Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario
Abstract
:1. Introduction
Why Do We Still Have to Do Derivatization?
- Derivatized analytes have an increased instrumental response factor. Thiols analysis with LC-MS is emblematic; the sulphur group allows these molecules to be easily ionized using ESI sources, but, due to their ultra-low concentration in beverages, derivatization boosts the analytical response, improving the limits of detection and quantitation down to ng·L−1 [39].
- Derivatized analytes have an increased extraction efficiency. Some molecules are quite hydrophilic, so their flavor is mostly due to their olfactory response rather than abundancy in the vapors. It means that after derivatization, it is possible to achieve a less polar compound with higher volatility and a stronger affinity to extraction solvents, cartridges, or fibers [40].
- Derivatization can modify chemical and structural molecular characteristics to improve extraction selectivity [41]. A reduction in complexity, matrix effects, and purification steps needed is achieved.
- Derivatized analytes have a different reactivity, so derivatization can be intended also as a preservative process for unstable compounds [42]. This argument can be extended also to strongly volatile compounds, which can be stuck and stabilized in derivatized form into the samples.
2. Derivatization of VOAs in Wine and Beer Analysis
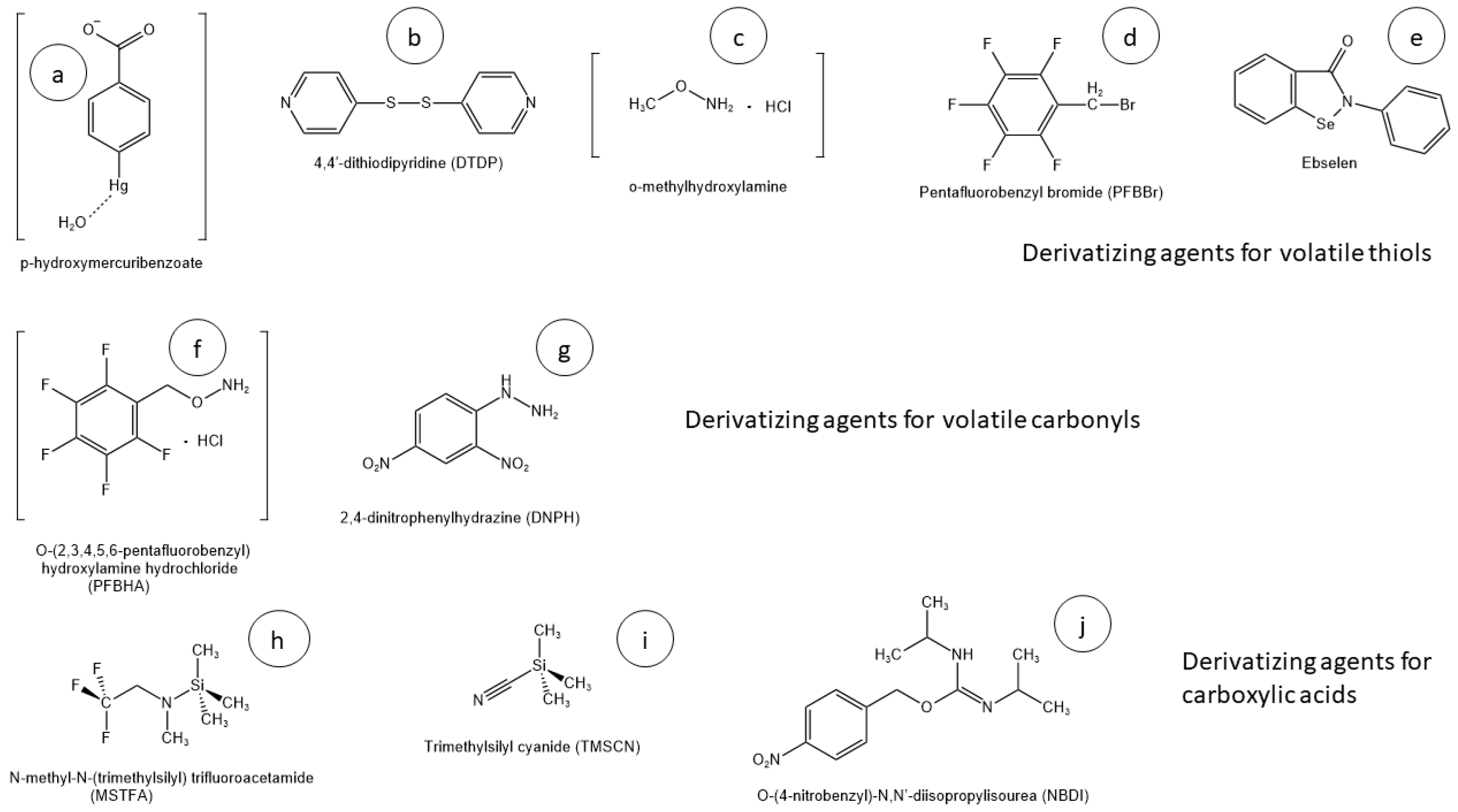
2.1. Volatile Thiols
| Article | Year | Matrix | Ext. Volume | Ext. Technique | Der. Agent | Instrumentation | Pro & Cons |
|---|---|---|---|---|---|---|---|
| [48] | 2003 | White wine | 500 mL | LLE + N2 concentration + preparative column | p-HMB | GC-EI-MS | + 5000 concentration factors − 100 mL of hazardous solvent |
| [53] | 2006 | White wine | 20 mL | HS-SPME with on-fiber derivatization | PFBBr | GC-NCI-MS | + Solvent-free − Time-consuming derivatizing process |
| [55] | 2007 | White wine | 6 mL | LLE with benzene | PFBBr | GC-NCI-MS | + No equipment required − Time-consuming, hazardous solvent |
| [54] | 2008 | White wine | 20 mL | SPE and SIDA | PFBBr | GC-NCI-MS | + Good performance − Disposable cartridge, use of solvents |
| [56] | 2014 | White wine | 3 mL | HS-SPME with in-situ derivatization | o-methyl-hydroxylamine hydrochloride | GC-EI-MS/MS | + Low LOD, high automation, low sample volume − Only 4-MSP |
| [57] | 2015 | Beer, hops, wort | 10 mL | SBSE-PDMS with in-situ derivatization | Ethyl propiolate | GC-EI-MS/MS + GC-EI-QTof | + Low LODs, many analytes, solvent-free, safe reagents − Instrumentation complexity |
| [58] | 2015 | White wine | 20 mL | SPE with Bond-Elut C18, and SIDA | DTDP | LC-MS/MS | + Relevant VTs, accuracy − Disposable cartridge |
| [59] | 2018 | Wine (all) | 20 mL | SPE with Bond-Elut C18, and SIDA | DTDP | LC-HRMS | + Enantiomer analysis − Disposable cartridge |
| [60] | 2018 | Red wine | 20 mL | SPE with Supelclean ENVI-18 | DTDP | GC-MS/MS | + Greener chromatography − Disposable cartridge, complexity |
| [61] | 2015 | Wine, beer | 20 mL | LLE with 4 mL of CH2Cl2 | Ebselen | LC-HRMS | + No equipment required, flexibility, performance − CH2Cl2, time-consuming |
| [62] | 2018 | White wine | 35 mL | LLE with ethanol | Ebselen | LC-HRMS | + No equipment required, safe solvent − high sample volume, filtration |
| [63] | 2017 | White wine | 100 mL | SPE, 20 mg Li-Chrolut EN | Ebselen | LC-HRMS | + Minimized cartridge, accuracy − High sample volume |
| [39] | 2022 | White wine | 35 mL | Micro LLE + 0.22 µm filtration | Ebselen | LC-MS/MS | + Performance, reduced volumes − Low automatability |
2.2. Volatile Carbonyls
2.3. Carboxylic Acids
2.4. Other VOAs
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOAs | volatile odor-active molecules |
| GC | gas chromatography |
| LC | liquid chromatography |
| MS | mass spectrometry |
| LLE | liquid-liquid extractions |
| SPE | solid-phase extractions |
| SPME | solid-phase micro-extraction |
| OSD | on-solution derivatization |
| OFD | on-fiber derivatization |
| SBSE | stir bar sorptive extraction |
| EI | electron ionization |
| API | atmospheric pressure ionization sources |
| APCI | atmospheric pressure chemical ionization |
| ESI | electrospray ionization |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| ODT | odor detection threshold |
| VTs | volatile thiols |
| VSCs | volatile sulphur compounds |
| VCCs | volatile carbonyl compounds |
| VPs | volatile phenols |
| p-HMB | p-hydroxymercuribenzoate |
| PFBBr | p-entafluorobenzyl bromide |
| ETP | ethyl propiolate |
| DTDP | 4,4′-dithiodipyridine |
| ebselen | 2-phenyl-1,2-benzisoselenazol-3-one |
| PFBHA | O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride |
| DNPH | 2,4-dinitrophenylhydrazine |
| MSTFA | N-methyl-N-(trimethylsilyl) trifluoroacetamide |
| TMSCN | trimethylsilyl cyanide |
| NBDI | used O-(4-nitrobenzyl)-N,N’-diisopropylisourea |
| BSTFA | bis(trimethylsilyl)trifluoroacetamide |
| TMCS | trimethylchlorosilane |
| TMS | trimethylsilyl |
| PA | polyacrylate |
| PDMS | polydimethylsiloxane |
| DVB | divinylbenzene |
| CAR | carboxen |
References
- Ibáñez, E.; Cifuentes, A. Green extraction techniques 2015. TrAC Trends Anal. Chem. 2015, 71, 1. [Google Scholar] [CrossRef] [Green Version]
- Özay, H.; Çakır, A.; Ecevit, M.C. Retronasal Olfaction Test Methods: A Systematic Review. Balk. Med. J. 2019, 36, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Morrin, M.; Tepper, B.J. Multisensory marketing: Effects of environmental aroma cues on perception, appetite, and consumption of foods and drinks. Curr. Opin. Food Sci. 2021, 40, 204–210. [Google Scholar] [CrossRef]
- Martins, R.O.; de Araújo, G.L.; de Freitas, C.S.; Silva, A.R.; Simas, R.C.; Vaz, B.G.; Chaves, A.R. Miniaturized sample preparation techniques and ambient mass spectrometry as approaches for food residue analysis. J. Chromatogr. A 2021, 1640, 461949. [Google Scholar] [CrossRef] [PubMed]
- Soares Maciel, E.V.; de Toffoli, A.L.; Lanças, F.M. Recent trends in sorption-based sample preparation and liquid chromatography techniques for food analysis. Electrophoresis 2018, 39, 1582–1596. [Google Scholar] [CrossRef]
- Van Opstaele, F.; De Causmaecker, B.; Aerts, G.; De Cooman, L. Characterization of Novel Varietal Floral Hop Aromas by Headspace Solid Phase Microextraction and Gas Chromatography–Mass Spectrometry/Olfactometry. J. Agric. Food Chem. 2012, 60, 12270–12281. [Google Scholar] [CrossRef]
- Karlsson, P.B. World Wine Production in 2021: Almost Record Low, France Drops to Third Place. Forbes 2021. Available online: https://www.forbes.com/sites/karlsson/2021/12/30/wine-production-in-the-world-in-2020-a-detailed-look/?sh=108ee1e364a0 (accessed on 20 September 2022).
- Statista. Wine Market Revenue Worldwide from 2012 to 2025 (in Million U.S. Dollars). 2021. Available online: https://www.statista.com/statistics/922403/global-wine-market-size/ (accessed on 20 September 2022).
- Association, B. No Title 2022. Available online: https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed on 20 September 2022).
- Lyu, J.; Chen, S.; Nie, Y.; Xu, Y.; Tang, K. Aroma release during wine consumption: Factors and analytical approaches. Food Chem. 2020, 346, 128957. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.E.; Calvo, E.; Cejudo, M.J.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Volatile compounds as markers of ageing in Tempranillo red wines from La Mancha D.O. stored in oak wood barrels. J. Chromatogr. A 2011, 1218, 4910–4917. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A. Analytical performance of three commonly used extraction methods for the gas chromatography–mass spectrometry analysis of wine volatile compounds. J. Chromatogr. A 2009, 1216, 7351–7357. [Google Scholar] [CrossRef]
- Davis, P.M.; Qian, M.C. Effect of Ethanol on the Adsorption of Volatile Sulfur Compounds on Solid Phase Micro-Extraction Fiber Coatings and the Implication for Analysis in Wine. Molecules 2019, 24, 3392. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Luzzini, G.; Solis, J.S.; Forte, F.; Ugliano, M. Two Sides to One Story—Aroma Chemical and Sensory Signature of Lugana and Verdicchio Wines. Molecules 2021, 26, 2127. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Sciarrone, D.; Panto, S.; Ragonese, C.; Dugo, P.; Mondello, L. Evolution and status of preparative gas chromatography as a green sample-preparation technique. TrAC Trends Anal. Chem. 2015, 71, 65–73. [Google Scholar] [CrossRef]
- Cucu, T.; David, F.; Devos, C.; Sandra, P. Untargeted flavor profiling of lager beers by stir bar sorptive extraction–capillary gas chromatography—Time-of-flight mass spectrometry: High analytical performance with a green touch. J. Chromatogr. A 2021, 1647, 462164. [Google Scholar] [CrossRef]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A. The history of electron ionization in LC-MS, from the early days to modern technologies: A review. Anal. Chim. Acta 2021, 1167, 338350. [Google Scholar] [CrossRef]
- Polášková, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef]
- Castro, C.C.; Martins, R.; Teixeira, J.A.; Ferreira, A.C.S. Application of a high-throughput process analytical technology metabolomics pipeline to Port wine forced ageing process. Food Chem. 2013, 143, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Fornells, E.; Hilder, E.F.; Breadmore, M.C. Preconcentration by solvent removal: Techniques and applications. Anal. Bioanal. Chem. 2019, 411, 1715–1727. [Google Scholar] [CrossRef]
- Souza-Silva, A.; Reyes-Garcés, N.; Gómez-Ríos, G.A.; Boyacı, E.; Bojko, B.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. TrAC Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Franc, C.; de Revel, G.; Marchand, S. Dual solid-phase and stir bar sorptive extraction combined with gas chromatography-mass spectrometry analysis provides a suitable tool for assaying limonene-derived mint aroma compounds in red wine. Anal. Chim. Acta 2018, 1001, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Metafa, M.; Economou, A. Chemometrical development and comprehensive validation of a solid phase microextraction/gas chromatography–mass spectrometry methodology for the determination of important free and bound primary aromatics in Greek wines. J. Chromatogr. A 2013, 1305, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Duan, J.; Yang, S.; Li, H.; Qin, D.; Shen, Y.; Li, H.; Sun, J.; Zheng, F.; Sun, B. Why the key aroma compound of soy sauce aroma type baijiu has not been revealed yet? LWT 2021, 154, 112735. [Google Scholar] [CrossRef]
- Bruins, A.P.; Niessen, W.M.A. Mass Spectrometry|Atmospheric Pressure Ionization Techniques, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 6. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [Green Version]
- Fracassetti, D.; Camoni, D.; Montresor, L.; Bodon, R.; Limbo, S. Chemical Characterization and Volatile Profile of Trebbiano di Lugana Wine: A Case Study. Foods 2020, 9, 956. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Famiglini, G.; Termopoli, V.; Palma, P.; Capriotti, F.; Cappiello, A. Rapid LC-MS method for the detection of common fragrances in personal care products without sample preparation. Electrophoresis 2013, 35, 1339–1345. [Google Scholar] [CrossRef]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Cappiello, A. Atmospheric Pressure Vaporization Mechanism for Coupling a Liquid Phase with Electron Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 2049–2056. [Google Scholar] [CrossRef]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Rocio-Bautista, P.; Ottaviani, M.F.; Cappiello, A.; Saeed, M.; Perry, S. Evaluation of a liquid electron ionization liquid chromatography–mass spectrometry interface. J. Chromatogr. A 2019, 1591, 120–130. [Google Scholar] [CrossRef]
- Tsizin, S.; Bokka, R.; Keshet, U.; Alon, T.; Fialkov, A.B.; Tal, N.; Amirav, A. Comparison of electrospray LC–MS, LC–MS with Cold EI and GC–MS with Cold EI for sample identification. Int. J. Mass Spectrom. 2017, 422, 119–125. [Google Scholar] [CrossRef]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A.; Tsizin, S.; Seemann, B.; Alon, T.; Fialkov, A.B.; Amirav, A. Electron Ionization LC-MS: What Is It and Why Use It? Compr. Anal. Chem. 2018, 79, 1–28. [Google Scholar] [CrossRef]
- Carlin, S.; Piergiovanni, M.; Pittari, E.; Lisanti, M.T.; Moio, L.; Piombino, P.; Marangon, M.; Curioni, A.; Rolle, L.; Segade, S.R.; et al. The contribution of varietal thiols in the diverse aroma of Italian monovarietal white wines. Food Res. Int. 2022, 157, 111404. [Google Scholar] [CrossRef]
- Liem-Nguyen, V.; Bouchet, S.; Björn, E. Determination of Sub-Nanomolar Levels of Low Molecular Mass Thiols in Natural Waters by Liquid Chromatography Tandem Mass Spectrometry after Derivatization with p-(Hydroxymercuri) Benzoate and Online Preconcentration. Anal. Chem. 2014, 87, 1089–1096. [Google Scholar] [CrossRef]
- Ferreira, V.; Culleré, L.; Loscos, N.; Cacho, J. Critical aspects of the determination of pentafluorobenzyl derivatives of aldehydes by gas chromatography with electron-capture or mass spectrometric detection: Validation of an optimized strategy for the determination of oxygen-related odor-active aldehydes in wine. J. Chromatogr. A 2006, 1122, 255–265. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Gosetti, F.; Rocío-Bautista, P.; Termopoli, V. Aroma determination in alcoholic beverages: Green MS-based sample preparation approaches. Mass Spectrom. Rev. 2022. [Google Scholar] [CrossRef]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. ACS Symp. Ser. 2011, 1068, 3–31. [Google Scholar] [CrossRef]
- Hart, R.S.; Jolly, N.P.; Ndimba, B.K. Characterisation of hybrid yeasts for the production of varietal Sauvignon blanc wine—A review. J. Microbiol. Methods 2019, 165, 105699. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of Potent Odour-Active Volatile Thiols in Foods and Beverages with a Focus on Wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, L.; Ábrányi-Balogh, P.; Varga, P.R.; Imre, T.; Keserű, G.M. Comparative reactivity analysis of small-molecule thiol surrogates. Bioorganic Med. Chem. 2020, 28, 115357. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Darriet, P.; Dubourdieu, D. Identification de l’acetate de 3-mercaptohexanol, compose a forte odeur de buis, intervenant dans l’arome des vins de Sauvignon. Vitis 1996, 35, 207–210. [Google Scholar]
- Tominaga, T.; Murat, A.M.-L.; Dubourdieu, D. Development of a Method for Analyzing the Volatile Thiols Involved in the Characteristic Aroma of Wines Made from Vitis vinifera L. Cv. Sauvignon Blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Tominaga, T.; Masneuf, I.; Dubourdieu, D. Powerful Aromatic Volatile Thiols in Wines Made from Several Vitis Vinifera Grape Varieties and Their Releasing Mechanism; ACS Publications: Washington, DC, USA, 2003; pp. 314–337. [Google Scholar] [CrossRef]
- Vermeulen, C.; Bailly, S.; Collin, S. Occurrence of polyfunctional thiols in fresh and aged lager beers. Dev. Food Sci. 2006, 43, 245–248. [Google Scholar] [CrossRef]
- Tominaga, T.; Guimbertau, G.; Dubourdieu, D. Role of Certain Volatile Thiols in the Bouquet of Aged Champagne Wines. J. Agric. Food Chem. 2003, 51, 1016–1020. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Ferreira, V.; Cacho, J. Automated analysis of 2-methyl-3-furanthiol and 3-mercaptohexyl acetate at ngL−1 level by headspace solid-phase microextracion with on-fibre derivatisation and gas chromatography–negative chemical ionization mass spectrometric determination. J. Chromatogr. A 2006, 1121, 1–9. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Improved solid-phase extraction procedure for the isolation and in-sorbent pentafluorobenzyl alkylation of polyfunctional mercaptans: Optimized procedure and analytical applications. J. Chromatogr. A 2008, 1185, 9–18. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Quantitative determination of wine polyfunctional mercaptans at nanogram per liter level by gas chromatography–negative ion mass spectrometric analysis of their pentafluorobenzyl derivatives. J. Chromatogr. A 2007, 1146, 242–250. [Google Scholar] [CrossRef]
- Dagan, L.; Reillon, F.; Roland, A.; Schneider, R. Development of a routine analysis of 4-mercapto-4-methylpentan-2-one in wine by stable isotope dilution assay and mass tandem spectrometry. Anal. Chim. Acta 2014, 821, 48–53. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; Kishimoto, T. Development of a Method for the Quantitation of Three Thiols in Beer, Hop, and Wort Samples by Stir Bar Sorptive Extraction with in Situ Derivatization and Thermal Desorption–Gas Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6698–6706. [Google Scholar] [CrossRef]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple Quantitative Determination of Potent Thiols at Ultratrace Levels in Wine by Derivatization and High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS) Analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Chiral analysis of 3-sulfanylhexan-1-ol and 3-sulfanylhexyl acetate in wine by high-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2018, 998, 83–92. [Google Scholar] [CrossRef]
- Mafata, M.; Stander, M.A.; Thomachot, B.; Buica, A. Measuring Thiols in Single Cultivar South African Red Wines Using 4,4-Dithiodipyridine (DTDP) Derivatization and Ultraperformance Convergence Chromatography-Tandem Mass Spectrometry. Foods 2018, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Vichi, S.; Cortés-Francisco, N.; Caixach, J. Analysis of volatile thiols in alcoholic beverages by simultaneous derivatization/extraction and liquid chromatography-high resolution mass spectrometry. Food Chem. 2015, 175, 401–408. [Google Scholar] [CrossRef]
- Román, T.; Tonidandel, T.; Larcher, R.; Celotti, E.; Nicolini, G. Importance of polyfunctional thiols on semi-industrial Gewürztraminer wines and the correlation to technological treatments. Eur. Food Res. Technol. 2017, 244, 379–386. [Google Scholar] [CrossRef]
- Lv, Z.; You, J.; Lu, S.; Sun, W.; Ji, Z.; Sun, Z.; Song, C.; Chen, G.; Li, G.; Hu, N.; et al. Sensitive determination of thiols in wine samples by a stable isotope-coded derivatization reagent d0/d4-acridone-10-ethyl-N-maleimide coupled with high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry analysis. J. Chromatogr. A 2017, 1491, 98–107. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Y.; Tang, B.; Laskin, J.; Roach, P.J.; Chen, H. Study of Highly Selective and Efficient Thiol Derivatization Using Selenium Reagents by Mass Spectrometry. Anal. Chem. 2010, 82, 6926–6932. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Glutathione Peroxidase (GPx)-like Antioxidant Activity of the Organoselenium Drug Ebselen: Unexpected Complications with Thiol Exchange Reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. [Google Scholar] [CrossRef]
- Vichi, S.; Cortés-Francisco, N.; Caixach, J. Determination of volatile thiols in lipid matrix by simultaneous derivatization/extraction and liquid chromatography–high resolution mass spectrometric analysis. Application to virgin olive oil. J. Chromatogr. A 2013, 1318, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Jerí, Y.; Cortés-Francisco, N.; Palacios, O.; Caixach, J. Determination of volatile thiols in roasted coffee by derivatization and liquid chromatography–high resolution mass spectrometric analysis. Food Res. Int. 2014, 64, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Meireles, S.; Brandão, T.; de Pinho, P.G. Optimization of the HS-SPME–GC–IT/MS method using a central composite design for volatile carbonyl compounds determination in beers. Talanta 2013, 117, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in alcoholic beverages: The profiles of perception, constituents, and contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Escudero, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Clues about the Role of Methional As Character Impact Odorant of Some Oxidized Wines. J. Agric. Food Chem. 2000, 48, 4268–4272. [Google Scholar] [CrossRef]
- Bueno, M.; Marrufo-Curtido, A.; Carrascon, V.; Fernandez-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and Accumulation of Acetaldehyde and Strecker Aldehydes during Red Wine Oxidation. Front. Chem. 2018, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Ugliano, M. Oxygen Contribution to Wine Aroma Evolution during Bottle Aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef]
- Ferreira, A.C.S.; Barbe, J.-C.; Bertrand, A. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone: A Key Odorant of the Typical Aroma of Oxidative Aged Port Wine. J. Agric. Food Chem. 2003, 51, 4356–4363. [Google Scholar] [CrossRef]
- Escudero, A.; Asensio, E.; Cacho, J.; Ferreira, V. Sensory and chemical changes of young white wines stored under oxygen. An assessment of the role played by aldehydes and some other important odorants. Food Chem. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and identification of odorants generated in wine during its oxidation: A gas chromatography-olfactometric study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine—Diacetyl—Desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef]
- Gabrielli, M.; Fracassetti, D.; Romanini, E.; Colangelo, D.; Tirelli, A.; Lambri, M. Oxygen-induced faults in bottled white wine: A review of technological and chemical characteristics. Food Chem. 2020, 348, 128922. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine science in the metabolomics era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Jackowetz, J.; de Orduña, R.M. Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control 2013, 32, 687–692. [Google Scholar] [CrossRef]
- Mayr, C.M.; Capone, D.L.; Pardon, K.H.; Black, C.A.; Pomeroy, D.; Francis, I.L. Quantitative Analysis by GC-MS/MS of 18 Aroma Compounds Related to Oxidative Off-Flavor in Wines. J. Agric. Food Chem. 2015, 63, 3394–3401. [Google Scholar] [CrossRef]
- Aly, A.A.; Górecki, T. Green Approaches to Sample Preparation Based on Extraction Techniques. Molecules 2020, 25, 1719. [Google Scholar] [CrossRef]
- Olivero, S.J.P.; Trujillo, J.P.P. A New Method for the Determination of Carbonyl Compounds in Wines by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography−Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 12976–12985. [Google Scholar] [CrossRef]
- Schmarr, H.-G.; Potouridis, T.; Ganß, S.; Sang, W.; Köpp, B.; Bokuz, U.; Fischer, U. Analysis of carbonyl compounds via headspace solid-phase microextraction with on-fiber derivatization and gas chromatographic–ion trap tandem mass spectrometric determination of their O-(2,3,4,5,6-pentafluorobenzyl)oxime derivatives. Anal. Chim. Acta 2008, 617, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Araújo, A.M.; Rogerson, F.; Vasconcelos, I.; De Freitas, V.; de Pinho, P.G. Development and optimization of a HS-SPME-GC-MS methodology to quantify volatile carbonyl compounds in Port wines. Food Chem. 2019, 270, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Zapata, J.; Ferreira, V. Simultaneous determination of free and bonded forms of odor-active carbonyls in wine using a headspace solid phase microextraction strategy. J. Chromatogr. A 2014, 1369, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dennenlöhr, J.; Thörner, S.; Maxminer, J.; Rettberg, N. Analysis of Selected Staling Aldehydes in Wort and Beer by GC-EI-MS/MS Using HS-SPME with On-Fiber Derivatization. J. Am. Soc. Brew. Chem. 2020, 78, 284–298. [Google Scholar] [CrossRef]
- Hernandes, K.C.; Souza-Silva, E.A.; Assumpção, C.F.; Zini, C.A.; Welke, J.E. Validation of an analytical method using HS-SPME-GC/MS-SIM to assess the exposure risk to carbonyl compounds and furan derivatives through beer consumption. Food Addit. Contam. Part A 2019, 36, 1808–1821. [Google Scholar] [CrossRef]
- Peng, Z.; Luo, Y.; Song, C.; Zhang, Y.; Sun, S.; Yu, A.; Zhang, W.; Zhao, W.; Zhang, S.; Xie, J. A novel methodology and strategy to detect low molecular aldehydes in beer based on charged microdroplet driving online derivatization and high resolution mass spectrometry. Food Chem. 2022, 383, 132380. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L. Chemical derivatization in LC-MS-based metabolomics study. TrAC Trends Anal. Chem. 2020, 131, 115988. [Google Scholar] [CrossRef]
- Tie, C.; Hu, T.; Jia, Z.-X.; Zhang, J.-L. Derivatization Strategy for the Comprehensive Characterization of Endogenous Fatty Aldehydes Using HPLC-Multiple Reaction Monitoring. Anal. Chem. 2016, 88, 7762–7768. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bioprotection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Thompson Witrick, K.; Duncan, S.E.; Hurley, K.E.; O’Keefe, S.F. Acid and Volatiles of Commercially-Available Lambic Beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Troton, D.; Charpentier, M. Evolution of the lipid contents of Champagne wine during the second fermentation of Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1989, 40, 175–182. [Google Scholar]
- Wang, X.; Xu, X.; Wang, Q.; Huang, Z.; He, J.; Qiu, T. Fatty Acid Methyl Ester Synthesis through Transesterification of Palm Oil with Methanol in Microchannels: Flow Pattern and Reaction Kinetics. Energy Fuels 2020, 34, 3628–3639. [Google Scholar] [CrossRef]
- Gallart, M.; Francioli, S.; Viu-Marco, A.; López-Tamames, E.; Buxaderas, S. Determination of free fatty acids and their ethyl esters in musts and wines. J. Chromatogr. A 1997, 776, 283–291. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Identification of Uncommon Plant Metabolites Based on Calculation of Elemental Compositions Using Gas Chromatography and Quadrupole Mass Spectrometry. Anal. Chem. 2000, 72, 3573–3580. [Google Scholar] [CrossRef]
- Borden, S.A.; Damer, H.N.; Krogh, E.T.; Gill, C.G. Direct quantitation and characterization of fatty acids in salmon tissue by condensed phase membrane introduction mass spectrometry (CP-MIMS) using a modified donor phase. Anal. Bioanal. Chem. 2018, 411, 291–303. [Google Scholar] [CrossRef]
- Gullberg, J.; Jonsson, P.; Nordström, A.; Sjöström, M.; Moritz, T. Design of experiments: An efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal. Biochem. 2004, 331, 283–295. [Google Scholar] [CrossRef]
- Khakimov, B.; Motawia, M.S.; Bak, S.; Engelsen, S.B. The use of trimethylsilyl cyanide derivatization for robust and broad-spectrum high-throughput gas chromatography–mass spectrometry based metabolomics. Anal. Bioanal. Chem. 2013, 405, 9193–9205. [Google Scholar] [CrossRef]
- Khakimov, B.; Bakhytkyzy, I.; Fauhl-Hassek, C.; Engelsen, S.B. Non-volatile molecular composition and discrimination of single grape white wines of Chardonnay, Riesling, Sauvignon Blanc and Silvaner using untargeted GC-MS analysis. Food Chem. 2021, 369, 130878. [Google Scholar] [CrossRef]
- Ripari, V.; Tomassetti, M.; Cecchi, T.; Berardi, E. First Study of Sourdough Beer Aging Via the Chemical Fingerprint of Volatile Markers. Food Anal. Methods 2019, 12, 2459–2468. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadawallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of Two Extraction Techniques, Solid-Phase Microextraction Versus Continuous Liquid-Liquid Extraction/Solvent-Assisted Flavor Evaporation, for the Analysis of Flavor Compounds in Gueuze Lambic Beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef] [PubMed]
- Witrick, K.; Pitts, E.R.; O’Keefe, S.F. Analysis of Lambic Beer Volatiles during Aging Using Gas Chromatography–Mass Spectrometry (GCMS) and Gas Chromatography–Olfactometry (GCO). Beverages 2020, 6, 31. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O.; Faria, M.A.; Ferreira, I.V.; Ferreira, M.A. Quantification of Organic Acids in Grape Musts and Port Wines Cuantificación De Ácidos Orgánicos En Mostos Y Vinos De Oporto Cuantificación De Ácidos Orgánicos En Mostos E Viños De Porto. Cienc. Tecnol. Aliment. 2002, 3, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Badoud, R.; Pratz, G. Improved high-performance liquid chromatographic analysis of some carboxylic acids in food and beverages as their p-nitrobenzyl esters. J. Chromatogr. A 1986, 360, 119–136. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Lan, Y.-B.; Zhu, B.-Q.; Xiang, X.-F.; Duan, C.-Q.; Shi, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography-mass spectrometry. J. Sci. Food Agric. 2017, 98, 104–112. [Google Scholar] [CrossRef]
- Ghaste, M.; Narduzzi, L.; Carlin, S.; Vrhovsek, U.; Shulaev, V.; Mattivi, F. Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 2015, 188, 309–319. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Different Woods in Cooperage for Oenology: A Review. Beverages 2018, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Schieber, A.; Wüst, M. Volatile Phenols—Important Contributors to the Aroma of Plant-Derived Foods. Molecules 2020, 25, 4529. [Google Scholar] [CrossRef]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile profile of Madeira wines submitted to traditional accelerated ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Karumanchiri, A.A.; Goldberg, D.M. A Multiresidue Derivatization Gas Chromatographic Assay for Fifteen Phenolic Constituents with Mass Selective Detection. Anal. Chem. 1997, 69, 4405–4409. [Google Scholar] [CrossRef]
- Minuti, L.; Pellegrino, R.M.; Tesei, I. Simple extraction method and gas chromatography–mass spectrometry in the selective ion monitoring mode for the determination of phenols in wine. J. Chromatogr. A 2006, 1114, 263–268. [Google Scholar] [CrossRef]
- Minuti, L.; Pellegrino, R. Determination of phenolic compounds in wines by novel matrix solid-phase dispersion extraction and gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1185, 23–30. [Google Scholar] [CrossRef]
- Allen, D.; Bui, A.D.; Cain, N.; Rose, G.; Downey, M. Analysis of free and bound phenolics in wine and grapes by GC–MS after automated SPE. Anal. Bioanal. Chem. 2013, 405, 9869–9877. [Google Scholar] [CrossRef]
- Cravero, M.C. Musty and Moldy Taint in Wines: A Review. Beverages 2020, 6, 41. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Optimization of headspace sampling using solid-phase microextraction for chloroanisoles in cork stoppers and gas chromatography-ion-trap tandem mass spectrometric analysis. J. Sep. Sci. 2003, 26, 369–375. [Google Scholar] [CrossRef]
- Pizarro, C.; Pérez-Del-Notario, N.; González-Sáiz, J. Optimisation of a headspace solid-phase microextraction with on-fiber derivatisation method for the direct determination of haloanisoles and halophenols in wine. J. Chromatogr. A 2007, 1143, 26–35. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Ardiana, F.; Indrayanto, G. Ezetimibe, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 36. [Google Scholar] [CrossRef]
- Rocha, L.P.; Cabral, L.M.; Pinto, E.C.; De Sousa, V.P. Ezetimibe: A Review of Analytical Methods for the Drug Substance, Pharmaceutical Formulations and Biological Matrices. Crit. Rev. Anal. Chem. 2022, 52, 1078–1093. [Google Scholar] [CrossRef]


| Article | Year | Matrix | Ext. Volume | Ext. Technique | Der. Agent | Instrumentation | Pro & Cons |
|---|---|---|---|---|---|---|---|
| [86] | 2008 | Wine | 10 mL | HS-OFD-SPME – 65 µm PDMS/DVB | PFBHA | GC-IT-MS | + Broad range of carbonyls, no salt addition − Large sample volume, no real application presented |
| [85] | 2010 | Wine | 2 mL | HS-ISD-SPME – 50/30 µm DVB/CAR/PDMS | PFBHA | GC-IT-MS | + Performance, robust validation, automatable − Limited range of carbonyls |
| [87] | 2019 | Wine | 2 mL | HS-ISD-SPME – 65 µm PDMS/DVB | PFBHA | GC-MS/MS | + Wide range of VCCs, robust validation, efficient, reliable − No diketone was quantified, used in analyte-rich matrix |
| [68] | 2013 | Beer | 2 mL | HS-ISD-SPME – 65 µm PDMS/DVB | PFBHA | GC-IT-MS | + Strong validation, efficient, reliable − Proof of application with a reduced number of samples |
| [89] | 2019 | Beer | 1 mL | HS-SPME – 50/30 µm DVB/CAR/PDMS | PFBHA | GC-MS | + Wide range of polar analytes − Long extraction time, reduced productivity |
| [90] | 2022 | Beer | - | - | DNPH | LC-HRMS | + No sample prep, huge innovation − Performance under HS-SPME with PFBHA |
| Article | Year | Matrix | Ext. Volume | Ext. Technique | Der. Agent | Instrumentation | Pro & Cons |
|---|---|---|---|---|---|---|---|
| [98] | 1989 | Spark. wine | 1 mL | 6 mL MeOH + 2.5% v/v H2SO4 | MeOH + acid catalysis—70 °C, 90 min | GC-FID | + Easy, no expensive agent required − Unsuitable for free fatty acids fraction |
| [100] | 1997 | Wine, must | 50 mL | 3 × 5 mL hexane + concentration under N2 stream | 1 mL MeOH + 3% v/v H2SO4 Room T°, 180 min | GC-FID | + Allows determination of free fraction − Many steps, complex, time-consuming |
| [111] | 2018 | Red wine | 100 µL | Lyophilization + drying/dissolution | 70 µL MSTFA—37 °C, 30 min | GC-EI-MS | + Miniaturized volumes, suitable for other compounds − Lyophilization needed, many steps |
| [105] | 2022 | White wine | 5 µL | Drying/dissolution, methoxymation | 40 μL TMSCN—40 °C, 40 min | GC-EI-MS | + Miniaturized, negligible waste, efficient − Time-consuming, many steps |
| [109] | 2002 | Fortified wine, must | 5 mL | Double cationic resins clean-up | 500 µL NBDI (10 g·L−1)—80 °C, 60 min | HPLC-UV | + Based on HPLC-UV, robust, cheap − Time-consuming, many steps |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piergiovanni, M.; Termopoli, V. Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario. Chemistry 2022, 4, 1679-1695. https://doi.org/10.3390/chemistry4040109
Piergiovanni M, Termopoli V. Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario. Chemistry. 2022; 4(4):1679-1695. https://doi.org/10.3390/chemistry4040109
Chicago/Turabian StylePiergiovanni, Maurizio, and Veronica Termopoli. 2022. "Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario" Chemistry 4, no. 4: 1679-1695. https://doi.org/10.3390/chemistry4040109
APA StylePiergiovanni, M., & Termopoli, V. (2022). Derivatization Strategies in Flavor Analysis: An Overview over the Wine and Beer Scenario. Chemistry, 4(4), 1679-1695. https://doi.org/10.3390/chemistry4040109






