A Novel Highly Divergent Strain of Cell Fusing Agent Virus (CFAV) in Mosquitoes from the Brazilian Amazon Region
Abstract
1. Introduction
2. Materials and Methods
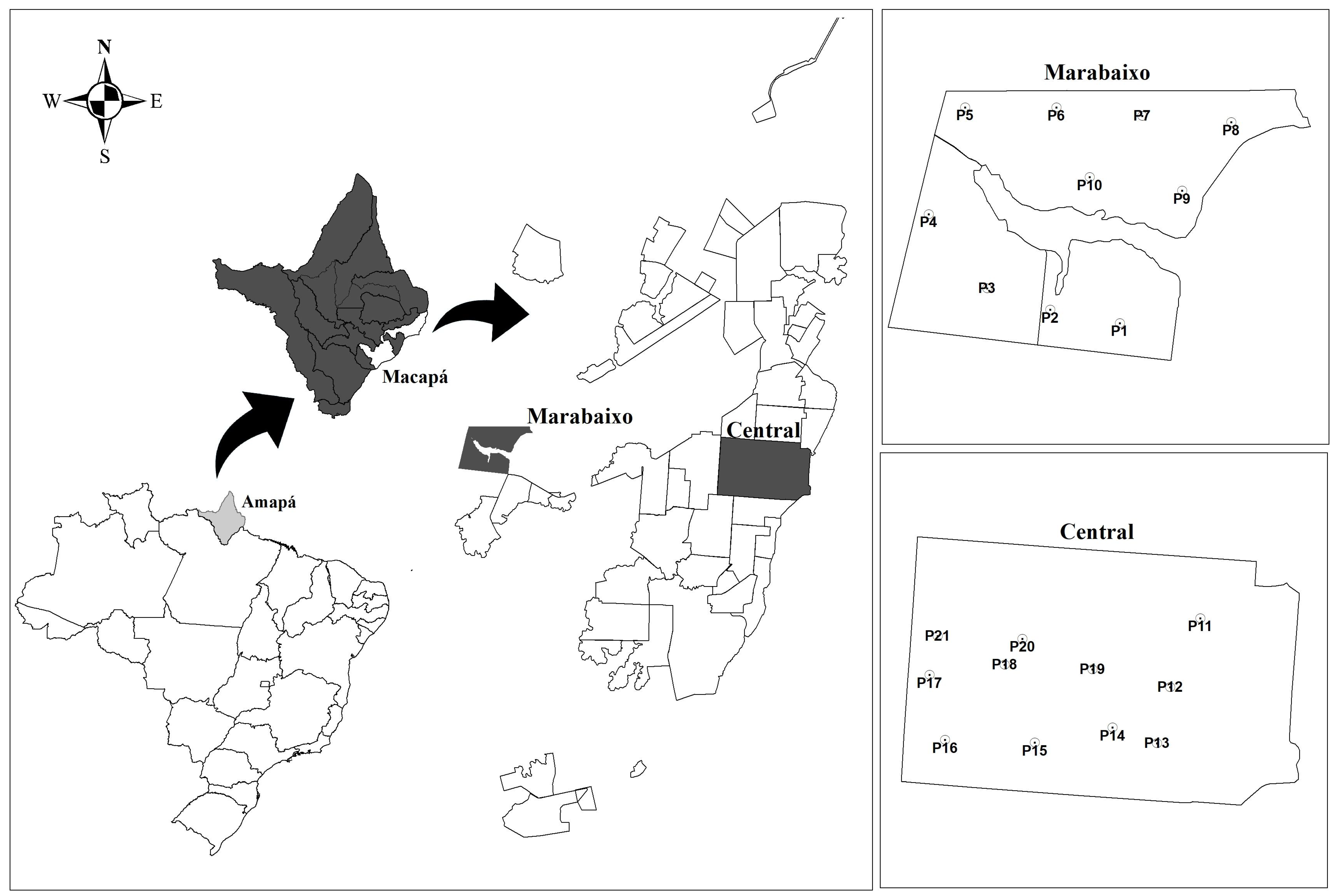
2.1. Location of Sample Collection
2.2. Collection and Identification of Mosquitoes
2.3. Sample Processing and Next Generation Sequencing (NGS)
2.4. Phylogenetic Analyses
3. Results
3.1. Phylogenetic Analysis of Genomes
3.2. Phylogenetic Trees of CFAV Envelope (E) and NS5 Genes
3.3. Genetic Distances of the Brazilian Isolate Macapá 02
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Higgs, S. The Discovery of Arthropod-Specific Viruses in Hematophagous Arthropods: An Open Door to Understanding the Mechanisms of Arbovirus and Arthropod Evolution? Annu. Rev. Entomol. 2018, 7, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Stollar, V.; Thomas, V.L. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 1975, 64, 367–377. [Google Scholar] [CrossRef]
- Sang, R.C.; Gichogo, A.; Gachoya, J.; Dunster, M.D.; Ofula, V.; Hunt, A.R.; Crabtree, M.B.; Miller, B.R.; Dunster, L.M. Isolation of a new flavivirus related to cell fusing agent virus (CFAV) from field-collected flood-water Aedes mosquitoes sampled from a dambo in central Kenya. Arch. Virol. 2003, 148, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.B.; Sang, R.C.; Stollar, V.; Dunster, L.M.; Miller, B.R. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch. Virol. 2003, 148, 1095–1118. [Google Scholar] [CrossRef] [PubMed]
- Cammisa-Parks, H.; Cisar, L.A.; Kane, A.; Stollar, V. The complete nucleotide sequence of cell fusing agent (CFA): Homology between the nonstructural proteins encoded by CFA and the nonstructural proteins encoded by arthropod-borne flaviviruses. Virology 1992, 189, 511–524. [Google Scholar] [CrossRef]
- Cook, S.; Bennett, S.N.; Holmes, E.C.; De Chesse, R.; Moureau, G.; de Lamballerie, X. Isolation of a new strain of the flavivirus cell fusing agent virus in a natural mosquito population from Puerto Rico. J. Gen. Virol. 2006, 87, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Satho, T.; Eshita, Y.; Sakai, K.; Kotaki, A.; Takasaki, T.; Rongsriyam, Y.; Komalamisra, N.; Srisawat, R.; Lapcharoen, P.; et al. Rapid determination of viral RNA sequences in mosquitoes collected in the field. J. Virol. Methods 2007, 146, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Thongrungkiat, S.; Ramasoota, P.; Konishi, E. Genetic and evolutionary analysis of cell-fusing agent virus based on Thai strains isolated in 2008 and 2012. Infect. Genet. Evol. 2013, 19, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Isawa, H.; Tsuda, Y.; Sawabe, K.; Kobayashi, M. Isolation and characterization of a new insect flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology 2009, 391, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Gomez, F.; Lopez-Lemus, A.U.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Newton-Sanchez, A.O.; Chavez-Flores, E.; Delgado-Enciso, I. Detection of sequences from a potentially novel strain of cell fusing agent virus in Mexican Stegomyia (Aedes) aegypti mosquitoes. Arch. Virol. 2011, 156, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, H.; Higa, Y.; Futami, K.; Lutiali, P.A.; Njenga, S.M.; Nabeshima, T.; Minakawa, N. Mosquito arbovirus survey in selected areas of Kenya: Detection of insect-specific virus. Trop. Med. Health 2018, 46, 19. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Isawa, H.; Tsuda, Y.; Yano, K.; Sasaki, T.; Yuda, M.; Takasaki, T.; Kobayashi, M.; Sawabe, K. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology 2007, 359, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Morales-Betoulle, M.E.; Pineda, M.M.; Sosa, S.M.; Panella, N.; Cordon-Rosales, C.; Komar, N.; Powers, A.; Johnson, B.W. Culex flavivirus isolates from mosquitoes in Guatemala. J. Med. Entomol. 2008, 45, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Ale, J.A.; Loroño-Pino, M.A.; Garcia-Rejon, J.E.; Hovav, E.; Powers, A.M.; Lin, M.; Dorman, K.S.; Platt, K.B.; Bartholomay, L.C.; Soto, V.; et al. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Ale, J.A.; Loroño-Pino, M.A.; Garcia-Rejon, J.E.; Soto, V.; Lin, M.; Staley, M.; Dorman, K.S.; Bartholomay, L.C.; Hovav, E.; Blitvich, B.J. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector Borne Zoon. Dis. 2010, 10, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Saiyasombat, R.; Dorman, K.S.; Garcia-Rejon, J.E.; Loroño-Pino, M.A.; Farfan-Ale, J.A.; Blitvich, B.J. Isolation and sequence analysis of Culex flavivirus from Culex interrogator and Culex quinquefasciatus in the Yucatan Peninsula of Mexico. Arch. Virol. 2010, 155, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Guzman, H.; Bueno, R., Jr.; Dennett, J.A.; Auguste, A.J.; Carrington, C.V.; Popov, V.L.; Weaver, S.C.; Beasley, D.W.; Tesh, R.B. Characterization of Culex flavivirus (Flaviviridae) strains isolated from mosquitoes in the United States and Trinidad. Virology 2009, 386, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Lin, M.; Dorman, K.S.; Soto, V.; Hovav, E.; Tucker, B.J.; Staley, M.; Platt, K.B.; Bartholomay, L.C. Genomic sequence and phylogenetic analysis of Culex flavivirus, an insect-specific flavivirus, isolated from Culex pipiens (Diptera: Culicidae) in Iowa. J. Med. Entomol. 2009, 46, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Eisen, L.; Moore, C.G.; Blair, C.D. Insect-specific flaviviruses from Culex mosquitoes in Colorado, with evidence of vertical transmission. Am. J. Trop. Med. Hyg. 2011, 85, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Crockett, R.K.; Burkhalter, K.; Mead, D.; Kelly, R.; Brown, J.; Varnado, W.; Roy, A.; Horiuchi, K.; Biggerstaff, B.J.; Miller, B.; et al. Culex flavivirus and West Nile virus in Culex quinquefasciatus populations in the southeastern United States. J. Med. Entomol. 2012, 49, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.; Cerutti, F.; Anderson, T.K.; Hamer, G.L.; Walker, E.D.; Kitron, U.D.; Ruiz, M.O.; Brawn, J.D.; Goldberg, T.L. Culex flavivirus and West Nile virus mosquito coinfection and positive ecological association in Chicago, United States. Vector Borne Zoon. Dis. 2011, 11, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Moureau, G.; Harbach, R.E.; Mukwaya, L.; Goodger, K.; Ssenfuka, F.; Gould, E.; Holmes, E.C.; de Lamballerie, X. Isolation of a novel species of flavivirus and new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J. Gen. Virol. 2009, 90, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.C.; Mondini, A.; dos Santos Santana, V.; Yonamine, P.T.; Neto, F.C.; de Andrade Zanotto, P.M.; Nogueira, M.L. First Identification of Culex flavivirus (Flaviviridae) in Brazil. Intervirology 2012, 55, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.N.; de Paula, M.B.; Araújo, A.B.; Gonçalves, E.F.; Romano, C.M.; Natal, D.; dos Santos Malafronte, R.; Marrelli, M.T.; Levi, J.E. Detection of Culex flavivirus and Aedes flavivirus nucleotide sequences in mosquitoes from parks in the city of São Paulo, Brazil. Acta Trop. 2016, 157, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Fu, S.; Liu, G.; Liu, H.; Gao, X.; Song, L.; Rayner, S.; Xu, A.; Liang, G. Isolation and identification of a distinct strain of Culex flavivirus from mosquitoes collected in Mainland China. Virol. J. 2012, 9, e73. [Google Scholar] [CrossRef]
- An, S.Y.; Liu, J.S.; Ren, Y.; Wang, Z.S.; Han, Y.; Ding, J.; Guo, J.Q. Isolation of the Culex flavivirus from mosquitoes in Liaoning Province, China. Bing Du Xue Bao 2012, 28, 511–516. [Google Scholar] [PubMed]
- Fang, Y.; Zhang, Y.; Zhou, Z.B.; Shi, W.Q.; Xia, S.; Li, Y.Y.; Wu, J.T.; Liu, Q.; Lin, G.Y. Co-circulation of Aedes flavivirus, Culex flavivirus, and Quang Binh virus in Shanghai, China. Infect. Dis. Poverty 2018, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lin, J.W.; Fan, Y.C.; Tu, W.C.; Chang, G.J.; Chiou, S.S. First detection of the Africa/Caribbean/Latin American subtype of Culex flavivirus in Asian country, Taiwan. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, S.; Fabbri, C.M.; Garcia, J.B.; Rondan, J.C.; Gardenal, N.; Calderon, G.E.; Enria, D.A.; Levis, S.M. New strains of Culex flavivirus isolated in Argentina. J. Med. Entomol. 2014, 51, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Kyaw Kyaw, A.; Tun, M.M.N.; Buerano, C.C.; Nabeshima, T.; Sakaguchi, M.; Ando, T.; Inoue, S.; Mya, Y.Y.; Hayasaka, D.; Thu, H.M.; et al. Isolation and genomic characterization of Culex flaviviruses from mosquitoes in Myanmar. Virus Res. 2018, 247, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bittar, C.; Machado, D.C.; Vedovello, D.; Ullmann, L.S.; Rahal, P.; Araújo Junior, J.P.; Nogueira, M.L. Genome sequencing and genetic characterization of Culex flavirirus (CxFV) provides new information about its genotypes. Virol. J. 2016, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Available online: https://cidades.ibge.gov.br/brasil/ap/macapa/panorama (accessed on 3 September 2018).
- Natal, D.; Marucci, D. Aparelho de sucção tipo aspirador para captura de mosquitos. Rev Saude Publica 1984, 18, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Filho, W.S.; Prates Júnior, P.H.S. Collection Techniques and Insects Identification, 2nd ed.; EDIPUCRS: Porto Alegre, Brazil, 2005. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil, 1st ed.; Fiocruz: Rio de Janeiro, Brazil, 1994; ISBN 85-85676-03-5. [Google Scholar]
- Li, L.; Deng, X.; Mee, E.T.; Collot-Teixeira, S.; Anderson, R.; Schepelmann, S.; Minor, P.D.; Delwart, E. Comparing viral metagenomics methods using a highly multiplexed human viral pathogens reagent. J. Virol. Methods 2015, 213, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.C.; Thézé, J.; Komninakis, S.C.V.; Sanz-Duro, R.L.; Felinto, M.R.L.; Moura, L.C.C.; Barroso, I.M.O.; Santos, L.E.C.; Nunes, M.A.L.; Moura, A.A.; et al. Spread of chikungunya virus East/Central/South African genotype in Northeast Brazil. Emerg. Infect. Dis. 2017, 23, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next generation sequencing data. Nucleic Acids Res. 2015, 43, e46. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. Jmodeltest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Cywinska, A.; Hunter, F.F.; Hebert, P.D. Identifying Canadian mosquito species through DNA barcodes. Med. Vet. Entomol. 2006, 20, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Vadivalagan, C.; Karthika, P.; Panneerselvam, C.; Paulpandi, M.; Subramaniam, J.; Wei, H.; Aziz, A.T.; Alsalhi, M.S.; Devanesan, S.; et al. DNA barcoding and molecular evolution of mosquito vectors of medical and veterinary importance. Parasitol. Res. 2016, 115, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, E.V.; Angerami, R.N.; Luna, E.J.A. What to expect from the 2017 yellow fever outbreak in Brazil? Rev. Inst. Med. Trop. 2017, 59, e17. [Google Scholar] [CrossRef] [PubMed]
- Goldani, L.Z. Yellow fever outbreak in Brazil, 2017. Braz. J. Infect. Dis. 2017, 21, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Depoux, A.; Philibert, A.; Rabier, S.; Philippe, H.J.; Fontanet, A.; Flahault, A. A multi-faceted pandemic: A review of the state of knowledge on the Zika virus. Public Health Rev. 2018, 39, 10. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | Taxonomic Category | Collection Date (Epidemiological Week) | Collection Place | Neighborhood | Near-Complete Genome Sequence |
|---|---|---|---|---|---|
| Macapá 01 | Ae. aegypti | 10/2017 | P15 | Central | CFAV |
| Macapá 02 | Culex sp. | 10/2017 | P2 | Marabaixo | CFAV |
| Macapá 04 | Culex sp. | 10/2017 | P12 | Central | CFAV |
| Macapá 05 | Culex sp. | 6/2017 | P4 | Marabaixo | CxFV |
| Macapá 06 | Culex sp. | 6/2017 | P8 | Marabaixo | CxFV |
| Comparisons | Genome Region | |
|---|---|---|
| E | NS5 | |
| NC001564 (Galveston) versus Macapá 02 | 0.053 ± 0.008 | 0.049 ± 0.004 |
| KU936054 (Aag2 Bristol) versus Macapá 02 | 0.050 ± 0.007 | 0.056 ± 0.003 |
| AB813750 (E175-08) versus Macapá 02 | 0.055 ± 0.009 | 0.057 ± 0.004 |
| Macapá 01 versus Macapá 02 | 0.049 ± 0.008 | 0.055 ± 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natal Fernandes, L.; De Moura Coletti, T.; Julio Costa Monteiro, F.; Octavio da Silva Rego, M.; Soares D’Athaide Ribeiro, E.; De Oliveira Ribeiro, G.; Dos Santos Souza Marinho, R.; Vasconcelos Komninakis, S.; S. Witkin, S.; Deng, X.; et al. A Novel Highly Divergent Strain of Cell Fusing Agent Virus (CFAV) in Mosquitoes from the Brazilian Amazon Region. Viruses 2018, 10, 666. https://doi.org/10.3390/v10120666
Natal Fernandes L, De Moura Coletti T, Julio Costa Monteiro F, Octavio da Silva Rego M, Soares D’Athaide Ribeiro E, De Oliveira Ribeiro G, Dos Santos Souza Marinho R, Vasconcelos Komninakis S, S. Witkin S, Deng X, et al. A Novel Highly Divergent Strain of Cell Fusing Agent Virus (CFAV) in Mosquitoes from the Brazilian Amazon Region. Viruses. 2018; 10(12):666. https://doi.org/10.3390/v10120666
Chicago/Turabian StyleNatal Fernandes, Licia, Thaís De Moura Coletti, Fred Julio Costa Monteiro, Marlisson Octavio da Silva Rego, Edcelha Soares D’Athaide Ribeiro, Geovani De Oliveira Ribeiro, Robson Dos Santos Souza Marinho, Shirley Vasconcelos Komninakis, Steven S. Witkin, Xutao Deng, and et al. 2018. "A Novel Highly Divergent Strain of Cell Fusing Agent Virus (CFAV) in Mosquitoes from the Brazilian Amazon Region" Viruses 10, no. 12: 666. https://doi.org/10.3390/v10120666
APA StyleNatal Fernandes, L., De Moura Coletti, T., Julio Costa Monteiro, F., Octavio da Silva Rego, M., Soares D’Athaide Ribeiro, E., De Oliveira Ribeiro, G., Dos Santos Souza Marinho, R., Vasconcelos Komninakis, S., S. Witkin, S., Deng, X., Delwart, E., Cerdeira Sabino, E., Leal, É., & Charlys da Costa, A. (2018). A Novel Highly Divergent Strain of Cell Fusing Agent Virus (CFAV) in Mosquitoes from the Brazilian Amazon Region. Viruses, 10(12), 666. https://doi.org/10.3390/v10120666







