Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition
Abstract
1. Introduction
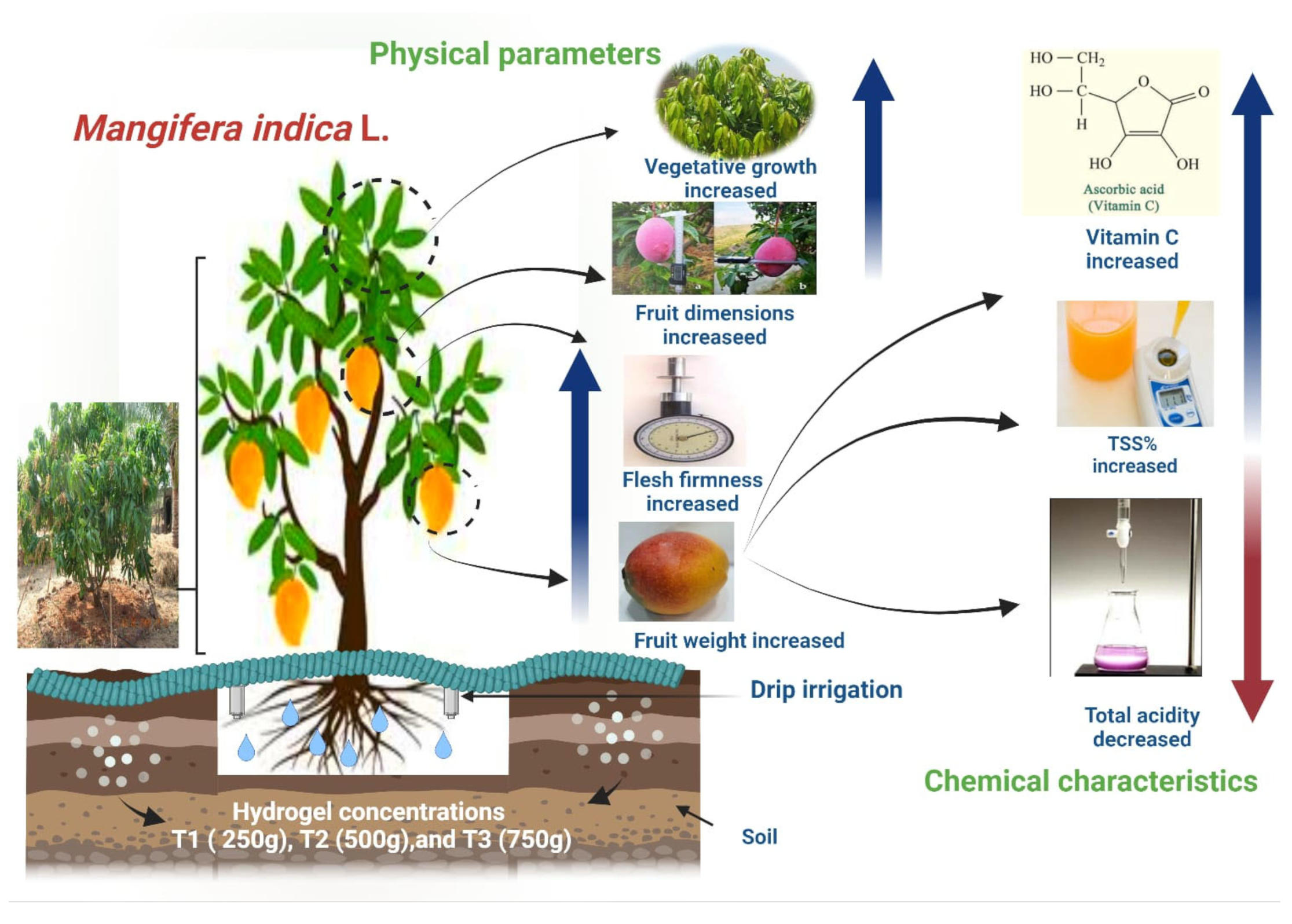
2. Results and Discussion
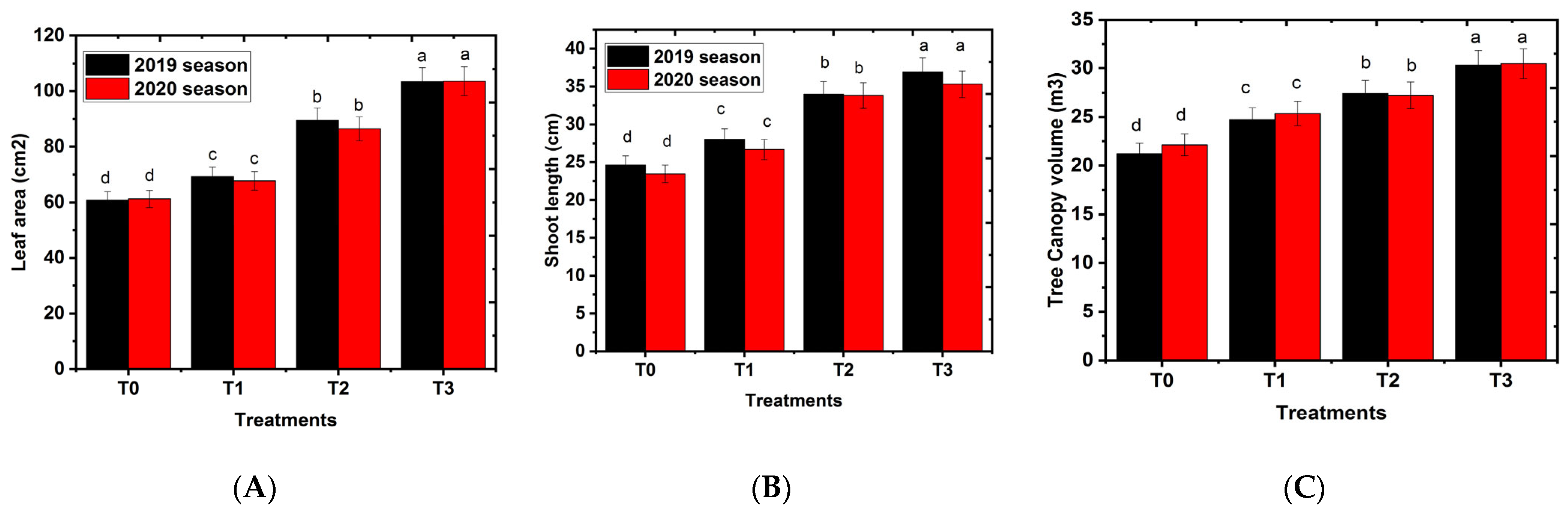
2.1. Effects of Hydrogel on Vegetative Growth Parameters
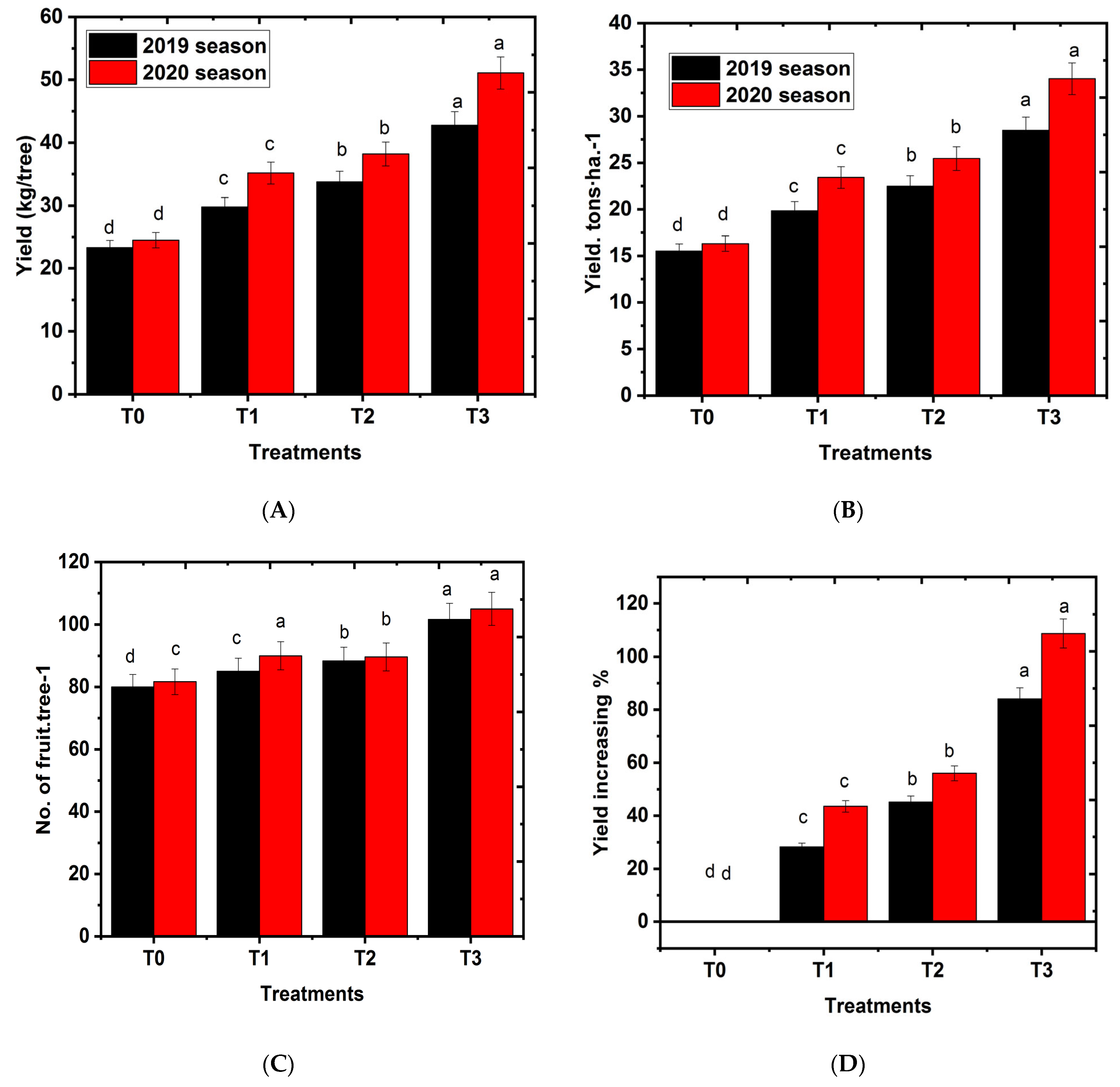
2.2. Effects of Hydrogel on Number of Fruits Per Tree
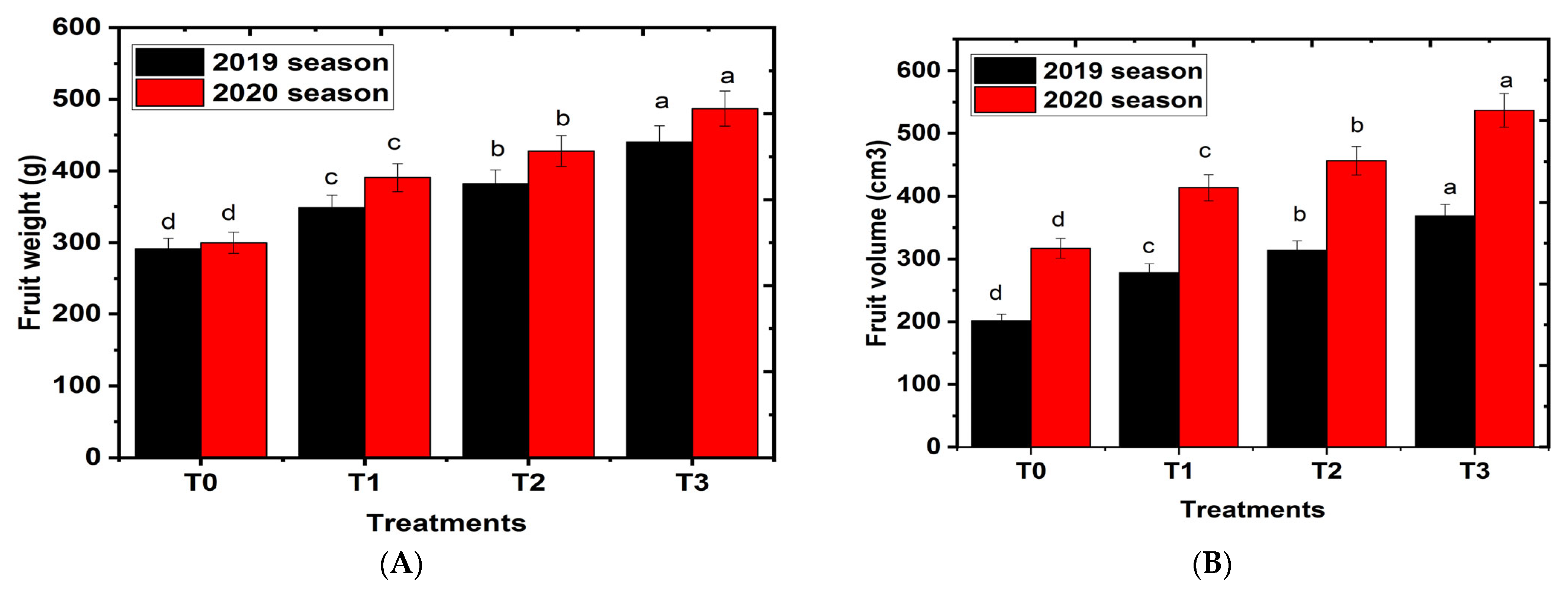
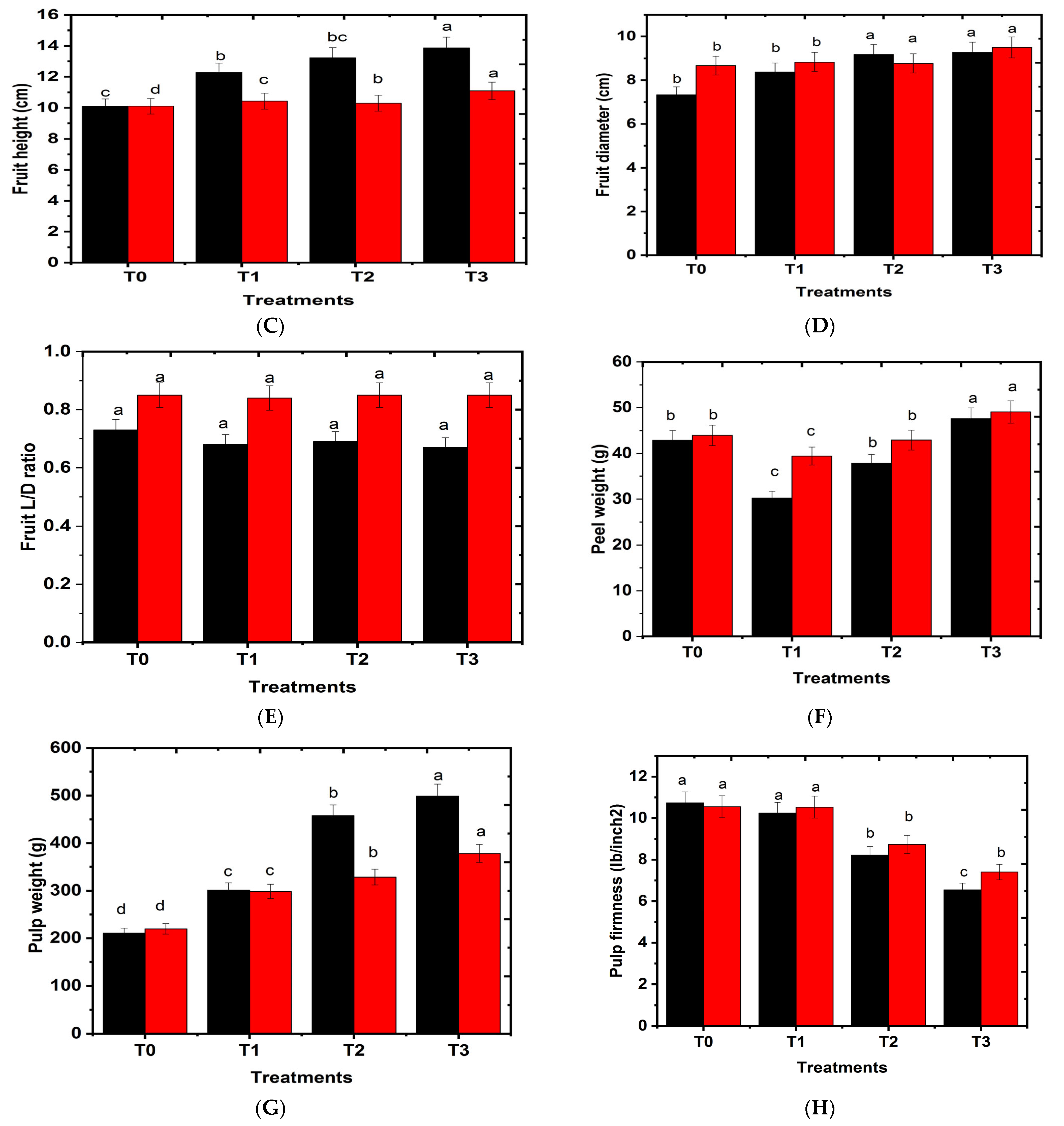
2.3. Effects of Hydrogel on Fruit Physical Characteristics
2.4. Effects of Hydrogel on Fruit Chemical Characteristics
3. Conclusions
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Experimental Measurements
4.2.1. Vegetative Growth
4.2.2. Tree Yield
4.3. Fruit physical Parameters
4.4. Fruit Chemical Characteristics
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viruel, M.A.; Escribano, P.; Barbieri, M.; Ferri, M.; Hormaza, J.I. Fingerprinting, embryo type and geographic differentiation in mango (Mangifera Indica L., Anacardiaceae) with microsatellites. Mol. Breed. 2005, 15, 383–393. [Google Scholar] [CrossRef]
- El-Sharony, T.F.; El-Gioushy, S.F.; Amin, O.A. Effect of foliar application with algae and plant extracts on growth, yield and fruit quality of fruitful mango trees cv. fagri kalan. J. Hortic. 2015, 2, 4. [Google Scholar] [CrossRef]
- Ledesma, N.; Campbell, R.J. The status of mango cultivars, market perspectives and mango cultivar improvement for the future. Acta Hortic. 2019, 1244, 23–27. [Google Scholar] [CrossRef]
- Otieno, H.M.O. A review of white mango scale (Aulacaspis Tubercularis Newstead; Hemiptera: Diaspididae) in Sub-Saharan Africa: Distribution, impact and management strategies. Pak. J. Agric. Res. 2021, 34, 227–238. [Google Scholar] [CrossRef]
- Zuazo, V.H.D.; García-Tejero, I.F.; Rodríguez, B.C.; Tarifa, D.F.; Ruiz, B.G.; Sacristán, P.C. Deficit irrigation strategies for subtropical mango farming. A review. Agron. Sustain. Dev. 2021, 41, 13. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant. Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Yassen, A.N.; Nam, W.H.; Hong, E.M. Impact of climate change on reference evapotranspiration in Egypt. Catena 2020, 194, 104711. [Google Scholar] [CrossRef]
- de Jong, P.; Tanajura, C.A.S.; Sánchez, A.S.; Dargaville, R.; Kiperstok, A.; Torres, E.A. Hydroelectric production from Brazil’s São Francisco river could cease due to climate change and inter-annual variability. Sci. Total Environ. 2018, 634, 1540–1553. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant. Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Spinelli, L.; Rizzolo, A.; Vanoli, M.; Grassi, M.; Zerbini, P.E.; Pimentel, R.; Torricelli, A. Optical properties of pulp and skin in brazilian mangoes in the 540–900 Nm spectral range: Implication for non-destructive maturity assessment by time-resolved reflectance spectroscopy. In Proceedings of the International Conference of Agricultural Engineering, CIGR-AgEng2012, Valencia, Spain, 8–12 July 2012; p. C–2096. [Google Scholar]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public. Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Testa, R.; Tudisca, S.; Schifani, G.; Trapani, A.M.D.; Migliore, G. Tropical fruits as an opportunity for sustainable development in rural areas: The case of mango in small-sized sicilian farms. Sustain. Switz. 2018, 10, 1436. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Liu, Z.; Li, W.; Liu, Y.; Li, J.; Wei, H.; Han, H. Fabrication of a water-retaining, slow-release fertilizer based on nanocomposite double-network hydrogels via ion-crosslinking and free radical polymerization. J. Ind. Eng. Chem. 2021, 93, 375–382. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, Z.T.; Zheng, X.L.; Jiang, G.B.; Fang, Y.S.; Mao, X.Y.; Liao, Z.W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2013, 92, 1367–1376. [Google Scholar] [CrossRef]
- Wf, A.; Sm, K. Influence of hydrogel composites soil conditioner on navel orange growth and productivity. J. Agric. Hortic. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Jamnická, G.; Ditmarová, L.; Kurjak, D.; Kmeť, J.; Pšidová, E.; Macková, M.; Gömöry, D.; Střelcová, K. The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant. Soil Environ. 2013, 59, 446–451. [Google Scholar] [CrossRef]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part. B Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Yang, F.; Cen, R.; Feng, W.; Liu, J.; Qu, Z.; Miao, Q. Effects of super-absorbent polymer on soil remediation and crop growth in arid and semi-arid areas. Sustain. Switz. 2020, 12, 7825. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Chehab, H.; Tekaya, M.; Mechri, B.; Jemai, A.; Guiaa, M.; Mahjoub, Z.; Boujnah, D.; Laamari, S.; Chihaoui, B.; Zakhama, H.; et al. Effect of the super absorbent polymer Stockosorb® on leaf turgor pressure, tree performance and oil quality of olive trees Cv. chemlali grown under field conditions in an arid region of Tunisia. Agric. Water Manag. 2017, 192, 221–231. [Google Scholar] [CrossRef]
- Hamdy, A.; Khalifa, S.; AbdEl-Aziz, H. Hydrogel as a soil conditioner affecting the growth, yield, and fruit quality of ‘murcott’ mandarin trees under arid and semi-arid lands. Al-Azhar J. Agric. Res. 2020, 45, 76–85. [Google Scholar] [CrossRef]
- Kapłan, M.; Lenart, A.; Klimek, K.; Borowy, A.; Wrona, D.; Lipa, T. Assessment of the possibilities of using cross-linked polyacrylamide (Agro Hydrogel) and preparations with biostimulation in building the quality potential of newly planted apple trees. Agronomy 2021, 11, 125. [Google Scholar] [CrossRef]
- Awad, F.; Kahl, L.; Kluge, R. Environmental aspects of sewage sludge and evaluation of super absorbent hydrogel under Egyptian conditions. In Iron Nutrition in Soils and Plants; Abadía, J., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 91–97. ISBN 978-94-010-4224-6. [Google Scholar]
- Cavalcante, A.G.; Cavalcante, L.F.; Cavalcante, A.C.P.; de L. Souto, A.G.; dos Santos, C.E.M.; de Araújo, D.L. Variation of thermal time, phyllochron and plastochron in passion fruit plants with irrigation depth and hydrogel. J. Agric. Sci. 2018, 10, 229. [Google Scholar] [CrossRef][Green Version]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Madigu, N.O.; Mathooko, F.M.; Onyango, C.A.; Kahangi, E.M.; Owino, W.O. Postharvest behaviour and quality characteristics of mango (Mangifera Indica L.) fruit grown under water deficit conditions. Acta Hortic. 2009, 837, 305–312. [Google Scholar] [CrossRef]
- Xu, H.; Watanabe, Y.; Ediger, D.; Yang, X.; Iritani, D. Rootstock-dependent damage-free yield of Ambrosia TM apple after sustained summer heat events. Plants 2022, 11, 1819. [Google Scholar] [CrossRef]
- Barakat, M.R.; El-Kosary, S.; Borham, T.I.; Abd-ElNafea, M.H. Effect of hydrogel soil addition under different irrigation levels on grandnain banana plants. J. Hortic. Sci. Ornam. Plants 2015, 7, 19–28. [Google Scholar]
- Austin, M.E.; Bondari, K. Hydrogel as a field medium amendment for blueberry plants. HortScience 1992, 27, 973–974. [Google Scholar] [CrossRef]
- Kassim, F.S.; El-Koly, M.F.; Hosny, S.S. Evaluation of super absorbent polymer application on yield, and water use efficiency of grand nain banana plant. Middle East. J. Agric. Res. 2017, 6, 188–198. [Google Scholar]
- Lipan, L.; Carbonell-Pedro, A.A.; Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Franco Tarifa, D.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S.; Muelas, R.; Sendra, E.; et al. Can sustained deficit irrigation save water and meet the quality characteristics of mango? Agriculture 2021, 11, 448. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Yang, Q.; Wang, X.; Cui, N. determining optimal deficit irrigation and fertilization to increase mango yield, quality, and WUE in a dry hot environment based on TOPSIS. Agric. Water Manag. 2021, 245, 106650. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Bisen, B.; Pandey, S. Effect of super absorbent polymer and irrigation scheduling on growth attributes in acid lime. Int. J. Chem. Stud. 2021, 9, 360–363. [Google Scholar] [CrossRef]
- Pattanaaik, S.K.; Wangchu, L.; Singh, B.; Hazarika, B.N.; Singh, S.M.; Pandey, A.K. Effect of hydrogel on water and nutrient management of citrus reticulata. Res. Crops 2015, 16, 98–103. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, X.; Gao, X.; Jia, R.; Yang, M.; Yang, X.; Wu, Y.; Siddique, K.H.M. Effect of natural factors and management practices on agricultural water use efficiency under drought: A meta-analysis of global drylands. J. Hydrol. 2021, 594, 125977. [Google Scholar] [CrossRef]
- Levin, A.G.; Peres, M.; Noy, M.; Love, C.; Gal, Y.; Naor, A. The response of field-grown mango (cv. keitt) trees to regulated deficit irrigation at three phenological stages. Irrig. Sci. 2018, 36, 25–35. [Google Scholar] [CrossRef]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in southern Italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- Spreer, W.; Nagle, M.; Neidhart, S.; Carle, R.; Ongprasert, S.; Müller, J. Effect of regulated deficit irrigation and partial rootzone drying on the quality of mango fruits (mangifera indica l., cv. ‘chok anan’). Agric. Water Manag. 2007, 88, 173–180. [Google Scholar] [CrossRef]
- Ahmad, I.; Bibi, F.; Ullah, H.; Munir, T. Mango fruit yield and critical quality parameters respond to foliar and soil applications of zinc and boron. Plants 2018, 7, 97. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Nissi, F.; Lakshmi, M.; Swami, D.; Krishna, U.; Salomi, D.; Dilip, B. Effect of soil conditioners on growth and fruit parameters of sweet orange (Citrus Sinensis (L.) osbeck) in andhra pradesh. Pharma Innov. J. 2021, 10, 582–586. [Google Scholar]
- Barakat, M.R.; El-Kosary, S.; Abd-ElNafea, M.H. Enhancing williams banana cropping by using some organic fertilization treatments. J. Hortic. Sci. Ornam. Plants 2011, 3, 29–37. [Google Scholar]
- Abdelraouf, R.E.; Ragab, R. Applying partial root drying drip irrigation in the presence of organic mulching. Is that the best irrigation practice for arid regions? Field and modelling study using the saltmed model: Partial root drying, drip irrigation, organic mulching, SALTMED model. Irrig. Drain. 2018, 67, 491–507. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Leng, X.; Yang, Q.; Chen, H.; Wang, X.; Cui, N. Exploring the optimisation of mulching and irrigation management practices for mango production in a dry hot environment based on the entropy weight method. Sci. Hortic. 2022, 291, 110564. [Google Scholar] [CrossRef]
- Anjum, M.A.; Hussain, S.; Arshad, P.; Hassan, A. Irrigation water of different sources affects fruit quality attributes and heavy metals contents of un-grafted and commercial mango cultivars. J. Environ. Manage. 2021, 281, 111895. [Google Scholar] [CrossRef]
- Costa, S.C.; Soares, A.A.; Sediyama, G.C.; de Araújo Viana, T.V.; de Oliveira Moreira, F.V. Variação de parâmetros físicos e químicos de frutos em bananeira ‘pacovan’ submetida a diferentes lâminas de irrigação e doses de potássio na chapada do apodi—Limoeiro do norte-ce. IRRIGA 2011, 16, 82. [Google Scholar] [CrossRef]
- El-rhman, A. Effect of magnetic iron and potassium humate on growth, yield and fruit quality of pomegranate trees in Siwa Oasis, Egypt. Int. J. Environ. 2017, 6, 103–113. [Google Scholar]
- Wei, J.; Liu, G.; Liu, D.; Chen, Y. Influence of irrigation during the growth stage on yield and quality in mango (Mangifera indica L.). PLoS ONE 2017, 12, e0174498. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- El-Aziz, G.H.A.; Ibrahim, A.S.; Fahmy, A.H. Using environmentally friendly hydrogels to alleviate the negative impact of drought on plant. Open J. Appl. Sci. 2022, 12, 111–133. [Google Scholar] [CrossRef]
- Farag, A.M.; Sokker, H.H.; Zayed, E.M.; Nour Eldien, F.A.; Abd Alrahman, N.M. Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 2018, 120, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.F.; Morsy, M.H. A new method for measuring leaf area in different fruit species. Minia J. Agric. Res. Dev. Egypt 1999, 19, 7–105. [Google Scholar]
- Zekri, M. Original article citrus rootstocks affect scion nutrition, fruit quality, growth, yield and economical return. Fruits 2000, 55, 231–239. [Google Scholar]
- Lavi, U.; Kashkush, K.; Sa’ada, D.; Shats, H.; Ravid, U.; Tomer, E. Mango breeding and the potential of modern biology. Acta Hortic. 2004, 645, 51–59. [Google Scholar] [CrossRef]
- Abd El-Naby, S.K.M.; Ahmed Mohamed, A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and Naa on vegetative growth, yield and quality of ‘canino’ apricot fruits. Acta Sci. Pol. Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemist. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2016; p. 18. [Google Scholar]
- Chow, P.S.; Landhäusser, S.M. A Method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Ridgman, W.J. Statistical Methods, 8th ed.; Snedecor, G.W., Cochran, W.G., Eds.; Xx + 503 Pp; Iowa State University Press: Ames, IA, USA, 1989; ISBN 0-8138-1561-6. [Google Scholar] [CrossRef]
- Stern, R. Review of Co-Stat-Statistical Software. Exp. Agric. 1991, 27, 87. [Google Scholar] [CrossRef]


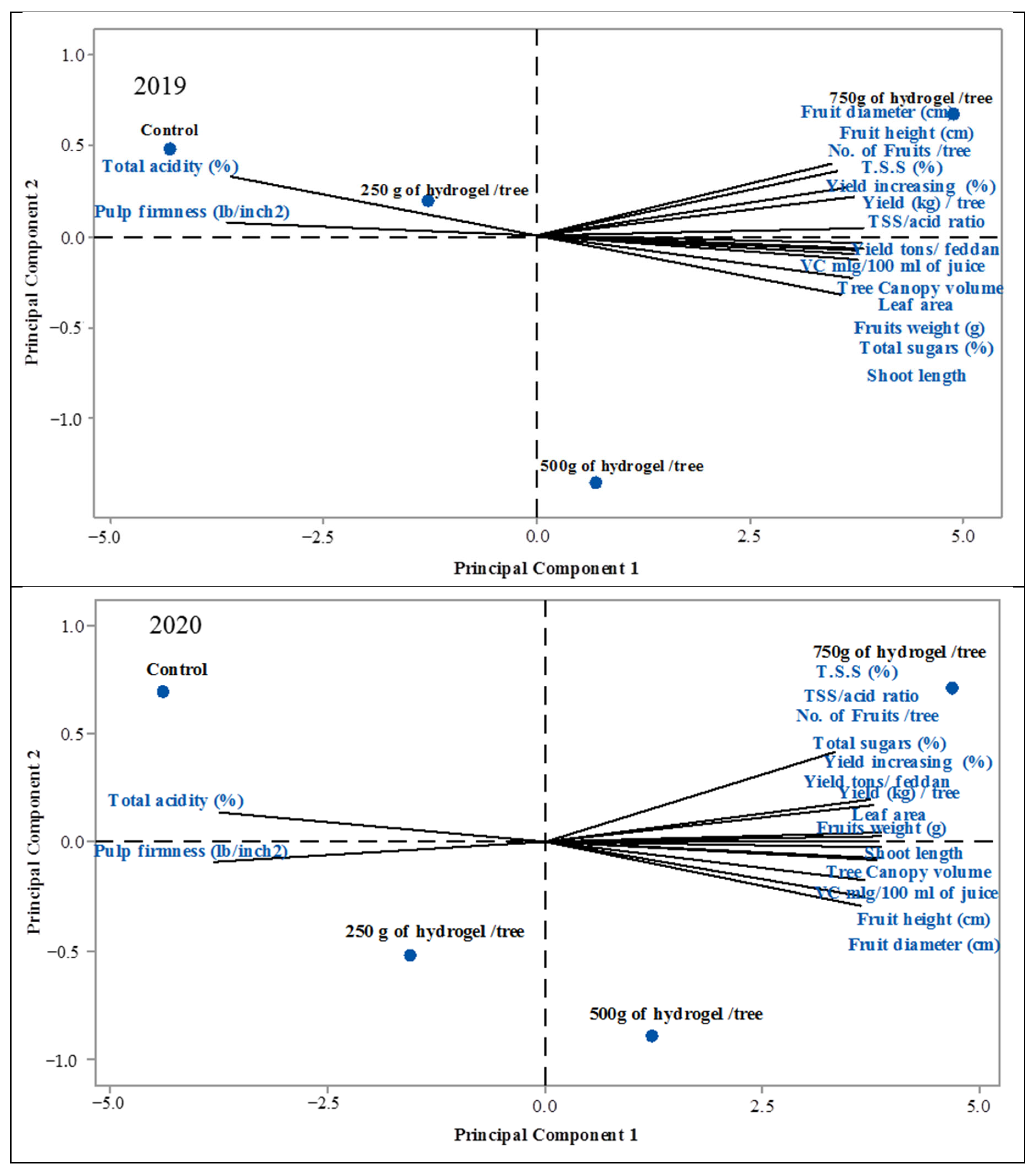

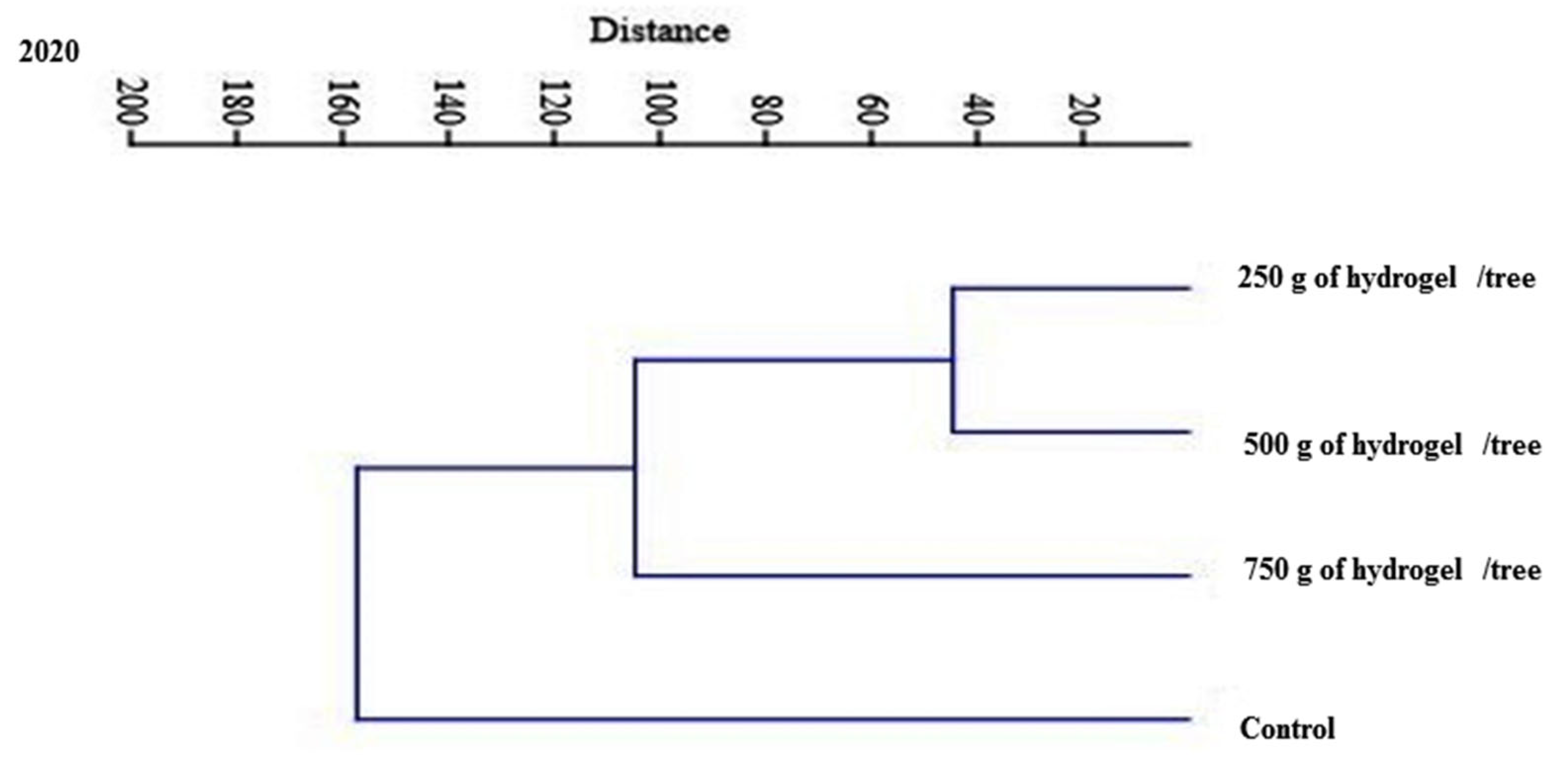
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
|---|---|---|---|---|---|---|
| 2019 | 2020 | |||||
| Eigenvalue | 14.02 | 1.58 | 0.388 | 14.82 | 0.850 | 0.324 |
| Variance | 87.78 | 9.94 | 0.024 | 92.75 | 5.30 | 0.020 |
| Cumulative | 87.78 | 97.64 | 1.000 | 92.75 | 98.00 | 1.000 |
| Components loadings | ||||||
| Leaf area | 0.264 | 0.106 | −0.166 | 0.254 | −0.09 | −0.347 |
| Shoot length | 0.264 | 0.031 | −0.226 | 0.242 | −0.375 | −0.205 |
| Tree Canopy volume | 0.267 | −0.027 | 0.001 | 0.259 | −0.074 | 0.090 |
| No. of Fruits /tree | 0.266 | −0.007 | 0.132 | 0.251 | 0.261 | 0.130 |
| Yield (kg)/tree | 0.265 | −0.081 | 0.103 | 0.258 | 0.050 | 0.159 |
| Yield increasing (%) | 0.258 | −0.207 | −0.055 | 0.258 | 0.050 | 0.158 |
| Yield tons/feddan | 0.255 | −0.195 | −0.279 | 0.258 | 0.051 | 0.160 |
| Fruits weight (g) | −0.226 | 0.348 | −0.481 | 0.254 | 0.050 | 0.266 |
| Fruit height (cm) | 0.096 | 0.741 | −0.043 | 0.239 | −0.148 | 0.122 |
| Fruit diameter (cm) | 0.261 | −0.02 | −0.342 | 0.233 | 0.421 | −0.113 |
| Pulp firmness (lb/inch2) | 0.266 | −0.007 | 0.132 | −0.245 | 0.470 | 0.574 |
| T.S.S. (%) | 0.221 | 0.365 | 0.517 | 0.246 | 0.088 | −0.241 |
| Total acidity (%) | −0.260 | −0.001 | 0.365 | −0.242 | 0.318 | −0.048 |
| TSS/acid ratio | 0.256 | 0.218 | 0.070 | 0.257 | 0.389 | −0.24 |
| VC mL/100 mL of juice | 0.257 | −0.196 | 0.195 | 0.251 | −0.039 | 0.416 |
| Total sugars (%) | 0.265 | 0.099 | −0.044 | 0.251 | −0.114 | 0.132 |
| Soil Depth (cm) | Soil Physical Analysis | EC (ds/m) | pH | Soil Chemical Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Soil Texture | Cations (meq/L) | Anions (meq/L) | |||||||||
| Ca++ | Mg++ | Na+ | K+ | So4= | CI− | HCo3− | Co3= | |||||||
| 0–30 | 94.72 | 3.00 | 2.28 | Sand | 0.50 | 7.0 | 2 | 1.11 | 1.5 | 0.21 | 0.82 | 2.0 | 2 | 00 |
| 30–60 | 93.72 | 5.00 | 1.28 | Sand | 0.47 | 7.2 | 2 | 0.9 | 1.17 | 0.22 | 0.29 | 2.0 | 2 | 00 |
| 60–90 | 92.15 | 4.65 | 3.2 | Sand | 0.40 | 7.4 | 2 | 0.8 | 1.04 | 0.20 | 0.34 | 1.6 | 2 | 00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshallash, K.S.; Sharaf, M.; Hmdy, A.E.; Khalifa, S.M.; Abdel-Aziz, H.F.; Sharaf, A.; Ibrahim, M.T.S.; Alharbi, K.; Elkelish, A. Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition. Gels 2022, 8, 602. https://doi.org/10.3390/gels8100602
Alshallash KS, Sharaf M, Hmdy AE, Khalifa SM, Abdel-Aziz HF, Sharaf A, Ibrahim MTS, Alharbi K, Elkelish A. Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition. Gels. 2022; 8(10):602. https://doi.org/10.3390/gels8100602
Chicago/Turabian StyleAlshallash, Khalid S., Mohamed Sharaf, Ashraf E. Hmdy, Sobhy M. Khalifa, Hosny F. Abdel-Aziz, Ahmed Sharaf, Mariam T. S. Ibrahim, Khadiga Alharbi, and Amr Elkelish. 2022. "Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition" Gels 8, no. 10: 602. https://doi.org/10.3390/gels8100602
APA StyleAlshallash, K. S., Sharaf, M., Hmdy, A. E., Khalifa, S. M., Abdel-Aziz, H. F., Sharaf, A., Ibrahim, M. T. S., Alharbi, K., & Elkelish, A. (2022). Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition. Gels, 8(10), 602. https://doi.org/10.3390/gels8100602











