Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Instrumentation
2.4. Preparation of the DESs
2.5. Conventional and the PEF-Based Extraction of the Polyphenols
2.6. Measurement of the Total Polyphenol Content (TPC)
2.7. HPLC-Based Identification and the Quantification of the Polyphenols
2.8. Determination of the Volatile Components
2.9. Statistical Analysis
3. Results and Discussion
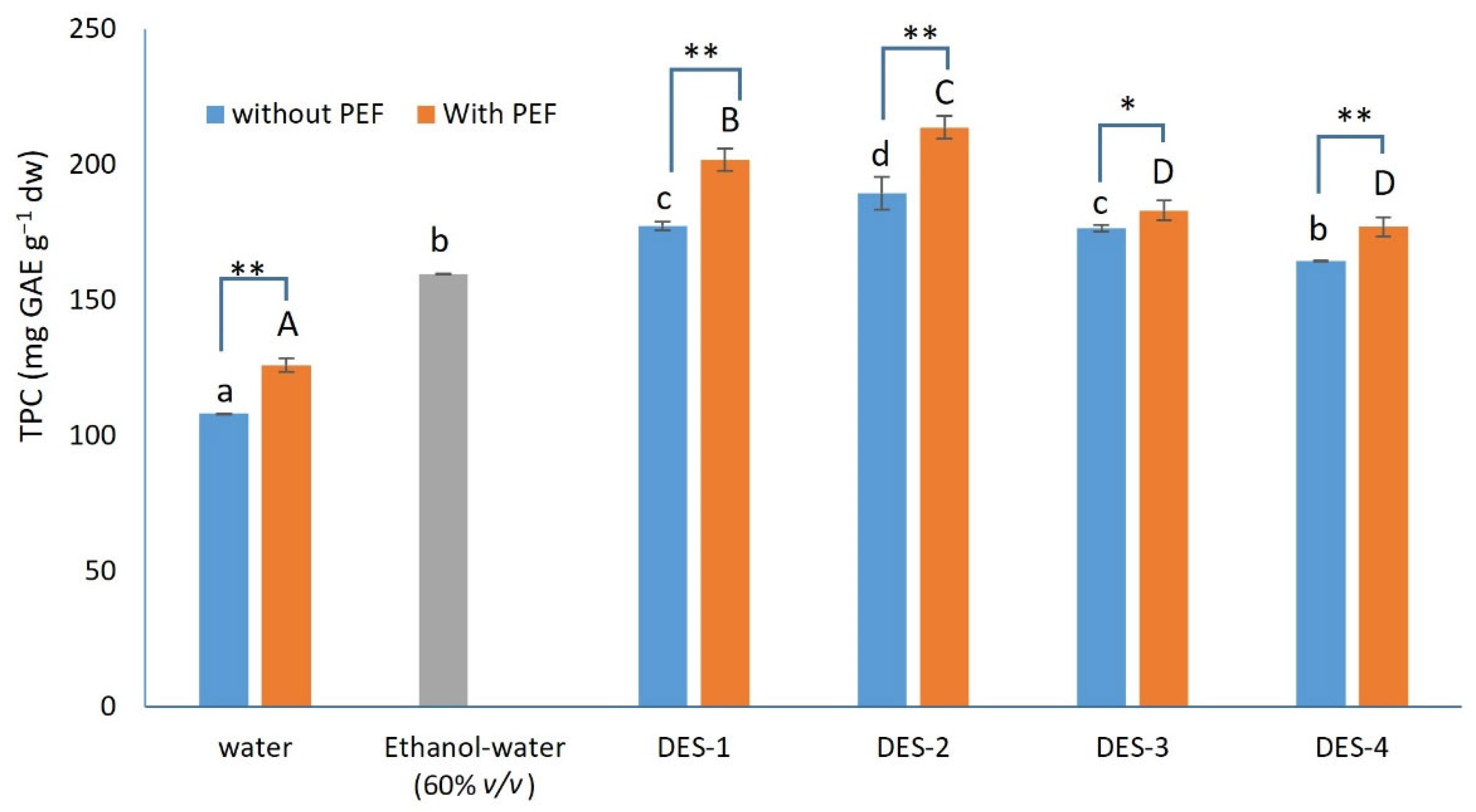
3.1. Effect of the DESs and the PEF Usage on the TPC of the Extracts
3.2. HPLC-Based Quantification of the Polyphenols
3.3. Volatile Profile of the Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Cistus incanus from strandja mountain as a source of bioactive antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Haida, S.; Bakkouche, K.; Kribii, A.R.; Kribii, A. Chemical Composition of Essential Oil, Phenolic Compounds Content, and Antioxidant Activity of Cistus monspeliensis from Northern Morocco. Biochem. Res. Int. 2021, 2021, 6669877. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Antioxidant activity and polyphenolic content of the Bulgarian wild herb Cistus incanus L. stored under different conditions. J. Chem. Technol. Metall. 2017, 52, 781–790. [Google Scholar]
- Rebaya, A.; Belghith, S.I.; Cherif, J.K.; Trabelsi-Ayadi, M. Total phenolic compounds and antioxidant potential of rokrose (Cistus salviifolius) leaves and flowers grown in Tunisia. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 327–331. [Google Scholar]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol diversity and antioxidant activity of european Cistus creticus L. (cistaceae) compared to six further, partly sympatric Cistus species. Plants 2021, 10, 615. [Google Scholar] [CrossRef]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. Incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Choudhary, P.; Guleria, S.; Sharma, N.; Salaria, K.H.; Chalotra, R.; Ali, V.; Vyas, D. Comparative phenolic content and antioxidant activity of some medicinal plant extracts prepared by choline chloride based green solvents and methanol. Curr. Res. Green Sustain. Chem. 2021, 4, 100224. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 8. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Dourtoglou, V.G.; Elhakem, A.; Sami, R.; Ashour, A.A.; Shafie, A.; et al. Combination of Pulsed Electric Field and Ultrasound in the Extraction of Polyphenols and Volatile Compounds from Grape Stems. Appl. Sci. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef] [PubMed]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Dourtoglou, V.G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering 2022, 4, 54. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [Green Version]
- Slim, Z.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: Effect of organic anion carbon chain length. Chem. Eng. Commun. 2018, 205, 1494–1506. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process Optimization and Stability of Waste Orange Peel Polyphenols in Extracts Obtained with Organosolv Thermal Treatment Using Glycerol-Based Solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Fadjare Frempong, T.; Owusu Boadi, N.; Badu, M. Optimization of extraction conditions for polyphenols from the stem bark of Funtumia elastica (Funtum) utilizing response surface methodology. AAS Open Res. 2021, 4, 46. [Google Scholar] [CrossRef]
- Zhumakanova, B.S.; Korona-Głowniak, I.; Skalicka-Woźniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.K.; Józefczyk, A.; Zhaparkulova, K.A.; Sakipova, Z.B.; Malm, A. Phytochemical fingerprinting and in vitro antimicrobial and antioxidant activity of the aerial parts of Thymus marschallianus willd. And thymus seravschanicus klokov growing widely in Southern Kazakhstan. Molecules 2021, 26, 3193. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the bioactive potential of brewers spent grain ohmic extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of Polyphenols from Olive Leaves Employing Deep Eutectic Solvents: The Application of Chemometrics to a Quantitative Study on Antioxidant Compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of antioxidant and antimicrobial activities of two cultivated cistus species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Ghalia, S.; Adawia, K.; Waed, A. Evaluation of radical scavenging activity, total phenolics and total flavonoids contents of Cistus species in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1071–1077. [Google Scholar]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and glycerol-based deep eutectic mixtures as emerging green solvents for polyphenol extraction: The evidence so far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Lanjekar, K.; Gokhale, S.; Rathod, V. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent (Ua-Nades). SSRN Electron. J. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Peng, X.; Yao, X.H.; Wei, Z.F.; Luo, M.; Wang, W.; Zhao, C.J.; Fu, Y.J.; Zu, Y.G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, W.; Niu, D.; Wang, R.; Xu, F.Y.; Chen, B.R.; Lin, J.W.; Tang, Z.S.; Zeng, X.A. Efficient and green strategy based on pulsed electric field coupled with deep eutectic solvents for recovering flavonoids and preparing flavonoid aglycones from noni-processing wastes. J. Clean. Prod. 2022, 368, 133019. [Google Scholar] [CrossRef]
- Cicci, A.; Bravi, M. Leveraging novel green solvents to drive conceptual and practical biorefinery innovation. Stud. Surf. Sci. Catal. 2019, 179, 243–259. [Google Scholar]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
| Identified Polyphenol | Without PEFs | With PEFs | ||
|---|---|---|---|---|
| Water | DES-2 | Water | DES-2 | |
| Luteolin glucoside derivative | 0.159 ± 0.005 b | 0.090 ± 0.003 c | 0.182 ± 0.012 a | 0.101 ± 0.004 c |
| Luteolin glucoside p-coumaroyl | 0.077 ± 0.002 c | 0.767 ± 0.018 b | 0.089 ± 0.004 c | 0.866 ± 0.026 a |
| 1-Myricetin glucoside | 0.321 ± 0.011 d | 0.527 ± 0.019 b | 0.387 ± 0.016 c | 0.602 ± 0.021 a |
| Myricetin rhamnoside | 1.302 ± 0.041 d | 1.891 ± 0.069 b | 1.512 ± 0.046 c | 2.258 ± 0.137 a |
| 1-Quercetin glucoside derivative | 0.274 ± 0.009 d | 0.425 ± 0.008 b | 0.323 ± 0.017 c | 0.485 ± 0.028 a |
| Rutin | 0.038 ± 0.001 d | 0.057 ± 0.002 b | 0.044 ± 0.002 c | 0.064 ± 0.002 a |
| 2-Quercetin glucoside derivative | 0.158 ± 0.005 d | 0.258 ± 0.007 b | 0.187 ± 0.019 c | 0.296 ± 0.019 a |
| Quercetin rhamnoside derivative | 0.513 ± 0.014 d | 0.862 ± 0.022 b | 0.599 ± 0.022 c | 0.975 ± 0.046 a |
| 2-Myricetin glucoside | 0.051 ± 0.002 c | 0.122 ± 0.003 b | 0.058 ± 0.001 c | 0.141 ± 0.012 a |
| Total extraction yield | 2.893 | 4.999 | 3.381 | 5.788 |
| Compounds | DES-2 without PEFs | DES-2 with PEFs |
|---|---|---|
| Methylene chloride | nd | 1.65 |
| 2-Pentenal | 0.57 | 0.58 |
| 1-Chloropentane | nd | 0.13 |
| 2-Penten-1-ol | 4.25 | 4.16 |
| cis-3-Hexenal | 0.45 | 0.29 |
| Caproaldehyde | 1.86 | 1.77 |
| 2-Hexenal | 1.69 | 2.14 |
| 3-Hexen-1-ol | 3.51 | 3.39 |
| 1R-α-Pinene * | 21.60 | 22.23 |
| Camphene | nd | 1.02 |
| Methylene chloride | nd | 1.65 |
| 2-Bornene | 0.84 | nd |
| γ-Terpinene | nd | 1.15 |
| β-Terpinene | 0.20 | nd |
| (-)-β-Pinene | 0.12 | nd |
| Limonene | 1.02 | 0.63 |
| trans-β-Terpineol | nd | 0.65 |
| cis-Sabinene hydrate | 1.39 | nd |
| Pelargonaldehyde | 0.89 | nd |
| β-Linalool | 0.47 | 0.70 |
| L-Camphor | 0.63 | 0.65 |
| trans-Pinocarveole | nd | 0.73 |
| Neomenthone | nd | 2.61 |
| L-Menthone | 2.41 | nd |
| α-Pinocarvone | 0.73 | nd |
| Camphol | 1.17 | 1.14 |
| Isomenthon | 0.28 | 0.33 |
| L-Borneol * | 6.46 | 5.93 |
| Terpinen-4-ol | 2.39 | 2.20 |
| (-)-Myrtenal | 0.27 | 0.27 |
| α-Terpinol * | 8.60 | 7.24 |
| α-Cubenene * | 8.04 | 7.32 |
| 2,5-Dimethylhex-5-en-3-yn-2-ol | nd | 0.76 |
| α-Copaene | nd | 0.22 |
| β-Cubebene | 3.08 | 2.83 |
| β-Caryophyllene | 1.49 | 2.00 |
| δ-Cadinene | 0.79 | 0.32 |
| β-Copaene | 0.21 | nd |
| β-Selinene | nd | 0.62 |
| (+)-epi-Bicyclosesquiphellandrene | 0.55 | 0.49 |
| cis-Muurola-3,5-diene | 2.27 | 2.52 |
| L-Calamenene | 3.46 | 3.90 |
| β-Cadinene | 3.81 | 3.47 |
| Cadinadiene-1,4 | 2.12 | 2.34 |
| Caryophyllene oxide * | 5.73 | 5.62 |
| Patchoulene | 0.84 | nd |
| Globulol | nd | 1.99 |
| Ledol | 2.32 | nd |
| Cadinadiene-1,4 | 3.20 | 2.49 |
| 3,7(11)-Selinadiene | 0.28 | 0.33 |
| Phthalic acid, isobutyl non-5-yn-3-yl ester | nd | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields. Compounds 2022, 2, 311-320. https://doi.org/10.3390/compounds2040026
Palaiogiannis D, Athanasiadis V, Bozinou E, Chatzimitakos T, Makris DP, Lalas SI. Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields. Compounds. 2022; 2(4):311-320. https://doi.org/10.3390/compounds2040026
Chicago/Turabian StylePalaiogiannis, Dimitrios, Vassilis Athanasiadis, Eleni Bozinou, Theodoros Chatzimitakos, Dimitris P. Makris, and Stavros I. Lalas. 2022. "Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields" Compounds 2, no. 4: 311-320. https://doi.org/10.3390/compounds2040026
APA StylePalaiogiannis, D., Athanasiadis, V., Bozinou, E., Chatzimitakos, T., Makris, D. P., & Lalas, S. I. (2022). Extraction of Polyphenolic and Volatile Compounds from Cistus creticus Using Deep Eutectic Solvents and Pulsed Electric Fields. Compounds, 2(4), 311-320. https://doi.org/10.3390/compounds2040026










