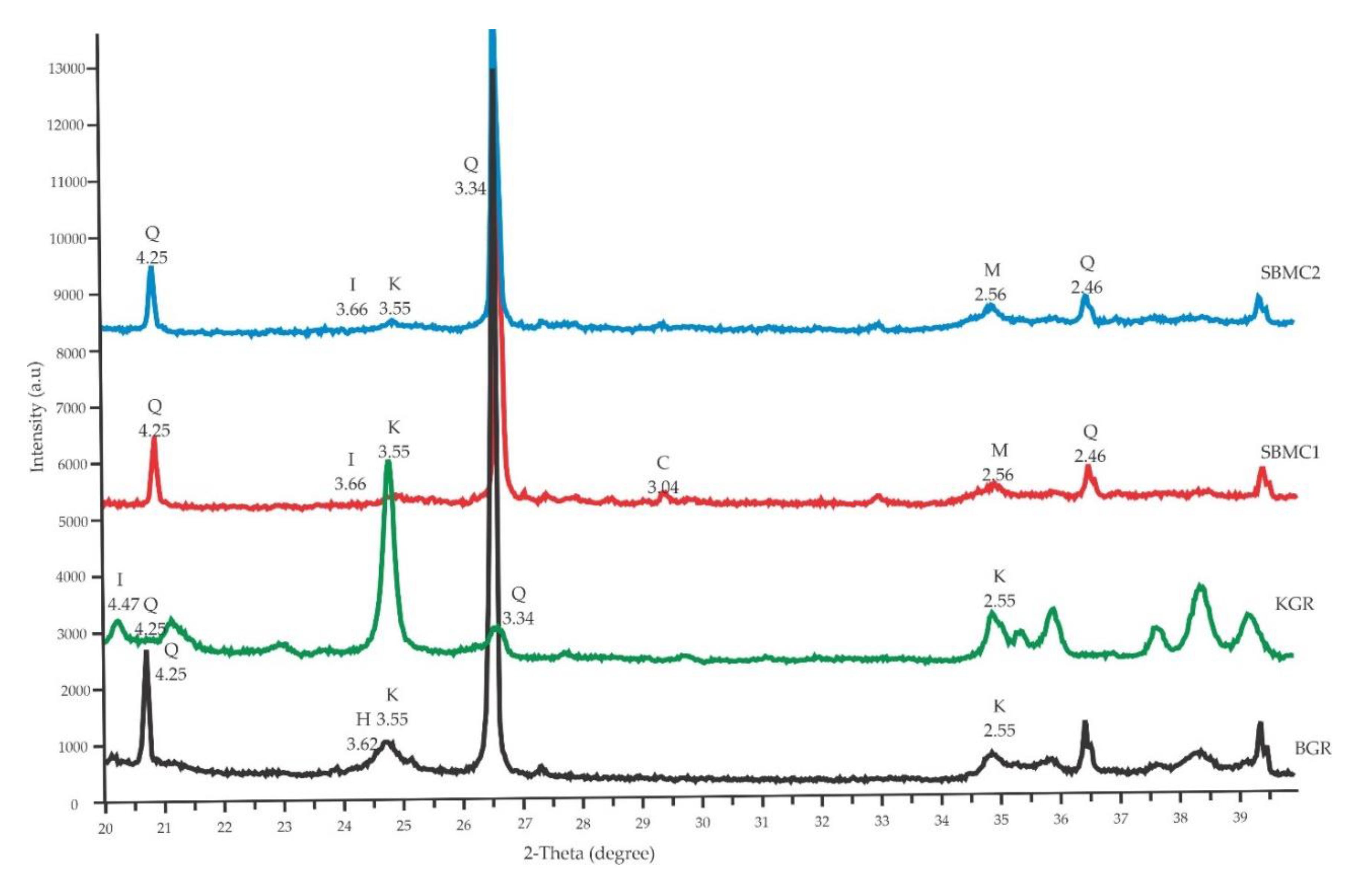
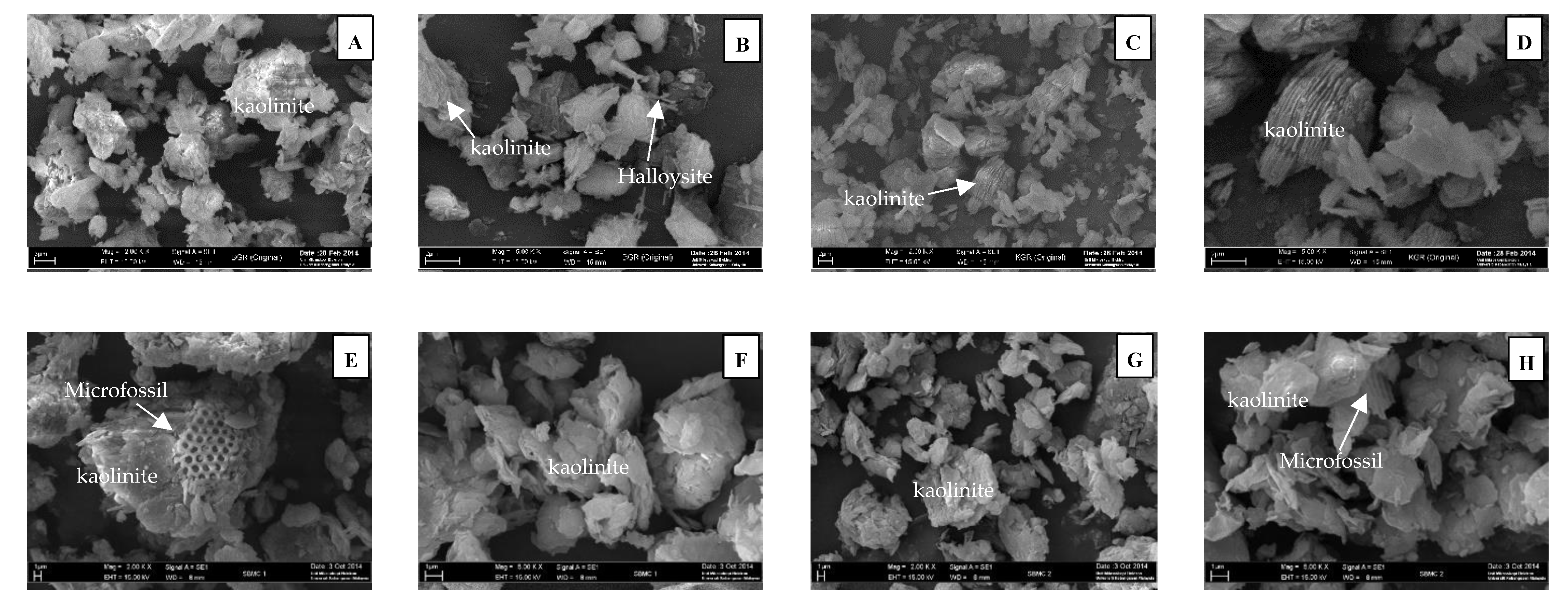
3.1. Soil Characterizations
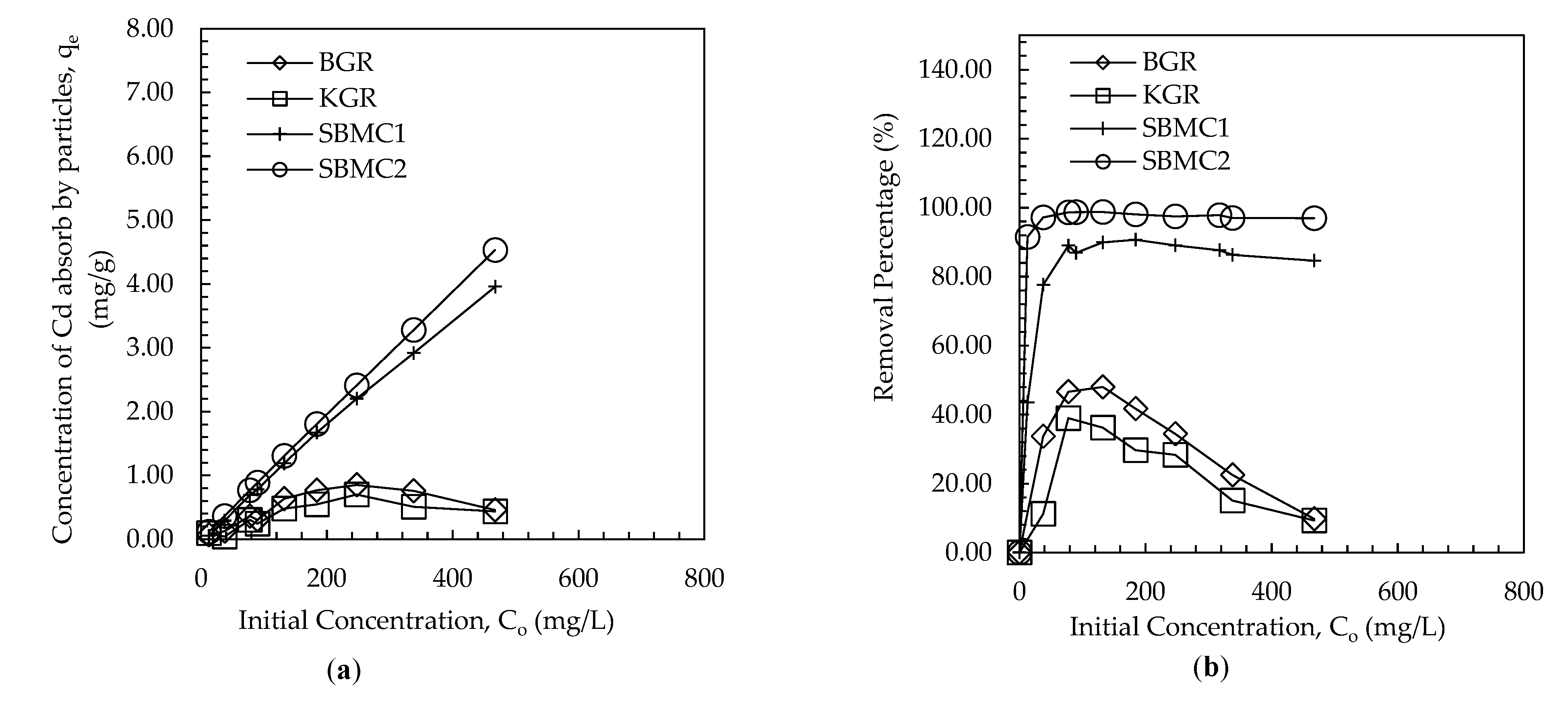
3.2. Batch Equilibrium Study
3.3. Column Infiltration
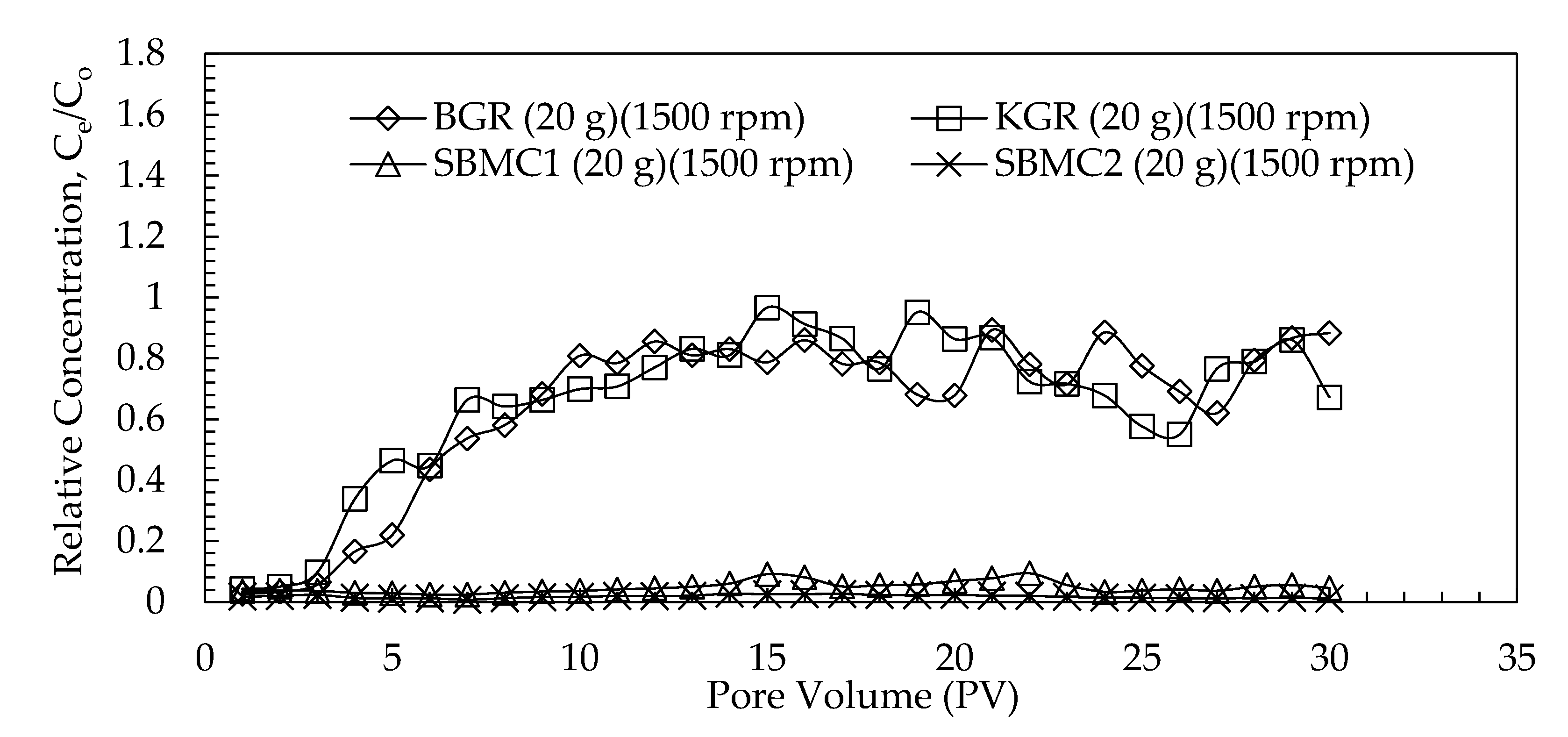
3.3.1. Migration Profiles
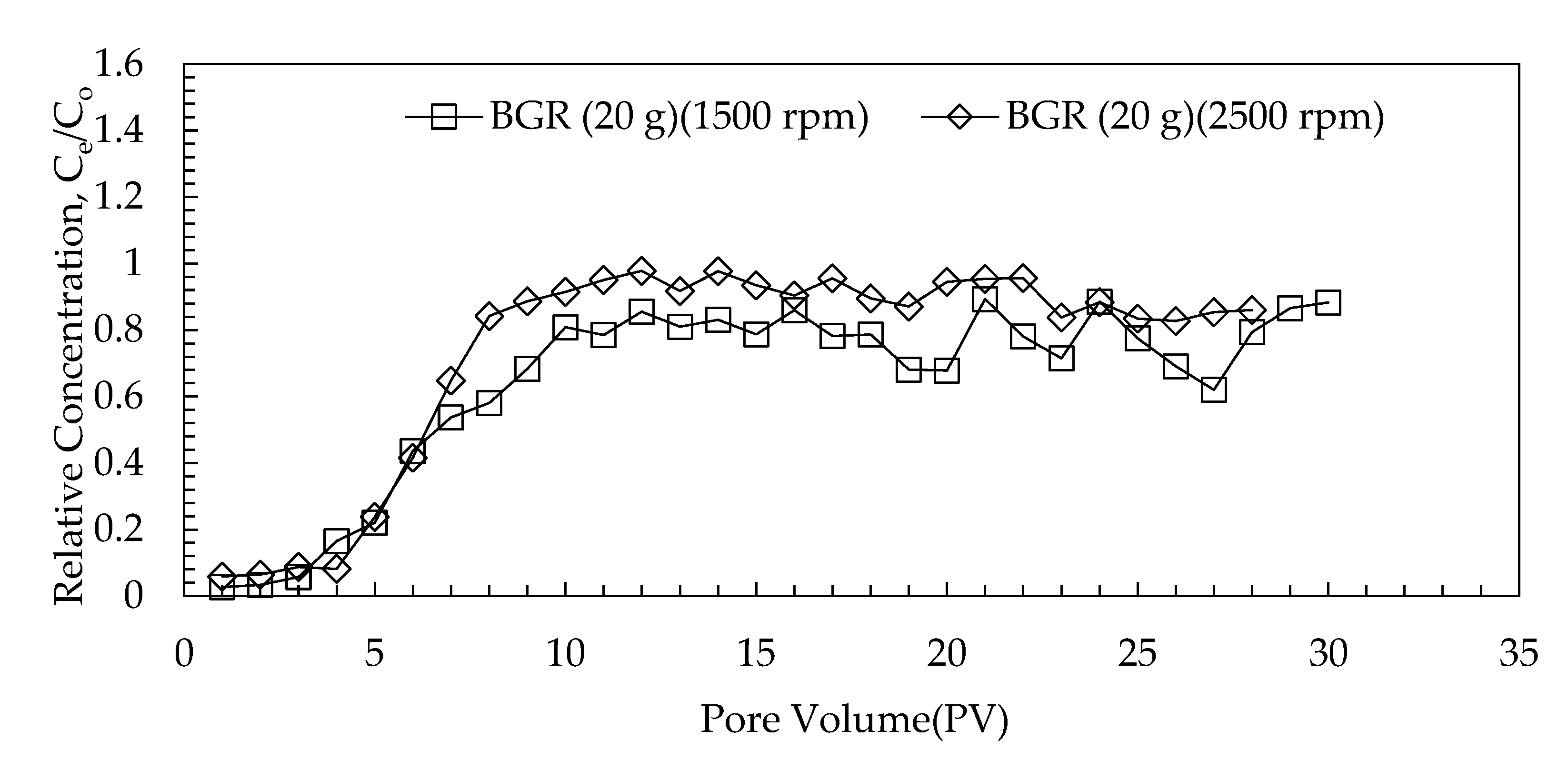
3.3.2. Effect of G-Force
3.3.3. The Effect of Soil Thickness
3.3.4. Buffering Capacity
3.3.5. Partition Coefficient (Kd)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalermyanont, T.; Arrykul, S.; Charoenthaisong, S.N. Potential use of lateritic and marine clay soils as landfill liners to retain heavy metals. Waste Manag. 2009, 29, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Heavy Metal Removal by Fixed-Bed Column-A Review. ChemBioEng Rev. 2018, 5, 173–179. [Google Scholar] [CrossRef]
- Tiku, D.K.; Kumar, A.; Sawhney, S.; Singh, V.P.; Kumar, R. Effectiveness of Treatment Technologies for Wastewater Pollution Generated by Indian Pulp Mills. Environ. Monit. Assess. 2007, 132, 453–466. [Google Scholar] [CrossRef]
- Singh, R.P. Effect of wastewater disposal and extent of industrial pollution in and around Kanpur, Uttar Pradesh, India. Bull. Eng. Geol. Environ. 2001, 60, 31–35. [Google Scholar] [CrossRef]
- Kwon, J.; Yun, S.; Lee, J.; Kim, S.; Young, H. Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic (III) from aqueous solutions using scoria: Kinetics and equilibria of sorption. J. Hazard. Mater. 2010, 174, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, Y.; Li, J.; Chen, D.; He, G.; Parkin, I.P. Natural Clay-Based Materials for Energy Storage and Conversion Applications. Adv. Sci. 2021, 8, 2004036. [Google Scholar] [CrossRef]
- Rahman, Z.A.; Yaacob, W.Z.W.; Rahim, S.A.; Lihan, T.; Idris, W.M.R.; Mohd Sani, W.N.F. Geotechnical characterization of marine clay as potential liner material. Sains Malays. 2013, 42, 1081–1089. [Google Scholar]
- Yong, B.P.; Mohamed, R.N.; Warkentin, A.M.O. Principles of Contaminant Transport in Soils; Elsevier: New York, NY, USA, 1992. [Google Scholar]
- Yong, R.N.; Yaacob, W.Z.W.; Bentley, S.P.; Harris, C.; Tan, B.K. Partitioning of heavy metals on soil samples from column tests. Eng. Geol. 2001, 60, 307–322. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2009, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Negrea, A.; Mihailescu, M.; Mosoarca, G.; Ciopec, M.; Duteanu, N.; Negrea, P.; Minzatu, V. Estimation on fixed-bed column parameters 453 of breakthrough behaviors for gold recovery by adsorption onto modified/functionalized amberlite xad7. Int. J. Environ. Res. Public Health 2020, 17, 6868. [Google Scholar] [CrossRef]
- Msaky, J.J.; Calvet, R. Adsorption behavior of copper and zinc in soils, influence of pH on adsorption characteristic. Soil Sci. 1990, 150, 513–522. [Google Scholar] [CrossRef]
- Alshaebi, F.Y.; Yaacob, W.Z.W.; Samsudin, A.R. Removal of Arsenic from Contaminated Water by Selected Geological Natural Materials. Aust. J. Basic Appl. Sci. 2010, 4, 4413–4422. [Google Scholar]
- Antoniadis, V.; Mckinley, J.D. Leaching tests in a laboratory centrifuge on zinc migration in London Clay. In Proceedings of the International Symposium on Physical Modelling and Testing in Environmental Geotechnics, La Baule, France, 15–17 May 2000. [Google Scholar]
- Kumar, P.R. Contaminant Transport Through Geotechnical Centrifuge Models. Environ. Monit. Assess. 2006, 117, 215–233. [Google Scholar] [CrossRef]
- Negahdar, A.; Nikghalbpour, M. Geotechnical properties of sandy clayey soil contaminated with lead and zinc. SN Appl. Sci. 2020, 2, 1331. [Google Scholar] [CrossRef]
- Mitchell, R. Centrifuge techniques for testing clay liner samples. Can. Geotech. J. 1994, 31, 577–583. [Google Scholar] [CrossRef]
- Cerato, A.B.; Lutenegger, A.J. Determination of surface area of fine-grained soils by the ethylene glycol monoethyl ether (EGME) method. Geotech. Test. J. 2002, 25, 315–321. [Google Scholar] [CrossRef]
- Cheyns, K.; Mertens, J.; Diels, J.; Smolders, E.; Springael, E. Monod kinetics rather than a first-order degradation model explains atrazine fate in soil mini-columns: Implications for pesticide fate modelling. Environ. Pollut. 2010, 158, 1405–1411. [Google Scholar] [CrossRef]
- Alther, G. Using organoclays to enhance carbon filtration. Waste Manag. 2002, 22, 507–513. [Google Scholar] [CrossRef]
- Shackelford, C.D. Fundamental considerations for column testing of engineered, clay-based barriers Fundamental considerations for column testing of engineered, clay-based barriers. Jpn. Geotech. Soc. Spec. Publ. 2021, 9, 441–460. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gasses on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1382. [Google Scholar] [CrossRef] [Green Version]
- Borna, M.O.; Pirsaheb, M.; Niri, M.V.; Mashizie, R.K.; Kakavandi, B.; Zare, M.R.; Asadi, A. Batch and column studies for the adsorption of chromium (VI) on low-cost Hibiscus Cannabinus kenaf, a green adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 68, 80–89. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, D.; Zheng, Y.; Liang, S.; Liu, Z. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef]
- Grim, R.E. Applied Clay Mineralogy; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1962. [Google Scholar]
- Freundlich, H. Über die adsorption in lösungen (adsorption in solution). Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Antoniadis, V.; Mckinley, J.D.; Yaacob, W.Z.W. Single-Element and Competitive Metal Mobility Measured with Column Infiltration and 486 Batch Tests. J. Environ. Qual. 2007, 60, 53–60. [Google Scholar] [CrossRef]
- Atanassova, I.D. Adsorption and desorption of Cu at high equilibrium concentrations by soil and clay samples from Bulgaria. Environ. Pollut. 1995, 87, 17–21. [Google Scholar] [CrossRef]
- United State Department of Agriculture. Elementary Soil Engineering; United State Department of Agriculture: Washington, DC, USA, 1990; pp. 261–468. [Google Scholar]
- Murray, E.J.; Rix, D.W.; Humprey, D. Clay linings to landfill sites. Quat. J. Eng. Geol. Hydrogeol. 1992, 25, 371–376. [Google Scholar] [CrossRef]
- Sastre, J.; Rauret, G.; Vidal, M. Sorption-desorption tests to assess the risk derived from metal contamination in mineral and organic soils. Environ. Int. 2007, 33, 246–256. [Google Scholar] [CrossRef]
- Hooda, P.S.; Alloway, B.J. Cadmium and lead sorption by selected English and Indian soils. Geoderma 1998, 84, 121–135. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Geochemistry; Holt, Rinehart and Winston: New York, NY, USA, 1967. [Google Scholar]
- De Plessis, P.; Gazley, M.F.; Tay, S.L.; Trunfull, E.F.; Knorsch, M.; Branch, T.; Fourie, L.F. Quantification of Kaolinite and Halloysite Using Machine Learning from FTIR, XRF, and Brightness Data. Minerals 2021, 11, 1350. [Google Scholar] [CrossRef]
- Robertson, I.D.M.; Eggleton, R.A. Weathering of Granitic Muscovite to Kaolinite and Halloysite and of Plagioclase-Derived Kaolinite to Halloysite Clays. Clay Miner. 1991, 39, 113–126. [Google Scholar] [CrossRef]
- Hanafi, M.; Ekinci, A.; Aydin, E. Triple-binder-stabilized marine deposit clay for better sustainability. Sustainability 2020, 12, 4633. [Google Scholar] [CrossRef]
- Patel, H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef]
- Veli, S.; Alyüz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, 1629–1638. [Google Scholar] [CrossRef]
- Aminu, I.; Gumel, S.M.; Ahmad, W.A.; Idris, A.A. Adsorption Isotherms and Kinetic Studies of Congo-Red Removal from Waste Water Using Activated Carbon Prepared from Jujube Seed. Am. J. Anal. Chem. 2020, 11, 47–59. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef]
- Gouran-orimi, R.; Mirzayi, B.; Nematollahzadeh, A.; Tardast, A. Journal of Environmental Chemical Engineering Competitive adsorption of nitrate in fi xed-bed column packed with bio-inspired polydopamine coated zeolite. J. Environ. Chem. Eng. 2018, 6, 2232–2240. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, F.; Cao, B.; Yin, H.; Al-Tabbaa, A. Simultaneous removal of Pb and MTBE by mixed zeolites in fixed-bed column tests Simultaneous removal of Pb and MTBE by mixed zeolites in fixed-bed column tests. J. Environ. Sci. 2022, 122, 41–49. [Google Scholar] [CrossRef]



| Samples | Soil Weight (g) | Soil Thickness (mm) | Parameters | ||
|---|---|---|---|---|---|
| Speed (rpm) | G-Force (g) | Spinning Time (min) | |||
| BGR/KGR | 10 | 10 | 1500 | 520 | 30 |
| 20 | 20 | 2500 | 1440 | 60 | |
| SBMC1/SBMC2 | 10 | 14 | 1500 | 520 | 80 |
| 20 | 28 | 2500 | 1440 | 180 | |
| Properties | BGR | KGR | SBMC1 | SBMC2 |
|---|---|---|---|---|
| Sand (%) | 60.0 ± 3.16 | 51.0 ± 2.83 | 3.6 ± 2.65 | 12.0 ± 3.29 |
| Silt (%) | 37.2 ± 3.25 | 44.6 ± 3.2 | 80.8 ± 4.66 | 71.0 ± 3.85 |
| Clay (%) | 2.8 ± 1.94 | 4.4 ± 1.50 | 15.6 ± 3.01 | 17.0 ± 2.61 |
| USCS classification | Sandy silt | Sandy elastic silt | Sandy elastic silt | Sandy elastic silt |
| USCS symbol | ML | MH | MH | MH |
| Plastic Limit (%) | 38.37 ± 0.25 | 35.57 ± 0.60 | 55.83 ± 3.72 | 47.39 ± 1.47 |
| Liquid Limit (%) | 49.06 ± 0.56 | 51.12 ± 0.37 | 82.16 ± 1.84 | 75.80 ± 2.01 |
| Plasticity Index (%) | 10.69 ± 0.70 | 15.55 ± 0.54 | 26.33 ± 5.20 | 28.41 ± 2.25 |
| Plasticity Chart | Intermediate | High | Very high | Very high |
| Specific Gravity | 2.54 ± 0.03 | 2.54 ± 0.07 | 2.23 ± 0.05 | 2.29 ± 0.05 |
| Max Dry Density, ρdmax (g/cm3) | 1.71 ± 0.05 | 1.70 ± 0.01 | 1.37 ± 0.01 | 1.39 ± 0.01 |
| Optimum Moisture Content, Wopt (%) | 18.57 ± 0.03 | 14.26 ± 2.58 | 36.56 ± 3.99 | 25.95 ± 1.61 |
| Permeability (m/s) | 2.08 × 10−6 | 1.43 × 10−6 | 6.56 × 10−7 | 5.64 × 10−7 |
| pH | 5.45 ± 0.08 | 5.63 ± 0.16 | 7.22 ± 0.16 | 7.59 ± 0.06 |
| Organic Matter (%) | 0.46 ± 0.04 | 0.27 ± 0.05 | 5.79 ± 0.26 | 5.72 ± 0.53 |
| SSA (m2/g) | 19.73 ± 1.64 | 26.36 ± 0.45 | 61.36 ± 0.86 | 73.73 ± 4.70 |
| CEC (meq/100 g) | 1.06 ± 0.23 | 1.33 ± 1.01 | 91.81 ± 0.44 | 76.91 ± 1.08 |
| Adsorption Isotherm | BGR | KGR | SBMC1 | SBMC2 | |
|---|---|---|---|---|---|
| Linear Equation | Kd (L/g) | 0.0062 | 0.0042 | 0.0703 | 0.3544 |
| R2 | 0.9723 | 0.978 | 0.9701 | 0.9614 | |
| Langmuir Equation | KL (L/g) | 0.0780 | 0.1378 | 0.0120 | 0.1236 |
| Am (mg/g) | 0.5590 | 0.4824 | 8.5799 | 6.4771 | |
| R2 | 0.8539 | 0.9398 | 0.8761 | 0.8964 | |
| RL | 0.0267 0.2544 | 0.0089 0.5520 | 0.5375 1.0000 | 0.3638 1.0000 | |
| Freundlich Equation | KF (L/g) | 0.0137 | 0.0454 | 0.1539 | 0.8202 |
| 1/n | 0.8343 | 0.5503 | 0.7805 | 0.6340 | |
| R2 | 0.9266 | 0.9266 | 0.9641 | 0.9813 | |
| Soils | Batch, Kd (L/Kg) | Column (20 g) Kd (L/Kg) | |
|---|---|---|---|
| 1500 rpm/52 | 2500 rpm/1440 | ||
| BGR | 6.20 | 0.88 | 0.45 |
| KGR | 4.20 | 0.59 | 0.28 |
| SBMC1 | 70.30 | 10.40 | 8.06 |
| SBMC2 | 354.40 | 23.58 | 21.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarime, N.A.; Solemon, B.; Wan Yaacob, W.Z.; Che Omar, R. The Migration of Cd in Granitic Residual Soil and Marine Clay: Batch and Column Studies. Minerals 2022, 12, 1106. https://doi.org/10.3390/min12091106
Zarime NA, Solemon B, Wan Yaacob WZ, Che Omar R. The Migration of Cd in Granitic Residual Soil and Marine Clay: Batch and Column Studies. Minerals. 2022; 12(9):1106. https://doi.org/10.3390/min12091106
Chicago/Turabian StyleZarime, Nur Aishah, Badariah Solemon, Wan Zuhairi Wan Yaacob, and Rohayu Che Omar. 2022. "The Migration of Cd in Granitic Residual Soil and Marine Clay: Batch and Column Studies" Minerals 12, no. 9: 1106. https://doi.org/10.3390/min12091106





