Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model of Hypertension
2.2. Tissue Extraction and Preparation
2.3. Histopathological Examination
2.4. Immunohistochemical Examination
2.5. Mechanical Tests
2.6. Statistical Analysis
3. Results
3.1. Blood Pressure Measurement
3.2. Histopathological Findings
3.3. Immunohistochemical Findings
3.4. Biaxial Mechanical Tests
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Kummer, B.R.; Diaz, I.; Wu, X.; Aaroe, A.E.; Chen, M.L.; Iadecola, C.; Kamel, H.; Navi, B.B. Associations between cerebrovascular risk factors and parkinson disease. Ann. Neurol. 2019, 86, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Seccia, T.; Caroccia, B.; Piazza, M.; Rossi, G.P. The key role of epithelial to mesenchymal transition (EMT) in hypertensive kidney disease. Int. J. Mol. Sci. 2019, 20, 3567. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Tafazzoli-Shadpour, M.; Shadmehr, M.B. Analysis of arterial wall remodeling in hypertension based on lamellar modeling. J. Am. Soc. Hypertens. 2015, 9, 735–744. [Google Scholar] [CrossRef]
- Taghizadeh, H. Mechanobiology of the arterial tissue from the aortic root to the diaphragm. Med. Eng. Phys. 2021, 96, 64–70. [Google Scholar] [CrossRef]
- Petelina, T.; Avdeeva, K.; Bykova, S.; Musikhina, N.; Gapon, L.; Gorbatenko, E.; Zueva, E.; Leonovich, S.; Lystsova, N. Nonspecific parameters of the immune inflammatory response as a link in the pathogenesis of remodeling of the vascular wall and destruction of bone tissue in women with arterial hypertension in postmenopausal women. Klin. Lab. Diagn. 2019, 64, 417–423. [Google Scholar] [CrossRef]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef]
- Brown, I.A.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gu, L. Implementation and validation of aortic remodeling in hypertensive rats. J. Biomech. Eng. 2014, 136, 091007. [Google Scholar] [CrossRef]
- Hu, J.-J.; Ambrus, A.; Fossum, T.W.; Miller, M.W.; Humphrey, J.D.; Wilson, E. Time courses of growth and remodeling of porcine aortic media during hypertension: A quantitative immunohistochemical examination. J. Histochem. Cytochem. 2008, 56, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Folkow, B. Structure and function of the arteries in hypertension. Am. Heart J. 1987, 114, 938–948. [Google Scholar] [CrossRef]
- de Mello, M.M.B.; Parente, J.M.; Schulz, R.; Castro, M.M. Matrix metalloproteinase (MMP)-2 activation by oxidative stress decreases aortic calponin-1 levels during hypertrophic remodeling in early hypertension. Vasc. Pharmacol. 2019, 116, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, H.; Tafazzoli-Shadpour, M. Characterization of mechanical properties of lamellar structure of the aortic wall: Effect of aging. J. Mech. Behav. Biomed. Mater. 2017, 65, 20–28. [Google Scholar] [CrossRef]
- Bashkaran, K.; Zunaina, E.; Bakiah, S.; Sulaiman, S.A.; Sirajudeen, K.; Naik, V. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: Experimental animal study. BMC Complement. Altern. Med. 2011, 11, 90. [Google Scholar] [CrossRef]
- Kono, Y.; Moriyasu, F.; Nada, T.; Suginoshita, Y.; Matsumura, T.; Kobayashi, K.; Nakamura, T.; Chiba, T. Gray scale second harmonic imaging of the liver: A preliminary animal study. Ultrasound Med. Biol. 1997, 23, 719–726. [Google Scholar] [CrossRef]
- Aksoy, B.; Atakan, N.; Aksoy, H.M.; Tezel, G.G.; Renda, N.; Özkara, H.A.; Önder, E. Effectiveness of topical zinc oxide application on hypertrophic scar development in rabbits. Burns 2010, 36, 1027–1035. [Google Scholar] [CrossRef]
- Council of the European Union. 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. Off. J. Eur Commun. 1986, 358, 1–28. [Google Scholar]
- Wiesel, P.; Mazzolai, L.; Nussberger, J.r.; Pedrazzini, T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 1997, 29, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Ketabchi, F.; Bajoovand, S.; Adlband, M.; Naseh, M.; Nekooeian, A.A.; Mashghoolozekr, E. Right ventricular pressure elevated in one-kidney, one clip Goldblatt hypertensive rats. Clin. Exp. Hypertens. 2017, 39, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Yershov, A.L.; Jordan, B.S.; Fudge, J.M.; Dubick, M.A. Influence of the mode of ventilation on ketamine/xylazine requirements in rabbits. Vet. Anaesth. Analg. 2007, 34, 157–163. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Tafazzoli-Shadpour, M.; Shadmehr, M.B.; Fatouraee, N. Evaluation of biaxial mechanical properties of aortic media based on the lamellar microstructure. Materials 2015, 8, 302–316. [Google Scholar] [CrossRef]
- Çomaklı, S.; Sevim, Ç.; Kontadakis, G.; Doğan, E.; Taghizadehghalehjoughi, A.; Özkaraca, M.; Aschner, M.; Nikolouzakis, T.K.; Tsatsakis, A. Acute glufosinate-based herbicide treatment in rats leads to increased ocular interleukin-1β and c-Fos protein levels, as well as intraocular pressure. Toxicol. Rep. 2019, 6, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Stemper, B.D.; Yoganandan, N.; Stineman, M.R.; Gennarelli, T.A.; Baisden, J.L.; Pintar, F.A. Mechanics of fresh, refrigerated, and frozen arterial tissue. J. Surg. Res. 2007, 139, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.O.V.; Delorme, S.; El-Ayoubi, R.; DiRaddo, R.; Hatzikiriakos, S.G. Effect of freezing on the passive mechanical properties of arterial samples. J. Biomed. Sci. Eng. 2010, 3, 645. [Google Scholar] [CrossRef]
- Pukacki, F.; Jankowski, T.; Gabriel, M.; Oszkinis, G.; Krasinski, Z.; Zapalski, S. The mechanical properties of fresh and cryopreserved arterial homografts. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 21–24. [Google Scholar] [CrossRef]
- Jensen, L.F.; Bentzon, J.F.; Albarrán-Juárez, J. The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues. Cells 2021, 10, 2209. [Google Scholar] [CrossRef]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef]
- Humphrey, J. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 2008, 50, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Humphrey, J.D. Modeling mechano-driven and immuno-mediated aortic maladaptation in hypertension. Biomech. Model. Mechanobiol. 2018, 17, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-L.; Lai, F.M.; Tong, P.C.; Zhong, D.-R.; Yang, D.; Tomlinson, B.; Chan, J.C. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes 2003, 52, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Petre, S.; Shah, I.; Gilani, N. Gastrointestinal amyloidosis–clinical features, diagnosis and therapy. Aliment. Pharmacol. Ther. 2008, 27, 1006–1016. [Google Scholar] [CrossRef]
- Baumbach, G.L.; Ghoneim, S. Vascular remodeling in hypertension. Scanning Microsc. 1993, 7, 15. [Google Scholar]
- Rizzoni, D.; De Ciuceis, C.; Porteri, E.; Paiardi, S.; Boari, G.E.; Mortini, P.; Cornali, C.; Cenzato, M.; Rodella, L.F.; Borsani, E.; et al. Altered structure of small cerebral arteries in patients with essential hypertension. J. Hypertens. 2009, 27, 838–845. [Google Scholar] [CrossRef]
- Doillon, C.J.; Dunn, M.G.; Bender, E.; Silver, F.H. Collagen fiber formation in repair tissue: Development of strength and toughness. Collagen Relat. Res. 1985, 5, 481–492. [Google Scholar] [CrossRef]
- Koohestani, F.; Braundmeier, A.G.; Mahdian, A.; Seo, J.; Bi, J.; Nowak, R.A. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells. PLoS ONE 2013, 8, e75844. [Google Scholar] [CrossRef]
- McNulty, M.; Mahmud, A.; Spiers, P.; Feely, J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J. Hum. Hypertens. 2006, 20, 867–873. [Google Scholar] [CrossRef]
- Schildmeyer, L.A.; Braun, R.; Taffet, G.; Debiasi, M.; Burns, A.E.; Bradley, A.; Schwartz, R.J. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle α-actin null mouse. FASEB J. 2000, 14, 2213–2220. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-M.; Wu, N. Aortic α-smooth muscle actin expressions in aortic disorders and coronary artery disease: An immunohistochemical study. Anatol. J. Cardiol. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Schmidt, J.; Ryschich, E.; Schumacher, H.; Allenberg, J.R. Increased apoptosis and decreased density of medial smooth muscle cells in human abdominal aortic aneurysms. Chin. Med. J. 2003, 116, 1549–1552. [Google Scholar] [PubMed]
- Dobrian, A.; Wade, S.S.; Prewitt, R.L. PDGF-A expression correlates with blood pressure and remodeling in 1K1C hypertensive rat arteries. Am. J. Physiol. 1999, 276, H2159–H2167. [Google Scholar] [CrossRef]
- Li, Y.; Shen, X.Z.; Li, L.; Zhao, T.V.; Bernstein, K.E.; Johnson, A.K.; Lyden, P.; Fang, J.; Shi, P. Brain Transforming Growth Factor-beta Resists Hypertension Via Regulating Microglial Activation. Stroke 2017, 48, 2557–2564. [Google Scholar] [CrossRef]
- Chen, P.; Tang, H.; Zhang, Q.; Xu, L.; Zhou, W.; Hu, X.; Deng, Y.; Zhang, L. Basic Fibroblast Growth Factor (bFGF) Protects the Blood-Brain Barrier by Binding of FGFR1 and Activating the ERK Signaling Pathway After Intra-Abdominal Hypertension and Traumatic Brain Injury. Med. Sci. Monit. 2020, 26, e922009. [Google Scholar] [CrossRef] [PubMed]
- Zeinali-Davarani, S.; Wang, Y.; Chow, M.J.; Turcotte, R.; Zhang, Y. Contribution of collagen fiber undulation to regional biomechanical properties along porcine thoracic aorta. J. Biomech. Eng. 2015, 137, 051001. [Google Scholar] [CrossRef]
- Loree, H.M.; Grodzinsky, A.J.; Park, S.Y.; Gibson, L.J.; Lee, R.T. Static circumferential tangential modulus of human atherosclerotic tissue. J. Biomech. 1994, 27, 195–204. [Google Scholar] [CrossRef]
- Zulliger, M.A.; Fridez, P.; Hayashi, K.; Stergiopulos, N. A strain energy function for arteries accounting for wall composition and structure. J. Biomech. 2004, 37, 989–1000. [Google Scholar] [CrossRef]
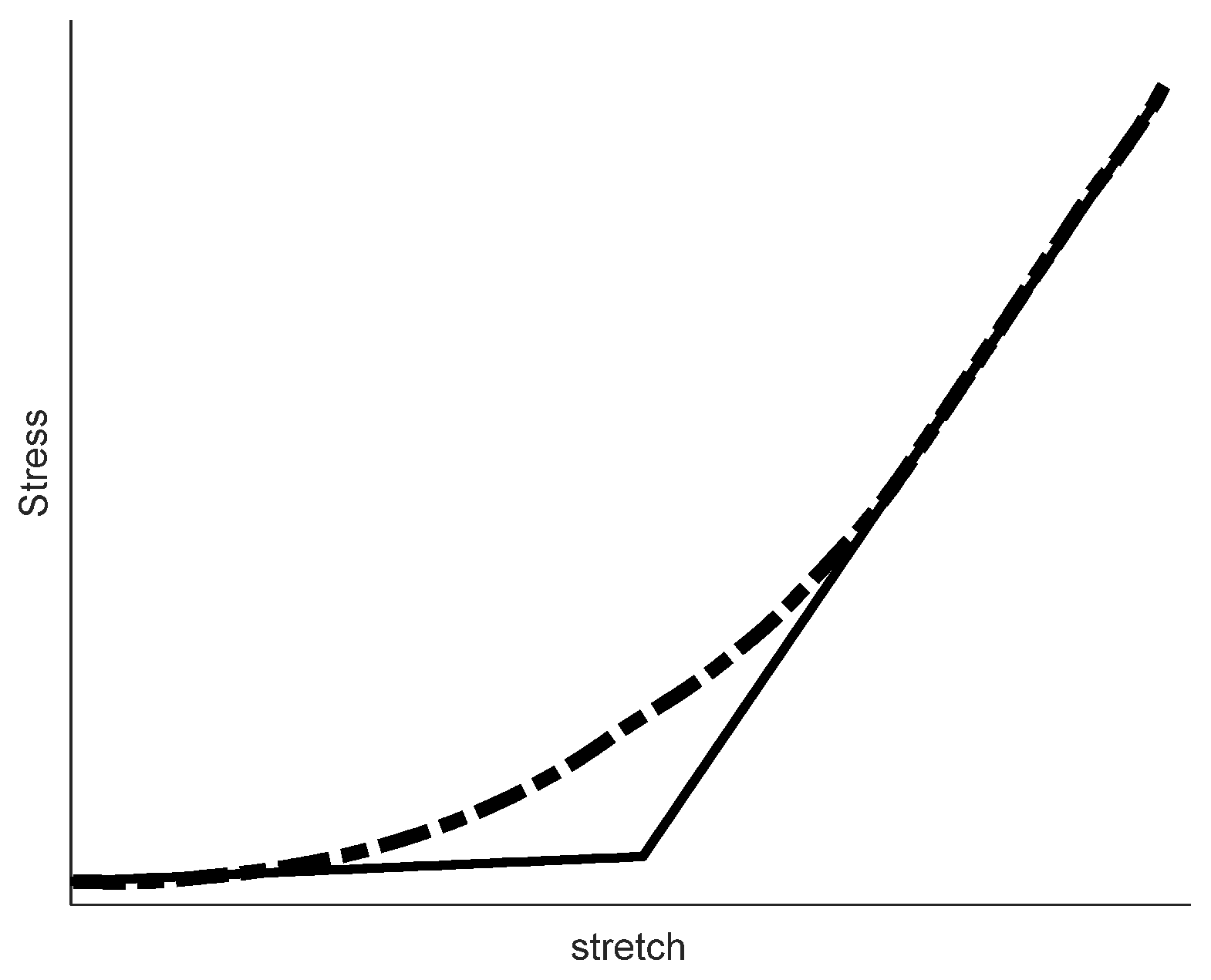
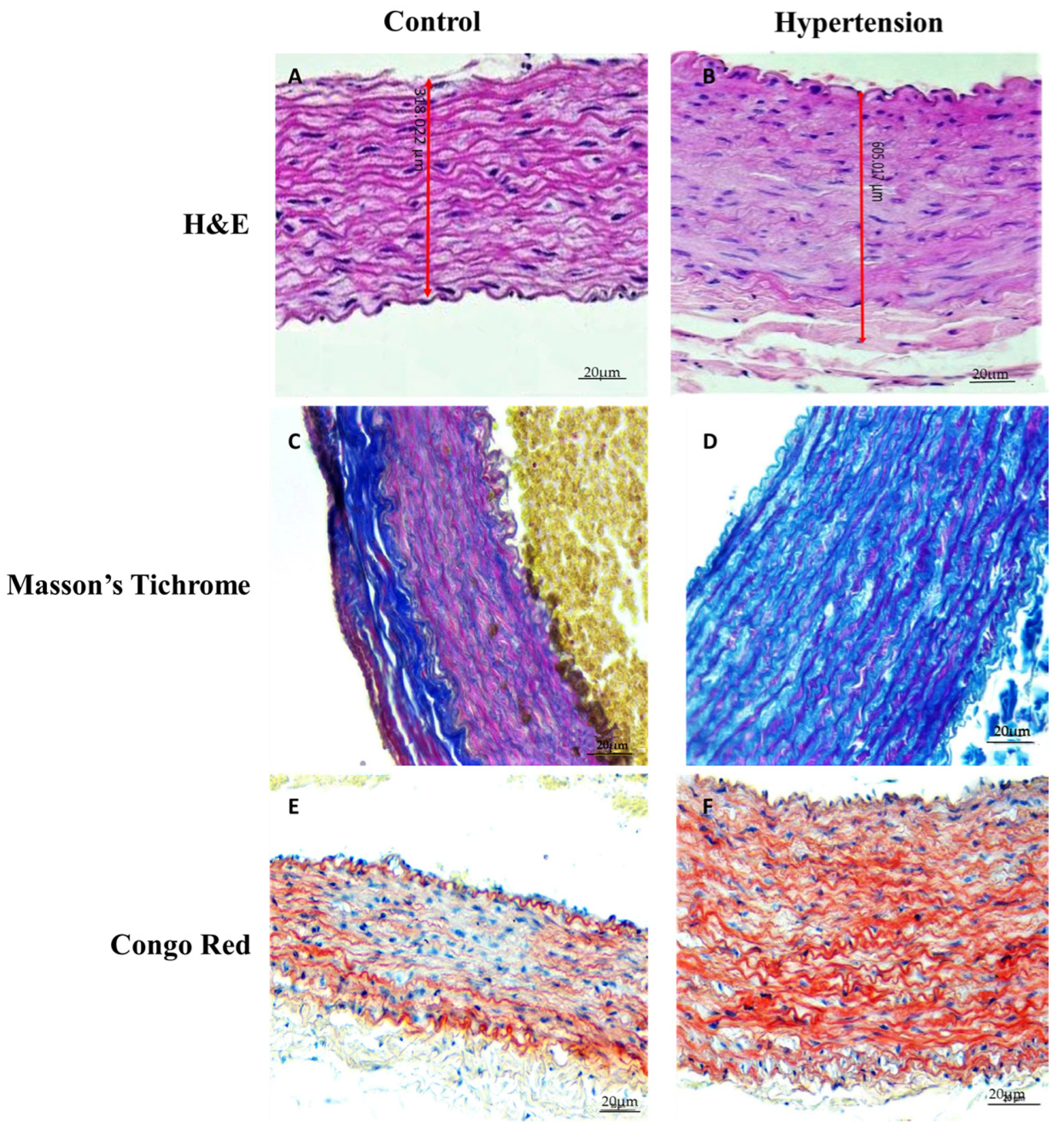


| Group | Background Values | 30th Day | 60th Day | |||
|---|---|---|---|---|---|---|
| Diastolic Pressure (mmHg) | Systolic Pressure (mmHg) | Diastolic Pressure (mmHg) | Systolic Pressure (mmHg) | Diastolic Pressure (mmHg) | Systolic Pressure (mmHg) | |
| Control group | 84.2 ± 4.71 | 115 ± 5.34 | 84.4 ± 7.05 | 115 ± 8.52 | 85.3 ± 5.54 | 118 ± 6.54 |
| Hypertension Group | 85.0 ± 3.23 | 115 ± 4.06 | 95.7 ± 9.04 | 133 ± 7.02 a | 98.0 ± 5.85 | 152 ± 7.51 a |
| Parameter | Aorta | Brain and Pancreatic Vasculature | ||
|---|---|---|---|---|
| Control | Hypertension | Control | Hypertension | |
| Collagen I | 64.11 ± 4.22 | 20.24 ± 6.45 * | 54.36 ± 5.71 | 19.23 ± 6.17 * |
| Collagen III | 28.18 ± 3.54 | 49.18 ± 6.13 * | 17.52 ± 5.18 | 35.0 ± 6.13 * |
| αSMA | 62.71 ± 5.18 | 31.18 ± 4.12 * | 40.13 ± 4.28 | 21.22 ± 5.18 * |
| PDGF | 29.56 ± 4.18 | 63.74 ± 5.18 * | 18.25 ± 6.75 | 60.11 ± 4.35 * |
| Sample No. | Control | Hypertension | ||||||
|---|---|---|---|---|---|---|---|---|
| Axial | Circ. | Axial | Circ. | |||||
| Einit (kPa) | Efin (kPa) | Einit (kPa) | Efin (kPa) | Einit (kPa) | Efin (kPa) | Einit (kPa) | Efin (kPa) | |
| 1 | 139.9 | 4983 | 493.1 | 7748 | 159.1 | 521.2 | 372.3 | 5704 |
| 2 | 201.5 | 4229 | 470.4 | 6696 | 97.62 | 882.9 | 261.3 | 5006 |
| 3 | 238.1 | 4991 | 379.2 | 7742 | 89.05 | 687.8 | 404.6 | 4821 |
| 4 | 172.5 | 5646 | 387.1 | 6734 | 52.05 | 553.7 | 429.4 | 5093 |
| 5 | 193.2 | N/A | 329.9 | N/A | 84.94 | 652.7 | 353.5 | 4936 |
| mean ± sd | 189.0 ± 36.29 | 4962 ± 579.0 | 411.8 ± 67.81 | 7230 ± 594.9 | 96.54 ± 39.02 | 659.7 ± 142.4 | 364.2 ± 64.52 | 5112 ± 345.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghizadeh, H.; Taghizadehghalehjoughi, A.; Yildirim, S.; Ozkaraca, M.; Genc, S.; Yeni, Y.; Mokresh, M.Y.; Hacimuftuoglu, A.; Tsatsakis, A.; Tsarouhas, K. Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature. J. Pers. Med. 2022, 12, 1602. https://doi.org/10.3390/jpm12101602
Taghizadeh H, Taghizadehghalehjoughi A, Yildirim S, Ozkaraca M, Genc S, Yeni Y, Mokresh MY, Hacimuftuoglu A, Tsatsakis A, Tsarouhas K. Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature. Journal of Personalized Medicine. 2022; 12(10):1602. https://doi.org/10.3390/jpm12101602
Chicago/Turabian StyleTaghizadeh, Hadi, Ali Taghizadehghalehjoughi, Serkan Yildirim, Mustafa Ozkaraca, Sidika Genc, Yesim Yeni, Muhammed Yasser Mokresh, Ahmet Hacimuftuoglu, Aristidis Tsatsakis, and Konstantinos Tsarouhas. 2022. "Deteriorated Vascular Homeostasis in Hypertension: Experimental Evidence from Aorta, Brain, and Pancreatic Vasculature" Journal of Personalized Medicine 12, no. 10: 1602. https://doi.org/10.3390/jpm12101602







